Abstract
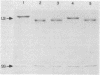
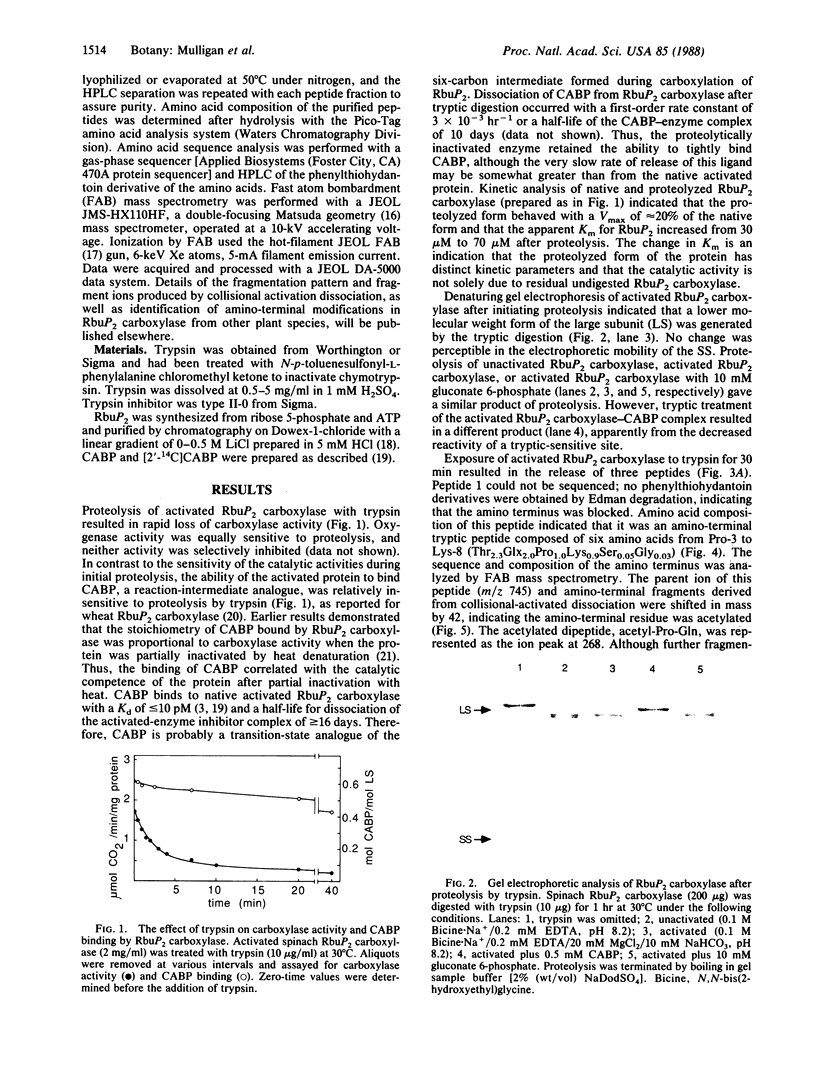
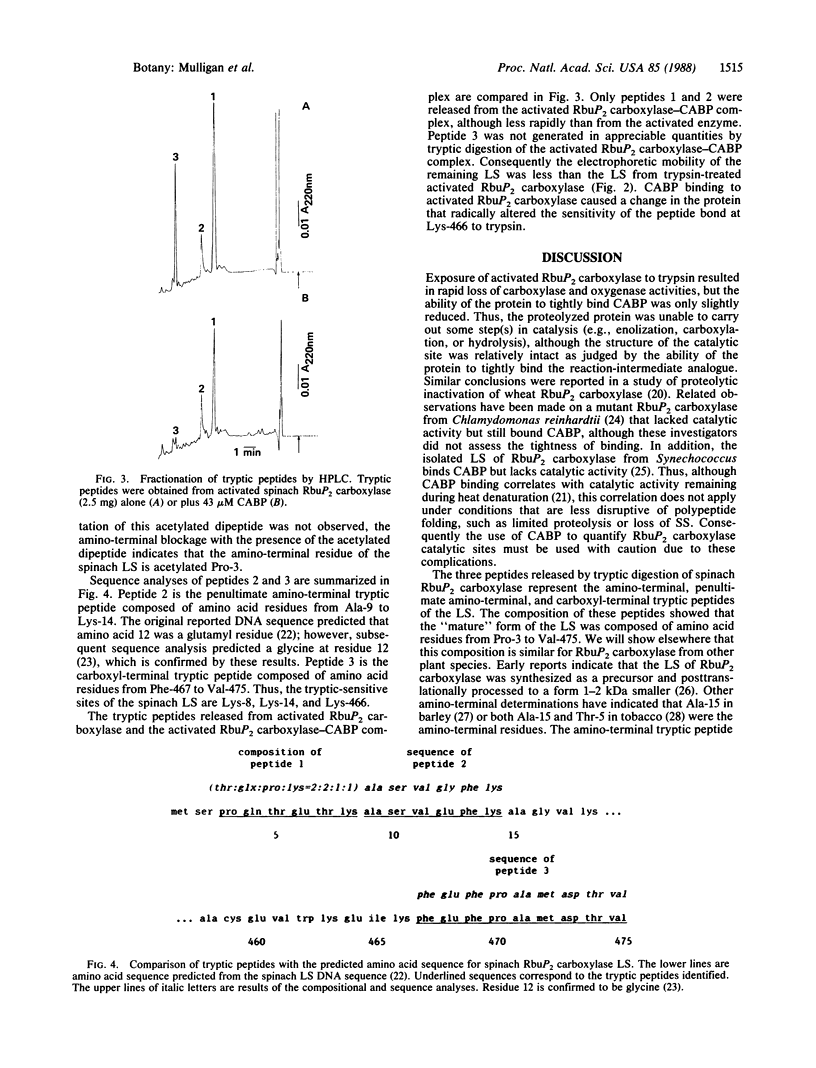
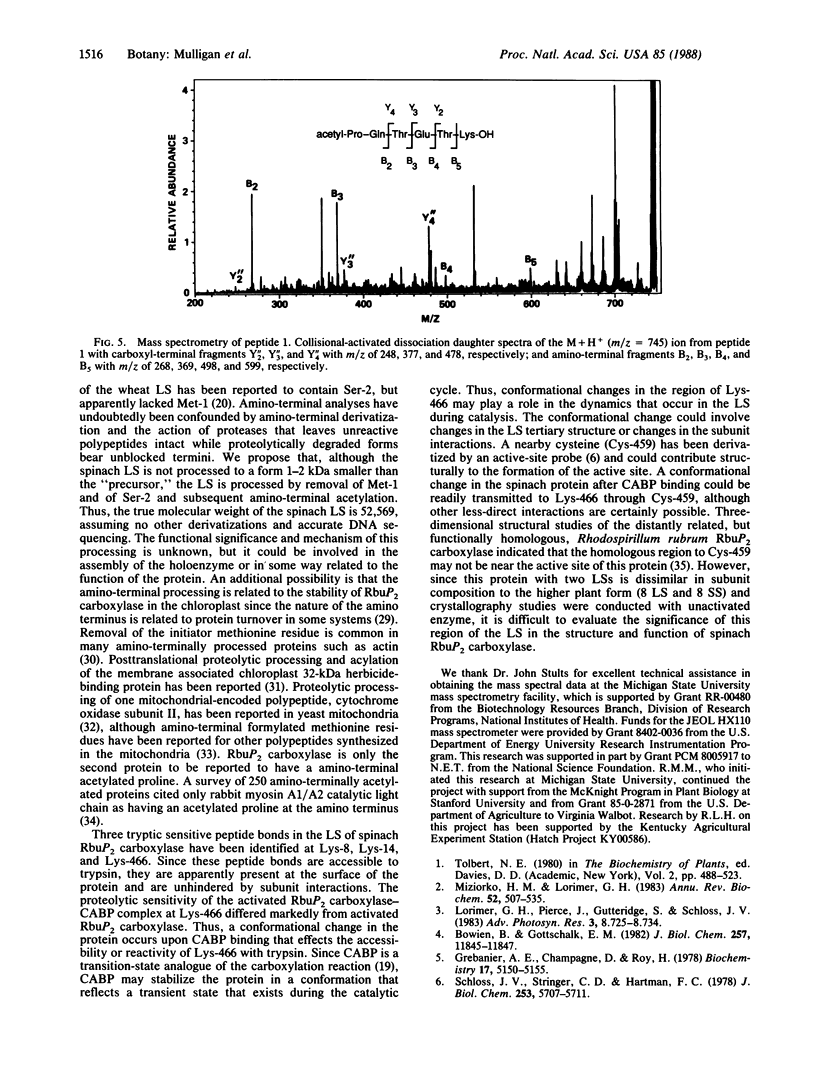
Trypsin rapidly inactivated the catalytic activities of spinach ribulose bisphosphate carboxylase/oxygenase [3-phospho-D-glycerate carboxy-lyase (dimerizing), EC 4.1.1.39], but the stoichiometry of binding of the reaction-intermediate analogue carboxyarabinitol bisphosphate was only slightly reduced after proteolysis. Electrophoretic analysis indicated that several forms of the large subunit were generated during proteolysis but that the small subunit was resistant. Three tryptic peptides were isolated and characterized after digestion of the activated enzyme; the tryptic-sensitive sites were identified at Lys-8, Lys-14, and Lys-466 of the large subunit. Tryptic digestion of the enzyme complexed with the reaction-intermediate analogue released only two peptides by hydrolysis at Lys-8 and at Lys-14. The loss of susceptibility of Lys-466 to trypsin may be the result of a conformational change that limits the accessibility of the carboxyl-terminal region after binding of the reaction-intermediate analogue. Analysis of the amino-terminal tryptic peptide by composition and fast atom bombardment mass spectrometry demonstrated that the actual amino-terminal residue is proline at position 3 of the DNA-deduced sequence and that this proline is blocked with an N-acetyl moiety. Thus, posttranslational processing of the chloroplast-encoded large subunit of the enzyme must occur to remove Met-1 and Ser-2 and to acetylate the amino terminus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Ballment B. Active-site carbamate formation and reaction-intermediate-analog binding by ribulosebisphosphate carboxylase/oxygenase in the absence of its small subunits. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3660–3664. doi: 10.1073/pnas.81.12.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmair A., Finley D., Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986 Oct 10;234(4773):179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- Bowien B., Gottschalk E. M. Influence of the activation state on the sedimentation properties of ribulose bisphosphate carboxylase from Alcaligenes eutrophus. J Biol Chem. 1982 Oct 25;257(20):11845–11847. [PubMed] [Google Scholar]
- Fearnley I. M., Walker J. E. Two overlapping genes in bovine mitochondrial DNA encode membrane components of ATP synthase. EMBO J. 1986 Aug;5(8):2003–2008. doi: 10.1002/j.1460-2075.1986.tb04456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebanier A. E., Champagne D., Roy H. Effects of Mg2+ and substrates on the conformation of ribulose-1,5-bisphosphate carboxylase. Biochemistry. 1978 Nov 28;17(24):5150–5155. doi: 10.1021/bi00617a013. [DOI] [PubMed] [Google Scholar]
- Hall N. P., Pierce J., Tolbert N. E. Formation of a carboxyarabinitol bisphosphate complex with ribulose bisphosphate carboxylase/oxygenase and theoretical specific activity of the enzyme. Arch Biochem Biophys. 1981 Nov;212(1):115–119. doi: 10.1016/0003-9861(81)90349-0. [DOI] [PubMed] [Google Scholar]
- Johal S., Partridge B. E., Chollet R. Structural characterization and the determination of negative cooperativity in the tight binding of 2-carboxyarabinitol bisphosphate to higher plant ribulose bisphosphate carboxylase. J Biol Chem. 1985 Aug 15;260(17):9894–9904. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., Edelman M. Intramembrane translocation and posttranslational palmitoylation of the chloroplast 32-kDa herbicide-binding protein. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1497–1501. doi: 10.1073/pnas.84.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry S. D., Gee R., Tolbert N. E. Ribulose-1,5-bisphosphate carboxylase/oxygenase from spinach, tomato, or tobacco leaves. Methods Enzymol. 1982;90(Pt E):515–521. doi: 10.1016/s0076-6879(82)90178-1. [DOI] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Persson B., Flinta C., von Heijne G., Jörnvall H. Structures of N-terminally acetylated proteins. Eur J Biochem. 1985 Nov 4;152(3):523–527. doi: 10.1111/j.1432-1033.1985.tb09227.x. [DOI] [PubMed] [Google Scholar]
- Pierce J. W., McCurry S. D., Mulligan R. M., Tolbert N. E. Activation and assay of ribulose-1,5-bisphosphate carboxylase/oxygenase. Methods Enzymol. 1982;89(Pt 500):47–55. doi: 10.1016/s0076-6879(82)89011-3. [DOI] [PubMed] [Google Scholar]
- Pierce J., Tolbert N. E., Barker R. Interaction of ribulosebisphosphate carboxylase/oxygenase with transition-state analogues. Biochemistry. 1980 Mar 4;19(5):934–942. doi: 10.1021/bi00546a018. [DOI] [PubMed] [Google Scholar]
- Schloss J. V., Stringer C. D., Hartman F. C. Identification of essential lysyl and cysteinyl residues in spinach ribulosebisphosphate carboxylase/oxygenase modified by the affinity label N-bromoacetylethanolamine phosphate. J Biol Chem. 1978 Aug 25;253(16):5707–5711. [PubMed] [Google Scholar]
- Schneider G., Lindqvist Y., Brändén C. I., Lorimer G. Three-dimensional structure of ribulose-1,5-bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum at 2.9 A resolution. EMBO J. 1986 Dec 20;5(13):3409–3415. doi: 10.1002/j.1460-2075.1986.tb04662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevarino K. A., Poyton R. O. Mitochondrial membrane biogenesis: identification of a precursor to yeast cytochrome c oxidase subunit II, an integral polypeptide. Proc Natl Acad Sci U S A. 1980 Jan;77(1):142–146. doi: 10.1073/pnas.77.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M. I., Lane M. D. Interaction of ribulose diphosphate carboxylase with 2-carboxyribitol diphosphate, an analogue of the proposed carboxylated intermediate in the CO 2 fixation reaction. Biochem Biophys Res Commun. 1972 Aug 7;48(3):508–516. doi: 10.1016/0006-291x(72)90377-4. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Perrot B., Bottomley W., Whitfeld P. R. The structure of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from spinach chloroplast DNA. Nucleic Acids Res. 1981 Jul 24;9(14):3251–3270. doi: 10.1093/nar/9.14.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]