Abstract
OBJECTIVE
Reductions in insulin sensitivity in conjunction with muscle mitochondrial dysfunction have been reported to occur in many conditions including aging. The objective was to determine whether insulin resistance and mitochondrial dysfunction are directly related to chronological age or are related to age-related changes in body composition.
RESEARCH DESIGN AND METHODS
Twelve young lean, 12 young obese, 12 elderly lean, and 12 elderly obese sedentary adults were studied. Insulin sensitivity was measured by a hyperinsulinemic-euglycemic clamp, and skeletal muscle mitochondrial ATP production rates (MAPRs) were measured in freshly isolated mitochondria obtained from vastus lateralis biopsy samples using the luciferase reaction.
RESULTS
Obese participants, independent of age, had reduced insulin sensitivity based on lower rates of glucose infusion during a hyperinsulinemic-euglycemic clamp. In contrast, age had no independent effect on insulin sensitivity. However, the elderly participants had lower muscle MAPRs than the young participants, independent of obesity. Elderly participants also had higher levels inflammatory cytokines and total adiponectin. In addition, higher muscle MAPRs were also noted in men than in women, whereas glucose infusion rates were higher in women.
CONCLUSIONS
The results demonstrate that age-related reductions in insulin sensitivity are likely due to an age-related increase in adiposity rather than a consequence of advanced chronological age. The results also indicate that an age-related decrease in muscle mitochondrial function is neither related to adiposity nor insulin sensitivity. Of interest, a higher mitochondrial ATP production capacity was noted in the men, whereas the women were more insulin sensitive, demonstrating further dissociation between insulin sensitivity and muscle mitochondrial function.
As the population ages, the prevalence of several chronic health problems such as obesity, type 2 diabetes, and cardiovascular disease has risen. Insulin resistance is recognized as a key factor contributing to the development of both type 2 diabetes and its related cardiometabolic disorders (1,2). Insulin resistance and impaired glucose tolerance are commonly observed phenomena among elderly adults. For example, the glucose excursion postprandially is substantially greater and remains elevated longer in nondiabetic elderly adults than in nondiabetic younger adults, which is indicative of age-related declines in insulin sensitivity and glucose tolerance (3). Aging is associated with detrimental changes in body composition, which persists even when elderly adults are matched to younger adults for BMI (4). Adiposity, in particular abdominal adiposity, is well accepted as a determinant of insulin resistance and therefore may be a key mediator for the development of age-related insulin resistance. Despite an inverse relationship between age and insulin sensitivity (4,5), it remains contentious whether chronological age is a primary determinant of insulin resistance or whether age-related elevations in adiposity and/or physical inactivity are the primary causes of age-related insulin resistance (6,7).
Aging is also associated with reductions in skeletal muscle mitochondrial function. In particular, skeletal muscle mitochondrial ATP production rates (MAPRs) in elderly people are reduced in vivo in the resting state (8) as well as in vitro in the maximally stimulated state (3). These age-related reductions in MAPRs have also been associated with concomitant reductions in skeletal muscle mitochondrial enzyme activities (9), protein synthesis and expression (3,10), and mtDNA abundance in humans (3,11) and rodents (12). Of interest, insulin resistance is closely associated with skeletal muscle mitochondrial dysfunction in some (3,5,13,14) but not in all conditions (15,16). This close association between muscle mitochondrial dysfunction and insulin resistance has led to the hypothesis that mitochondrial dysfunction could be the basis of insulin resistance (5). Another equally plausible hypothesis is that insulin resistance causes muscle mitochondrial dysfunction (16,17). In support of the later hypothesis is the demonstration that, in type 2 diabetic people, muscle MAPR fails to increase in response to physiologically high insulin levels, unlike in nondiabetic people (18). However, it should be recognized that the association between insulin resistance and mitochondrial dysfunction are not consistent. For example, we recently reported that Asian Indians in comparison with Northern European Americans matched for age, sex, and BMI are severely insulin resistant, while having higher muscle MAPR and mitochondrial DNA copy numbers (19). Furthermore, a recent report also indicated that while a low-calorie diet substantially enhanced insulin sensitivity (e.g., ∼30% increase in insulin-stimulated glucose disposal), it failed to increase skeletal muscle mitochondrial function in the absence of exercise (19). In contrast, in rats, a high-fat diet caused insulin resistance while enhancing mitochondrial biogenesis (15). Together, the results from the above studies indicate that the close association between insulin sensitivity and muscle mitochondrial function can be uncoupled, arguing against the hypothesis that insulin resistance causes muscle mitochondrial dysfunction or vice versa.
Age is not only associated with insulin resistance and muscle mitochondrial dysfunction but is also associated with changes in body composition, which likely contribute to the development of age-related insulin resistance (20). We therefore sought to determine whether the changes in insulin sensitivity and muscle mitochondrial function are secondary to age-related changes in body composition rather than being directly related to chronological age. We studied 48 lean and obese, young and elderly men and women. Insulin sensitivity was measured using hyperinsulinemic-euglycemic clamp and skeletal muscle mitochondrial function by measuring MAPRs from freshly prepared mitochondria obtained from muscle biopsy samples. The studies demonstrated the impact of not only age and body weight, but also sex on insulin sensitivity and muscle mitochondrial function in humans.
RESEARCH DESIGN AND METHODS
Twelve elderly lean (65–80 years and BMI <25 kg/m2), 12 elderly obese (65–80 years and BMI >30 kg/m2), 12 young lean (18–30 years and BMI <25 kg/m2), and 12 young obese (18–30 years and BMI >30 kg/m2) healthy sedentary adults completed this cross-sectional study (Table 1). There were six men and six women in each group. Participants underwent an initial screening that included a medical history, physical examination, resting electrocardiogram, incremental treadmill test, and biochemical tests of renal, hepatic, hematologic, and metabolic function. Participants with evidence of diabetes, cardiovascular disease, thyroid dysfunction, or a history of alcohol or substance abuse were excluded. Participants who reported using β-blockers were excluded. A list of medications is reported in supplemental Table 1 (available in an online-only appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0591/DC1). Participants who reported exercising ≥2 days per week or whose fasting glucose levels were ≥6.1 mmol/l (≥110 mg/dl) were also excluded. Activity levels were confirmed with a leisure-time activity questionnaire. The study was approved by the institutional review board of the Mayo Foundation, and all participants gave written informed consent.
TABLE 1.
Participant characteristics
| Young lean (n = 12) | Young obese (n = 12) | Elderly lean (n = 12) | Elderly obese (n = 12) |
P |
||||
|---|---|---|---|---|---|---|---|---|
| Overall | Age | Sex | BMI | |||||
| Age (years) | 22.0 (19, 29) | 24.5 (21, 28) | 67.5 (65, 80) | 70.5 (65, 80) | <0.001 | <0.001 | 0.65 | 0.32 |
| Body composition | ||||||||
| BMI (kg/m2) | 23.3 (20.2, 25.4) | 30.9 (27.7, 34.0) | 23.3 (19.1, 25.1) | 29.2 (27.9, 34.3) | <0.001 | 0.27 | 0.50 | <0.001 |
| Fat (%) | 29.8 (10.7, 37.6) | 44.3 (22.4, 53.5) | 29.7 (17.5, 43.0) | 40.4 (26.3, 50.0) | <0.001 | 0.93 | <0.001 | <0.001 |
| FFM (kg) | 45.8 (32.3, 65.5) | 53.2 (36.1, 78.1) | 44.0 (27.4, 61.5) | 47.8 (35.6, 64.2) | <0.001 | 0.013 | <0.001 | <0.001 |
| Fat mass (kg) | 18.6 (6.2, 24.1) | 35.3 (21.5, 59.6) | 16.5 (11.5, 28.8) | 32.4 (21.7, 38.6) | <0.001 | 0.014 | <0.001 | <0.001 |
| Truncal fat mass (kg) | 8.5 (3.0, 10.8) | 17.6 (10.9, 29.4) | 8.0 (5.6, 14.4) | 15.8 (11.5, 19.8) | <0.001 | 0.23 | 0.025 | <0.001 |
| Fitness | ||||||||
| Peak Vo2 (l/min) | 2.3 (1.37, 3.00) | 2.65 (1.62, 3.66) | 1.37 (0.76, 2.48) | 1.47 (0.92, 3.06) | <0.001 | <0.001 | <0.001 | 0.0189 |
| Peak Vo2 (ml · kg−1 FFM · min−1) | 46.0 (41.3, 58.0) | 46.6 (40.9, 55.6) | 31.1 (24.3, 44.3) | 30.3 (25.6, 47.6) | <0.001 | <0.001 | 0.17 | 0.79 |
| Leisure-time activity score* | 256 (24, 893) | 102 (13, 384) | 201 (34, 736) | 111 (40, 1,032) | 0.38 | 0.66 | 0.53 | 0.12 |
Data are medians (minimum, maximum).
*Data were log transformed prior to analysis.
Study design.
On a second outpatient visit, each participant underwent a dual-energy X-ray absorptiometry (DPX-L; Lunar, Madison, WI) examination to determine body composition and an incremental cycle ergometer exercise test to determine peak oxygen uptake (peak Vo2), as previously described (21). Participants were placed on a weight-maintaining diet (energy content as carbohydrate:protein:fat = 55:15:30%) provided by the Nutrition Unit of Mayo Center of Translational Science Activities for 3 consecutive days before the inpatient study period.
On the evening before each study day, participants were admitted to the Mayo Center of Translational Science Activities Clinical Research Unit at 1700 and stayed overnight until 1500 the following day. A retrograde catheter was inserted into a dorsal hand vein for sample collection, and the hand was kept in a heating pad overnight. A second intravenous catheter was placed in the contralateral forearm for infusions. After a standard dinner at 1800 and a standardized snack at 2200, a fasting state was maintained, except for water, until the end of the inpatient visit.
In the morning, the hand with the retrograde catheter was kept in a “hot box” at 60°C to obtain arterialized venous blood. A hyperinsulinemic-euglycemic clamp was performed infusing 1.5 mU · kg−1 fat-free mass (FFM) · min−1 of insulin while maintaining similar plasma glucose levels [∼5.0 mmol/l (90 mg/dl)] in every participant (22,23). In addition, a standard amino acid solution (10% Travasol) was infused (0.6 μmol leucine per kg of FFM per min) to maintain leucine concentrations near fasting levels during the insulin infusion, as previously described (22). Arterialized venous blood was used to measure glucose levels every 10 min with a Beckman glucose analyzer (Fullerton, CA). The glucose (40% solution) infusion rate was adjusted to maintain euglycemia during the insulin infusion.
Muscle biopsies.
Vastus lateralis muscle samples (300 mg each) were obtained under local anesthesia (lidocaine, 2%) with a percutaneous needle, as previously described (24). Baseline samples from the participants were obtained at 0700 (0 h) and after 3 and 8 h from the contralateral thigh. Muscle samples were immediately frozen in liquid nitrogen and kept at −80°C after keeping apart a 50-mg fresh muscle sample that was used to measure MAPR.
Hormones and substrates.
Plasma insulin was measured with a two-site immunoenzymatic assay (Access; Beckman Instruments, Chaska, MN). Glucose was measured with a Beckman Glucose Analyzer (Beckman Instruments, Fullerton, CA). Plasma levels of amino acids were measured by an HPLC system (HP 1090, 1046 fluorescence detector and cooling system) with precolumn o-phthalaldehyde derivatization (25). Total adiponectin and HMW adiponectin concentrations were measured by the human adiponectin double-antibody radioimmunoassay kit (Linco Research, St. Louis, MO). Highly sensitive C-reactive protein (hsCRP) concentrations were measured on the Hitachi 912 chemistry analyzer by a polystyrene particle–enhanced immunoturbidimetric assay from DiaSorin (Stillwater, MN). Tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and leptin concentrations were measured as previously described (26).
Mitochondrial ATP production rates.
We measured maximal muscle MAPRs as previously described (18). Briefly, fresh muscle tissue (150 mg) was minced on a chilled glass plate and washed in buffer A (100 mmol/l KCl, 50 mmol/l Tris base, 5 mmol/l MgCl2, 1.8 mmol/l ATP, 1 mmol/l EDTA, pH 7.2). The tissue was transferred to a glass mortar and homogenized in 20 volumes of buffer A with a motor-driven Teflon pestle. Samples were centrifuged at 1,020g for 10 min at 4°C, and the supernatant was removed and respun at the same speed. The supernatant was then centrifuged at 10,000g for 5 min at 4°C. The resulting pellet was resuspended in buffer A and respun at 9,000g for 5 min at 4°C. This final mitochondrial pellet was suspended in buffer B (180 mmol/l sucrose, 35 mmol/l KH2PO4, 10 mmol/l Mg acetate, 5 mmol/l EDTA) and used to measure MAPR with a bioluminescent technique as previously described (8,14,27,28). The reaction mixture included a luciferin–luciferase ATP monitoring reagent (BioThema, Haninge, Sweden), substrates for oxidation, and 35 μmol/l ADP. Substrates used were (in mmol/l final concentration): 20 succinate plus 0.1 rotenone (SR), 10 glutamate plus 1 malate (GM), 1 pyruvate plus 1 malate (PM), 0.05 palmitoyl-l-carnitine plus 1 malate (PCM), 10 α-ketoglutarate (KG), and 1 pyruvate plus 0.05 palmitoyl-l-carnitine plus 10 α-ketoglutarate plus 1 malate (PPKM), with blank tubes used for measuring background activity. Several substrates were used to allow for the potential detection of pathway-specific differences among study groups. The SR substrate, for example, delivers electrons to complex II of the respiratory chain, whereas all other substrates used transfer energy predominately to complex I. GM and PM rely on different transporters to enter mitochondrial and provide fuel to different points of the tricarboxylic acid (TCA) cycle, whereas KG enters the TCA after interconversion with glutamate. PCM processing uses the carnitine-palmitoyl transferase system to enter mitochondria, then undergoes fatty acid oxidation pathway before acetyl CoA units are directed to the TCA cycle. The PPKM substrate provides an energy-rich combination to provide substrates to multiple pathways. All reactions for a given sample were monitored simultaneously at 25°C for 20–25 min and calibrated with addition of an ATP standard using a BioOrbit 1251 luminometer (BioOrbit Oy, Turku, Finland).
Mitochondrial DNA (mtDNA) copy number.
Skeletal muscle mtDNA copy numbers were determined as previously described (16,18). Real-time PCR (Applied Biosystems 7900HT Sequence Detection System) was used to measure mtDNA copy numbers (3), using primer/probe sets targeted to mtDNA-encoded cytochrome B gene normalized to 28S ribosomal DNA, which was co-amplified within the same reaction well.
Statistical analysis.
All statistical analyses were conducted using SAS software (SAS Version 9.1, Cary, NC). All data examined for departures from normality and transformations were used as needed. Analyses evaluating the main effects of age (young versus elderly), BMI (lean versus obese), and sex (male versus female) were conducted using three-way ANOVA. Because no significant interactions were observed, the results are reported considering only the main effects. Pair-wise comparisons between groups were made using two sample t tests. Comparisons between baseline and ending values within groups were made using paired t tests. Multiple linear regression models were developed to determine the independent effects of age, BMI, and sex, after adjusting for each other, on insulin sensitivity (glucose infusion rate [GIR]) and MAPR. Finally, Spearman rank correlation analyses were conducted among peak Vo2, MAPR and mtDNA copy number.
RESULTS
Participant characteristics.
As per the study design, our young groups were younger than elderly, and our obese groups had a higher BMI than lean. Percent fat and FFM was higher in the obese groups than the lean (P < 0.001). Both absolute (l/min) and relative (ml · kg−1 FFM · min−1) peak Vo2 were significantly lower in the elderly participants compared with the younger participants (P < 0.001), whereas leisure-time activity scores were similar between the two age-groups. As expected, the absolute peak Vo2 was lower in women than men (P < 0.001), whereas the relative peak Vo2 was similar between men and women (P = 0.17) (Table 1).
Hormones and substrates.
Fasting glucose levels did not differ among the groups, as by study design, all participants fasting glucose levels had to be <6.1 mmol/l (<110 mg/dl) for inclusion. However, there was a sex effect on fasting glucose levels with women having lower glucose levels (P < 0.009) than men (Table 2). Fasting insulin levels were higher in the obese groups (P < 0.001), but there was no significant difference in fasting glucagon levels. The obese groups had lower levels of total and high molecular weight (HMW) adiponectin (P = 0.027 and P < 0.001, respectively), and as expected, higher concentrations of leptin (P < 0.001). The elderly groups had higher concentrations of IL-6 (P = 0.012), hsCRP (P = 0.051), and total adiponectin (P = 0.004). However, HMW adiponectin levels were not different between the young and elderly participants. Women had higher concentrations of HMW adiponectin (P = 0.022) and leptin (<0.001) than men.
TABLE 2.
Hormones and substrates
| Young lean (n = 12) | Young obese (n = 12) | Elderly lean (n = 12) | Elderly obese (n = 12) |
P |
||||
|---|---|---|---|---|---|---|---|---|
| Overall | Age | Sex | BMI | |||||
| Fasting glucose (mmol/l) | 4.9 (4.3, 5.5) | 5.1 (4.6, 6.1) | 5.2 (4.2, 5.8) | 52 (4.8, 5.9) | 0.005 | 0.055 | 0.009 | 0.061 |
| Fasting insulin (pmol/l) | 28.2 (20.4, 55.2) | 39.6 (18.6, 111.0) | 19.8 (9.6, 51.0) | 36.0 (21.0, 62.4) | 0.003 | 0.10 | 0.79 | <0.001 |
| Fasting glucagon (pg/ml) | 66 (34, 174) | 80 (50, 115) | 59 (31, 90) | 65 (45, 116) | 0.080 | 0.17 | 0.15 | 0.090 |
| CRP (mg/l)* | 0.6 (0.3, 2.4) | 0.6 (0.2, 17.0) | 1.5 (0.3, 3.4) | 1.8 (0.4, 11.5) | 0.078 | 0.051 | 0.46 | 0.15 |
| IL-6 (pg/ml)* | 1.2 (0.7, 3.1) | 1.1 (0.7, 11.7) | 1.9 (0.9, 5.2) | 2.9 (1.4, 8.4) | 0.011 | 0.012 | 0.31 | 0.040 |
| TNF-α (pg/ml)* | 1.1 (0.5, 2.1) | 0.9 (0.5, 1.7) | 0.9 (0.5, 2.0) | 1.2 (0.9, 2.0) | 0.22 | 0.51 | 0.10 | 0.26 |
| Adiponectin (μg/ml)* | 9.1 (5.3, 12.0) | 8.3 (4.4, 10.2) | 12.9 (7.7, 26.8) | 10.9 (3.6, 17.1) | 0.003 | 0.004 | 0.12 | 0.027 |
| HMW adiponectin (μg/ml)* | 3.1 (1.4, 5.6) | 2.4 (1.2, 4.5) | 4.9 (2.3, 10.8) | 2.5 (0.3, 5.5) | <0.001 | 0.19 | 0.022 | <0.001 |
| Leptin (μg/ml)* | 10.4 (1.6, 27.5) | 14.1 (4.4, 65.8) | 7.0 (1.3, 23.6) | 18.7 (4.2, 50.7) | <0.001 | 0.78 | <0.001 | <0.001 |
Data are medians (minimum, maximum). To convert values for glucose to milligram per deciliter, multiply by 18. To convert values for insulin to micro-units per milliliter, divide by 6.
*Data were log transformed prior to analysis.
Insulin sensitivity.
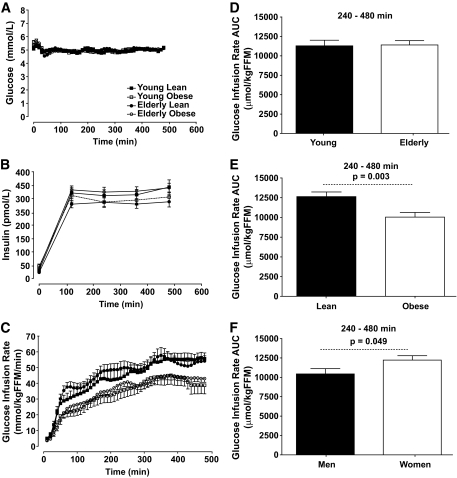
The GIR required to maintain similar glucose levels during the euglycemic-hyperinsulinemic clamp was significantly lower in obese participants compared with the lean participants (P < 0.01), indicating that obese participants were more insulin resistant than the lean participants. Notably, there was no age effect (P = 0.99) on GIR. In addition, men required lower GIR than women (P < 0.05), indicating that the women were more insulin sensitive than the men (Fig. 1).
FIG. 1.
Hyperinsulinemic-euglycemic clamp. A and B show the plasma insulin and glucose concentrations during the 8-h clamp, respectively. C shows that the GIRs required to maintain euglycemia during the 8-h clamp were higher in lean groups than in the obese groups with no effects of age. D–F show integrated area under the curve (AUC) for the GIR comparing the young and elderly participants, the lean and obese participants, and men and women, respectively.
Mitochondrial ATP production rates.
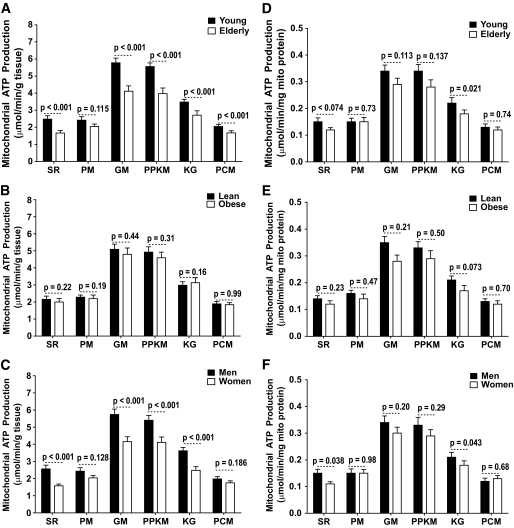
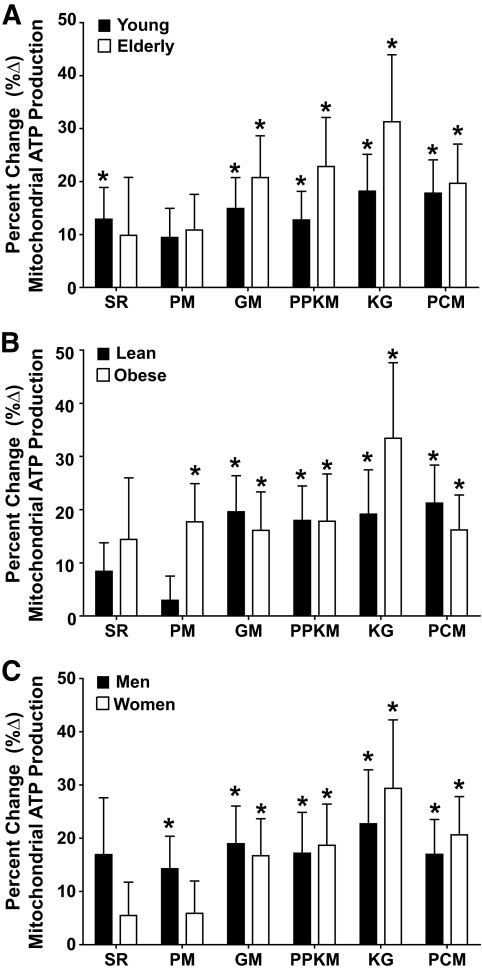
MAPRs normalized to tissue weight showed an age effect, with the elderly groups having lower MAPRs using substrate combinations of SR, GM, PPKM, KG, and PCM but not PM (Fig. 2A). When obese and lean groups were analyzed separately, we found that with all six substrates, both groups showed a significant age-related decline in MAPR or similar trend (supplementary Fig. 1 in the online appendix), although not all of these changes reached the level of statistical significance. Notably, there was no BMI effect on MAPR normalized to tissue weight. Figure 2C reveals that men had higher MAPRs normalized for tissue weight using all substrates except PCM. Similar trends were also observed when the MAPR data were expressed relative to mitochondrial protein content (Fig. 2D–F); however, these differences did not reach the level of statistical significance except when SR was used as a substrate. The 8-h infusion of insulin resulted in significant increases in MAPR independent of age (Fig. 3A), BMI (Fig. 3B), and sex (Fig. 3C). As previously noted, MAPRs measured using SR, GM, PPKM, and PCM were significantly (P < 0.05) increased in response to the 8-h insulin infusion in the young lean participants (Fig. 3A) (18).
FIG. 2.
Mitochondrial ATP production rates. Age effect: An age-related decline in mitochondrial ATP production was observed when expressed per gram of tissue using substrates succinate plus rotenone (SR), glutamate plus malate (GM), pyruvate plus palmitoyl-l-carnitine plus α-ketoglutarate plus malate (PPKM), α-ketoglutarate plus glutamate (KG), and palmitoyl-l-carnitine plus 1 malate (PCM) but not pyruvate plus malate (PM) (A). Similar trends were also observed when expressed per milligram of mitochondrial (mito) protein (D). BMI effect: No significant BMI effect was observed when the mitochondrial ATP production rate was expressed per gram of tissue (B) or when expressed per milligram of mitochondrial protein (E). Sex effect: Men had higher MAPRs than women using substrates SR, PM, GM, PPKM, and KG but not PCM when expressed per gram of tissue (C), but significant differences were only observed when using the SR and KG substrates when expressed per milligram of mitochondrial protein content (F). Data are presented as means ± SE.
FIG. 3.
Insulin-induced changes in MAPRs. A–C show the percent change (%Δ) in mitochondrial ATP production rates after an 8-h infusion of insulin stratified by age, BMI, and sex, respectively. The 8-h insulin infusion resulted in significant increases in mitochondrial ATP production rates independent of age (A), BMI (B), and sex (C). Data are presented as means ± SE. *P < 0.05 (within group).
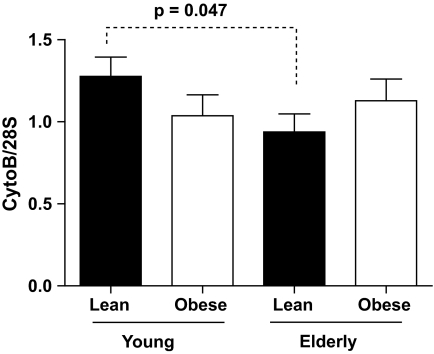
Mitochondrial DNA (mtDNA) copy number.
Skeletal muscle mtDNA copy numbers were lower in the elderly lean than the young lean participants (P = 0.047). There was no age-related reduction in mtDNA copy numbers among the obese participants. In addition, there was no sex effect on mtDNA copy numbers (data not shown) (Fig. 4).
FIG. 4.
Mitochondrial DNA copy number (mtDNA). Baseline mitochondrial mtDNA copy numbers assessed using primers and probes directed to cytochrome B normalized to 28 s. Data are presented as means ± SE.
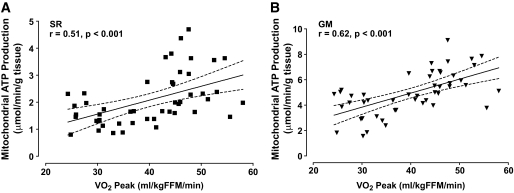
Regression analyses between peak Vo2 and MAPR and mtDNA copy numbers.
Peak Vo2 was positively associated with skeletal muscle MAPR (Fig. 5). In contrast, peak Vo2 was not associated with mtDNA copy number (data not shown).
FIG. 5.
Association between peak Vo2 and mitochondrial ATP production rates. A and B display the relationship between peak Vo2 and mitochondrial ATP production rates assessed using the succinate plus rotenone (SR) and glutamate plus malate (GM) substrate combinations, respectively. Data are presented as means and 95% CIs.
Multiple regression analyses.
Multiple regression models were developed to determine the independent effects of age, BMI, and sex on insulin sensitivity (GIR) and MAPR. Because no significant interactions were observed, the results are reported for the models considering only the main effects of age, BMI, and sex. Insulin sensitivity was not associated with age after adjusting for BMI and sex. In contrast, insulin sensitivity was lower in obese compared with lean subjects after adjusting for age and sex and was lower in men compared with women after adjusting for age and BMI. MAPR were lower in the elderly participants compared with the younger participants for five of the six substrate combinations after adjusting for BMI and sex. However, MAPRs were not associated with BMI after adjusting for age and sex. Interestingly, MAPRs were higher in men than in women after adjusting for age and sex. Multiple linear regression analysis also revealed that there was no association between truncal fat mass (by DPX) and MAPR after adjusting for age, BMI, and sex (all P < 0.10, data not shown) (Table 3).
TABLE 3.
Multivariate regression analyses
| Intercept | Intercept P value | Age | Age P value | BMI | BMI P value | Sex | Sex P value | |
|---|---|---|---|---|---|---|---|---|
| Insulin sensitivity | ||||||||
| GIR (μmol · kg−1 FFM · min−1) | 58.2 | <0.001 | 0.01 | 0.997 | − 11.57 | 0.00113 | − 7.83 | 0.023 |
| Mitochondrial ATP production | ||||||||
| SR* | 0.670 | <0.001 | − 0.403 | <0.001 | − 0.117 | 0.21935 | 0.448 | <0.001 |
| PM* | 0.809 | <0.001 | − 0.161 | 0.116 | − 0.134 | 0.19074 | 0.156 | 0.128 |
| GM | 5.12 | <0.001 | − 1.674 | <0.001 | − 0.272 | 0.44408 | 1.596 | <0.001 |
| PPKM | 5.09 | <0.001 | − 1.589 | <0.001 | − 0.357 | 0.30591 | 1.291 | <0.001 |
| KG | 3.12 | <0.001 | − 0.982 | <0.001 | − 0.370 | 0.16317 | 1.307 | <0.001 |
| PCM | 1.93 | <0.001 | − 0.374 | 0.0357 | 0.0032 | 0.98541 | 0.23218 | 0.186 |
Data were pooled for the multivariate regression analyses (n = 48). The model set young to 0 and elderly to 1; negative parameter estimate indicates elderly < young. The model set lean to 0 and obese to 1; negative parameter estimate indicates obese < lean. The model set women to 0 and men to 1; negative parameter estimate indicates men < women. Since no significant interactions were observed, the results are reported for the model considering only the main effects.
*Variables were log transformed for analysis.
DISCUSSION
The current study determined the effects of age and adiposity on insulin sensitivity and mitochondrial function to further explore whether age-related mitochondrial dysfunction and insulin resistance are direct effects of chronological age or are related to adiposity. The main findings of the current study are, first, that skeletal MAPRs were higher among the young compared with the elderly participants, independent of obesity or truncal fat. Second, in contrast to the age effect on muscle mitochondrial function, insulin sensitivity was lower in the obese compared with the lean participants, and there was no age effect. Finally, of great interest, it was also shown that women in general have higher insulin sensitivity than men, whereas they have lower muscle MAPRs than men.
There is considerable interest in the inverse relationship between age and insulin sensitivity, because of the age-related increase in the prevalence of type 2 diabetes (1) and its related comorbidities (2). Using the hyperinsulinemic-euglycemic clamp, the current results demonstrate that there were no differences in the insulin sensitivities measured between the young and the elderly participants, independent of obesity. Although numerous investigators have previously reported age-related declines in insulin sensitivity (4,29,30), our results are consistent with a growing body of evidence that indicates that age-related changes in adiposity and physical inactivity are the primary determinants of the age-related declines in insulin sensitivity rather than chronological age (6,7,31–33). The present results demonstrated that insulin sensitivity was substantially lower in the obese compared with the lean participants, independent of age, which is consistent with a recent report in a large number of people (7) that demonstrated that after adjusting for body composition and cardiorespiratory fitness, the age-related differences in insulin action were eliminated. We have also recently reported that there were no differences in insulin sensitivity between highly trained young and elderly lean adults, and the differences in insulin sensitivity were due to differences in exercise status (6). Importantly, this later study demonstrated that fat mass, abdominal adiposity, and BMI were significant predictors of skeletal muscle glucose disposal, independent of age (6). The present data, in context of other recent findings (6,32), strongly support the contention that the age-related reductions in insulin action (3) are likely secondary to changes in body composition and physical activity and not inherent to chronological aging.
The current study also helps clarify the independent effects of age and obesity on changes in mitochondrial function. Several prior studies have shown changes in mitochondrial function with age (3,8,10,14), obesity (14), and insulin resistance (3,5,10,14,18,34–36). The current study helps to tease out two otherwise integrated processes. Age-related decline in muscle mass and activities results in a decline in physical activities and energy expenditure, causing a relative increase in adiposity (37). Indeed, the present data indicate that aging and not age-related changes in body composition likely mediate the age-related skeletal muscle mitochondrial dysfunction. We found that irrespective of differences in body composition, there was a significant age effect on MAPRs. The age-related reduction in MAPRs was a consistent effect of age, since MAPR values were lower in the elderly groups using several different substrates combinations, which is in agreement with our previous reports that also indicated an age-related lowering of MAPR when normalized per gram of tissue (3,6). Here we also report MAPRs normalized for mitochondrial protein content, which also showed a similar trend to decline with age, although it did not reach the level of statistical significance. Moreover, at the end of the hyperinsulinemic-euglycemic clamp, we observed an increase in MAPRs, similar to prior studies from our group in nondiabetic participants (36), and this response was independent of age, sex, and BMI. We have previously shown that many mitochondrial protein concentrations, especially those involved in oxidative reactions, are reduced with age (3,6). The age-related decline in MAPRs is proportional to the decline in mitochondrial DNA abundance (3) and seems to be proportional to the decline in protein content, since the age-related changes are less evident when MAPR was normalized for the mitochondrial protein content. These results indicate that the decline in MAPR is largely due to the decline in mitochondrial content rather than related to decline in mitochondrial quality. We also measured muscle mitochondrial DNA abundance that confirmed that, in nonobese people, there is an age-related decline in muscle mitochondrial DNA abundance, as we have previously reported (3). However, we did not see a significant lowering of mitochondrial DNA abundance with age in our obese participants, and no clear explanation for this observation emerges from the current study. Of interest, MAPR showed significant age-related change or similar trend in both obese and lean groups (Fig. 2 and supplementary Fig. 1). It is possible that the lack of age-related decline in mtDNA abundance in the obese group may represent a type II error due to inadequate power for this outcome variable. The underlying mechanism for this discrepancy is unclear from the current study.
A rather intriguing and unexpected finding of the current study was that MAPR was lower in women than in men. These differences between men and women persists only minimally after normalizing for mitochondrial protein content, suggesting that, as in elderly people, the reduced MAPR in women is related to reduced mitochondrial protein content. The women may have had lower MAPR than the men due in part to lower levels of physical activity and/or fitness in the women. Another potential mechanism that could have resulted in lower MAPR in women than men could have been due to sex-related differences in fiber type distribution, particularly if women had a higher proportion of fast-twitch (glycolytic, type II) skeletal muscle fibers. However, based on normative data for mATPase-based fiber-type distributions in healthy untrained young men and women, slow-twitch (oxidative, type I) skeletal muscle fibers tend to occupy a greater area in the women than the men (38). Of interest, we also found that the women had higher insulin sensitivity than the men, which argues against lesser physical activity levels in women. We also found no differences in peak Vo2 per kilogram FFM between men and women. Women have lower absolute peak Vo2 that is proportional to their lean tissue mass. We have previously found a close association between peak Vo2 and muscle MAPR, but it is unclear whether the lower MAPR we observed in the current study contributed to lower absolute peak Vo2. In the current study, we observed that women have lower fasting glucose levels and are more insulin sensitive than men. Using positron emission tomography, it has been shown that women exhibit greater insulin sensitivity at the whole-body level, which was largely attributed to greater glucose uptake by skeletal muscle (39). Lower fasting glucose levels and greater postprandial glucose disappearance rates in elderly women than in men were also observed in another study (7). An important observation in the current study is the dissociation between insulin sensitivity and muscle mitochondrial function, with women being more insulin sensitive while having lower muscle MAPR.
Aging is often associated with the development of low-grade inflammation (40), which is an independent risk factor for both type 2 diabetes and cardiovascular disease. Indeed, the present data demonstrate that the concentrations of proinflammatory cytokines, IL-6 and CRP, were higher among the elderly than the young, independent of obesity. In addition, we also examined the effect of aging on adiponectin concentrations. Adiponectin has been suggested to have both anti-inflammatory and antidiabetic properties. The present results indicate that adiponectin concentrations were higher among the elderly than the young. Mechanistically, it has been suggested that adiponectin concentrations increase with age due in part to an age-related decline in renal clearance of adiponectin (41). However, prior studies have also shown conflicting results with respect to the age-related changes in adiponectin concentrations (42). Recent data have also indicated that an individual's sex may modify the association between adiponectin and age (43). However, the present study was not powered to detect a sex differences for adiponectin. The age-related reduction in MAPR that was observed in our study may be related to low-grade inflammation. Indeed, CRP concentrations were inversely related to MAPR for both complex I– and complex II–related substrates (r = −0.44 and r = −0.40, respectively). There is a clear association to the decline in mitochondrial copy number and MAPR (3).
Low-grade inflammation is frequently observed in obese adults, which has been proposed as a potential cause of their increased risk for type 2 diabetes and cardiovascular disease (44). As expected, obesity was associated with higher IL-6 and leptin concentrations. In contrast, adiponectin concentrations were lower among the obese participants than their lean counterparts. Moreover, the adiponectin concentrations were significantly correlated to improved insulin sensitivity (r = 0.40). Increased inflammatory cytokines and decreased total and HMW adiponectin concentrations have been associated with obesity (45,46), intra-abdominal fat accumulation (47), insulin resistance (48), the metabolic syndrome (49), and cardiovascular disease (50).
In conclusion, the current study supports a hypothesis that age-related decline in insulin sensitivity are secondary to age-related changes in body composition, whereas the age-related lowering in skeletal muscle mitochondrial ATP production are related to chronological age. Further studies are necessary to help elucidate the sex-associated changes in insulin sensitivity and MAPR, although changes in adipokines and inflammation may play a role.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants RO1 AG09531, UL1 RR024150-01, and KL2 RR084151 (to B.A.I.) and the David Murdock Dole Professorship (to K.S.N.).
No potential conflicts of interest relevant to this article were reported.
We acknowledge support from the Clinical Research Unit staff, Maureen Bigelow, RN, and Jill Schimke, Kate Klaus, Dawn Morse, and Jane Kahl for skilled support.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ford ES, Giles WH, Dietz WH: Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356–359 [DOI] [PubMed] [Google Scholar]
- 2.Facchini FS, Hua N, Abbasi F, Reaven GM: Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab 2001;86:3574–3578 [DOI] [PubMed] [Google Scholar]
- 3.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS: Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A 2005;102:5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS: Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 2003;52:1888–1896 [DOI] [PubMed] [Google Scholar]
- 5.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI: Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004;350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS: Endurance exercise as a countermeasure for aging. Diabetes 2008;57:2933–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA: Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes 2003;52:1738–1748 [DOI] [PubMed] [Google Scholar]
- 8.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI: Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003;300:1140–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO: Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol 1992;47:B71–B76 [DOI] [PubMed] [Google Scholar]
- 10.Rooyackers OE, Adey DB, Ades PA, Nair KS: Effect of age in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A 1996;93:15364–15369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH: Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci 2006;61:534–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barazzoni R, Short KR, Nair KS: Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem 2000;275:3343–3347 [DOI] [PubMed] [Google Scholar]
- 13.Simoneau JA, Kelley DE: Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol 1997;83:171. [DOI] [PubMed] [Google Scholar]
- 14.Kelley DE, He J, Menshikova EV, Ritov VB: Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002;51:2944–2950 [DOI] [PubMed] [Google Scholar]
- 15.Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO: High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci U S A 2008;105:7815–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nair KS, Bigelow ML, Asmann YW, Chow LS, Coenen-Schimke JM, Klaus KA, Guo ZK, Sreekumar R, Irving BA: Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes 2008;57:1166–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Short KR, Nair KS, Stump CS: Impaired mitochondrial activity and insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004;350:2419–2421 [DOI] [PubMed] [Google Scholar]
- 18.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS: Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci U S A 2003;100:7996–8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toledo FG, Menshikova EV, Azuma K, Radiková Z, Kelley CA, Ritov VB, Kelley DE: Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes 2008;57:987–994 [DOI] [PubMed] [Google Scholar]
- 20.Forbes GB: Human Body Composition: Growth, Aging, Nutrition, and Activity New York, Springer-Verlag, 1987 [Google Scholar]
- 21.Proctor DN, Beck KC: Delay time adjustments to minimize errors in breath-by-breath measurement of VO2 during exercise. J Appl Physiol 1996;81:2495–2499 [DOI] [PubMed] [Google Scholar]
- 22.Nygren J, Nair KS: Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes 2003;52:1377–1385 [DOI] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 24.Nair KS, Halliday D, Griggs RC: Leucine incorporation into mixed skeletal muscle protein in humans. Am J Physiol Endocrinol Metab 1988;254:E208–E213 [DOI] [PubMed] [Google Scholar]
- 25.Nair KS, Ford GC, Ekberg K, Fernqvist-Forbes E, Wahren J: Protein dynamics in whole body and in splanchnic and leg tissues in type I diabetic patients. J Clin Invest 1995;95:2926–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moller N, O'Brien P, Nair KS: Disruption of the relationship between fat content and leptin levels with aging in humans. J Clin Endocrinol Metab 1998;83:931–934 [DOI] [PubMed] [Google Scholar]
- 27.Short KR, Nygren J, Barazzoni R, Levine J, Nair KS: T3 increases mitochondrial ATP production in oxidative muscle despite increased expression of UCP2 and -3. Am J Physiol Endocrinol Metab 2001;280:E761–E769 [DOI] [PubMed] [Google Scholar]
- 28.Wibom R, Hultman E: ATP production rate in mitochondria isolated from microsamples of human muscle. Am J Physiol Endocrinol Metab 1990;259:E204–E209 [DOI] [PubMed] [Google Scholar]
- 29.DeFronzo RA: Glucose intolerance and aging. Diabetes Care 1981;4:493–501 [DOI] [PubMed] [Google Scholar]
- 30.Houmard JA, Weidner MD, Dolan PL, Leggett-Frazier N, Gavigan KE, Hickey MS, Tyndall GL, Zheng D, Alshami A, Dohm GL: Skeletal muscle GLUT4 protein concentration and aging in humans. Diabetes 1995;44:555–560 [DOI] [PubMed] [Google Scholar]
- 31.Amati F, Dubé JJ, Coen PM, Stefanovic-Racic M, Toledo FG, Goodpaster BH: Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care 2009;32:1547–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohrt WM, Kirwan JP, Staten MA, Bourey RE, King DS, Holloszy JO: Insulin resistance in aging is related to abdominal obesity. Diabetes 1993;42:273–281 [PubMed] [Google Scholar]
- 33.Seals DR, Hagberg JM, Allen WK, Hurley BF, Dalsky GP, Ehsani AA, Holloszy JO: Glucose tolerance in young and older athletes and sedentary men. J Appl Physiol 1984;56:1521–1525 [DOI] [PubMed] [Google Scholar]
- 34.Charlton M, Nair KS: Protein metabolism in insulin-dependent diabetes mellitus. J Nutr 1998;128:323S–327S [DOI] [PubMed] [Google Scholar]
- 35.Halvatsiotis P, Short KR, Bigelow M, Nair KS: Synthesis rate of muscle proteins, muscle functions, and amino acid kinetics in type 2 diabetes. Diabetes 2002;51:2395–2404 [DOI] [PubMed] [Google Scholar]
- 36.Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, Nair KS: Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 2006;55:3309–3319 [DOI] [PubMed] [Google Scholar]
- 37.Nair KS: Aging muscle: what causes it? E.V. McCollum Lecture 2004. Am J Clin Nutr 2005;81:953–963 [DOI] [PubMed] [Google Scholar]
- 38.Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K: Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem 2000;48:623–629 [DOI] [PubMed] [Google Scholar]
- 39.Nuutila P, Knuuti MJ, Mäki M, Laine H, Ruotsalainen U, Teräs M, Haaparanta M, Solin O, Yki-Järvinen H: Gender and insulin sensitivity in the heart and in skeletal muscles: studies using positron emission tomography. Diabetes 1995;44:31–36 [DOI] [PubMed] [Google Scholar]
- 40.Csiszar A, Wang M, Lakatta EG, Ungvari Z: Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol 2008;105:1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isobe T, Saitoh S, Takagi S, Takeuchi H, Chiba Y, Katoh N, Shimamoto K: Influence of gender, age and renal function on plasma adiponectin level: the Tanno and Sobetsu study. Eur J Endocrinol 2005;153:91–98 [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto Y, Hirose H, Saito I, Tomita M, Taniyama M, Matsubara K, Okazaki Y, Ishii T, Nishikai K, Saruta T: Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin Sci 2002;103:137–142 [DOI] [PubMed] [Google Scholar]
- 43.Adamczak M, Rzepka E, Chudek J, Wiecek A: Ageing and plasma adiponectin concentration in apparently healthy males and females. Clin Endocrinol 2005;62:114–118 [DOI] [PubMed] [Google Scholar]
- 44.Hotamisligil GS: Inflammation and metabolic disorders. Nature 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 45.Engeli S, Feldpausch M, Gorzelniak K, Hartwig F, Heintze U, Janke J, Möhlig M, Pfeiffer AF, Luft FC, Sharma AM: Association between adiponectin and mediators of inflammation in obese women. Diabetes 2003;52:942–947 [DOI] [PubMed] [Google Scholar]
- 46.Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G: Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes 2003;52:1779–1785 [DOI] [PubMed] [Google Scholar]
- 47.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE: Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 2003;46:459–469 [DOI] [PubMed] [Google Scholar]
- 48.Snijder MB, Heine RJ, Seidell JC, Bouter LM, Stehouwer CD, Nijpels G, Funahashi T, Matsuzawa Y, Shimomura I, Dekker JM: Associations of adiponectin levels with incident impaired glucose metabolism and type 2 diabetes in older men and women: the Hoorn study. Diabetes Care 2006;29:2498–2503 [DOI] [PubMed] [Google Scholar]
- 49.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K: Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006;116:1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB: Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004;291:1730–1737 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.