Abstract
Helicobacter pylori colonizes the stomach and induces strong, specific local and systemic humoral and cell-mediated immunity, resulting in the development of chronic gastritis in humans. Although H. pylori-induced chronic atrophic gastritis is characterized by marked infiltration of T helper type 1 (Th1) cytokine-producing CD4+ T cells, almost all of the inflamed gastric mucosae also contain focal lymphoid aggregates with germinal centers. In addition, typical H. pylori-induced chronic gastritis in children, called follicular gastritis, is characterized by B-cell follicle formation in the gastric mucosa. The aim of this study was to examine whether thymic stromal lymphopoietin (TSLP), an epithelial-cell-derived cytokine inducing a dendritic cell (DC)-mediated inflammatory Th2 response, is involved in Th2 responses triggering B-cell activation in H. pylori-induced gastritis. Here, we show that H. pylori triggered human gastric epithelial cells to produce TSLP, together with the DC-attracting chemokine MIP-3α and the B-cell-activating factor BAFF. After DCs were incubated with supernatants from H. pylori-infected epithelial cells, the conditioned cells expressed high levels of costimulatory molecules, such as CD80, and triggered naïve CD4+ T cells to produce high levels of the Th2 cytokines interleukin-4 and interleukin-13 and of the inflammatory cytokines tumor necrosis factor alpha and gamma interferon. In contrast, after incubation of the supernatants with the neutralizing antibodies to TSLP, the conditioned DCs did not prime T cells to produce high levels of Th2 cytokines. These results, together with the finding that TSLP was expressed by the epithelial cells of human follicular gastritis, suggest that H. pylori can directly trigger epithelial cells to produce TSLP. It also suggests that TSLP-mediated DC activation may be involved in Th2 responses triggering B-cell activation in H. pylori-induced gastritis.
Helicobacter pylori (H. pylori) infection in the stomach induces chronic gastritis associated with the development of peptic ulcer diseases, gastric adenocarcinoma, and mucosa-associated lymphoid tissue (MALT) lymphoma in humans (3, 8, 35). Although H. pylori-induced chronic atrophic gastritis is characterized by marked infiltration of T helper type 1 (Th1) cytokine-producing CD4+ T cells (3, 8, 35), almost all of the inflamed gastric mucosae also contain focal lymphoid aggregates with germinal centers (5, 11). In addition, typical H. pylori-induced chronic gastritis in children, called follicular gastritis, is characterized by B-cell follicle formation in the gastric mucosa (10, 11, 34). Th2 responses triggering B-cell activation appear to be involved in the development of lymphoid aggregates with germinal centers. However, molecular mechanisms to induce Th2 responses triggering B-cell activation are not clear.
In humans, an epithelial-cell-derived cytokine, thymic stromal lymphopoietin (TSLP), activates CD11c+ myeloid dendritic cells (DCs), and activated DCs strongly upregulate the expression of costimulatory molecules, such as CD80 and CD86 (23, 38, 43, 44). TSLP-activated DCs promote CD4+ T cells to differentiate into inflammatory Th2 cells that produce interleukin-4 (IL-4), IL-5, IL-13, and tumor necrosis factor alpha (TNF-α) while downregulating IL-10 and gamma interferon (IFN-γ) (23, 44). Interestingly, TSLP primes DCs to produce large amounts of IL-12 following CD40 ligand stimulation. In addition, DCs activated with TSLP and CD40 ligand induce the differentiation of naïve CD4+ T cells into effectors producing both Th1 and Th2 cytokines. These findings suggest that IL-12-mediated negative regulation of Th2 responses is not effective in TSLP-induced Th2 inflammation and that it leads to a mixed Th1 and Th2 profile (42).
Here, we show that H. pylori triggered human gastric epithelial cells to produce TSLP. Such cells produced a DC-attracting chemokine, macrophage inflammatory protein 3α (MIP-3α), and a B-cell-activating factor belonging to the TNF family (BAFF). After DCs were incubated with supernatants from H. pylori-infected epithelial cells, the conditioned cells expressed high levels of costimulatory molecules and triggered naïve CD4+ T cells to produce IL-4 and IL-13 with the inflammatory cytokines TNF-α and IFN-γ. In addition, TSLP was expressed by the epithelial cells of human follicular gastritis. These results suggest that H. pylori can directly trigger epithelial cells to produce TSLP and that TSLP-mediated DC activation may be involved in Th2 responses triggering B-cell activation in H. pylori-induced gastritis.
MATERIALS AND METHODS
H. pylori and H. felis.
H. pylori TN2GF4, isolated from a Japanese patient with a duodenal ulcer, was donated by M. Nakao (Pharmaceutical Research Division, Takeda Chemical Industries, Ltd., Osaka, Japan). It was maintained as described previously (26). The inoculated H. pylori strain, TN2GF4, was CagA and VacA positive, as described previously (46). Helicobacter felis (ATCC 49179) was purchased from the American Type Culture Collection (Rockville, MD). The bacteria were grown in brucella broth at a titer of 1 × 108 organisms/ml. The bacterial suspension was stored at −80°C until it was used.
Gastric epithelial cell culture.
Upon 80% confluence of the human gastric cancer cell line passages 20 to 30, AGS, MKN28, MKN45, MKN74, and KATOIII cells were trypsinized (trypsinethylenediaminetetraacetic acid; Gibco, Taastrup, Denmark). These cells were reseeded at 5.0 × 105 cells per well in six-well plates and maintained in RPMI 1640 medium (Gibco BRL, Grand Island, NY) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (Sigma, St. Louis, MO), penicillin G, and streptomycin (Gibco). Six hours after being seeded, the cells were washed with phosphate-buffered saline (PBS) and stimulated for 12 to 36 h in the presence of live H. pylori or H. felis at 1 cell per 150 bacteria or at the indicated cell/bacterium ratio. In some experiments, cells were stimulated with lipopolysaccharide (LPS) from Escherichia coli (1 μg/ml; Sigma) and cultured in a Transwell (Corning, NY).
Real-time quantitative RT-PCR.
Real-time quantitative reverse transcription (RT)-PCR was performed as described previously (45). Gastric epithelial cells were frozen in RNAlater (Qiagen, Valencia, CA) and stored at −80°C until they were used. Total RNA was extracted using an RNeasy minikit (Qiagen) according to the manufacturer's instructions. Single-stranded cDNA was synthesized with SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA). Real-time quantitative reactions were performed with an ABI Prism 7300 detection system (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Values are expressed as arbitrary units relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The following primers were used: GAPDH, 5′-CCACATCGCTCAGACACCAT-3′ and 5′-GGCAACAATATCCACTTTACCAGAGT-3′; TSLP, 5′-CCCAGGCTATTCGGAAACTCAG-3′ and 5′-CGCCACAATCCTTGTAATTGTG-3′; and BAFF, 5′-ACCGCGGGACTGAAAATCT-3′ and 5′-CACGCTTATTTCTGCTGTTCTGA-3′.
Cytokine production.
After 24 h of culture of gastric epithelial cells under the conditions described above, culture supernatants were collected and analyzed with protein enzyme-linked immunosorbent assay (ELISA) kits for TSLP, MIP-3α, MIP-1α, MIP-1β, and monocyte chemoattractant protein 1 (MCP-1) (all from R&D Systems).
DC purification and culture.
This study was approved by the Institutional Review Board for Human Research at the Graduate School of Medicine, Kyoto University. Peripheral blood mononuclear cells (PBMCs) were obtained from adult buffy coats of healthy donors (kindly provided by the Kyoto Red Cross Blood Center, Kyoto, Japan). CD11c+ DCs were isolated from PBMCs as described previously (43). CD11c+ lineage− cells were isolated with a FACS Aria (BD Biosciences, San Jose, CA) to >99% purity. CD11c+ DCs were cultured immediately after being sorted in RPMI 1640 medium supplemented with 5% human AB serum (Sigma), penicillin G, streptomycin, 10 mM HEPES, and 1 mM sodium pyruvate (Gibco BRL) (referred to as complete medium). Cells were seeded at a density of 1 × 106/ml in round-bottom 96-well plates in the presence of 15 ng/ml of TSLP (R&D Systems, Minneapolis, MN) or 50 μl of supernatant from the Helicobacter-colonized gastric epithelial cells. For neutralization of TSLP, supernatants from H. pylori-colonized gastric epithelial cells were incubated with 20 μg/ml of anti-human TSLP (R&D Systems). After 24 h of culture, viable DCs were counted by trypan blue exclusion of dead cells.
Analysis of cell surface markers of DCs.
To determine the cell surface markers characteristic of activated DCs, DCs were incubated with various stimuli for 24 h. The DCs were subsequently stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD80 (Immunotech). Finally, they were analyzed with a FACS Calibur (BD Biosciences).
DC-T-cell coculture.
After 24 h of culture, CD11c+ DCs were collected and washed three times to remove any cytokines. Viable DCs were counted by trypan blue exclusion of dead cells. CD4+ CD45RA+ naive T cells were isolated from PBMCs using a FACS Aria to reach >99% purity, as described previously (43). The remaining DCs were cocultured with 2.5 × 104 freshly purified allogeneic naive CD4+ T cells in round-bottom 96-well culture plates in complete medium. The cells were cultured in triplicate at a DC/T-cell ratio of 1:5. After 7 days of culture, viable cells were counted by trypan blue exclusion of dead cells.
T-cell cytokine production.
DC-primed CD4+ T cells were collected on day 7 of the coculture, washed twice, and restimulated with 50 ng/ml phorbol myristate acetate (PMA) (Sigma) plus 2 μg/ml ionomycin (Sigma) in flat-bottom 96- or 48-well plates at a concentration of 1 × 106 cells/ml. After 3.5 h, brefeldin A (Sigma) was added at 10 μg/ml. After 2.5 h, cells were collected and stained for cell surface molecules. The cells were fixed and permeabilized using a Fix & Perm Cell Permeabilization Kit (Caltag Laboratories, An Der Grub, Austria) and stained with phycoerythrin-conjugated monoclonal antibodies (MAbs) to IL-4, IL-13, TNF-α, and FITC-conjugated anti-IFN-γ (all from eBioscience). The stained cells were analyzed on a FACS Calibur.
Gastric mucosa samples.
Samples of mucosa in biopsy specimens were obtained from inflamed gastric mucosae in three patients with Helicobacter-induced follicular gastritis (male/female ratio, 1/2; mean age [range], 37.3 [32-44] years). Samples of healthy controls were taken from three patients with duodenal ulcers (male/female ratio, 2/1; mean age [range], 37.7 [35-43] years) in whom the absence of inflammation had been histopathologically confirmed.
Histological and immunohistological analyses.
Samples of mucosa in biopsy specimens were fixed in neutral buffered formalin and embedded in paraffin wax. Sections were stained with hematoxylin and eosin for histopathology. Fluorescence immunohistology was performed on frozen sections as described previously (18, 43, 45). In brief, 6-μm sections were cut from tissue blocks of frozen mucosal samples onto glass slides. The sections were air dried for 30 min, fixed in acetone for 5 min, and blocked with phosphate-buffered saline containing 10% nonfat dried milk for 30 min. The sections were stained with anti-human TSLP (43, 45), anti-CD11c (BD Pharmingen), or anti-DC-LAMP (Immunotech) antibodies (Abs) for 1 h, followed by staining using FITC-conjugated anti-immunoglobulin Abs. After the final wash, the slides were mounted with Vectashield (Vector Laboratories, Burlingame, CA) and examined under a fluorescence microscope.
Statistical analysis.
Statistical analysis was performed by the Student t test for pairwise comparisons and analysis of variance with the Tukey-Kramer test for multiple comparisons. P values below 0.05 were considered significant.
RESULTS
H. pylori colonization induces TSLP expression in human gastric epithelial cells.
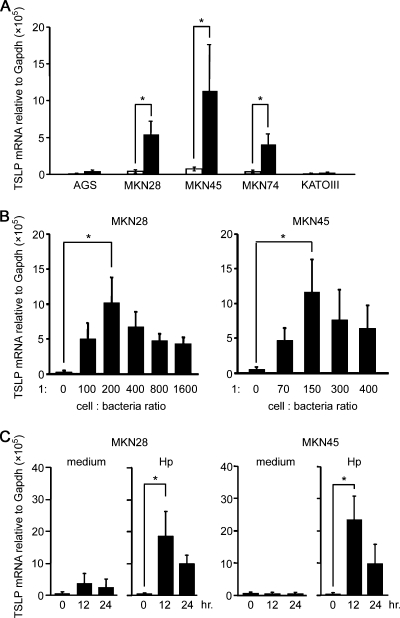
To test whether H. pylori colonization can induce expression of TSLP in gastric epithelial cells, various human gastric epithelial cell lines, such as AGS, MKN28, MKN45, MKN74, and KATOIII cells, were cultured for 24 h with H. pylori. Expression levels of mRNA encoding TSLP were measured using real-time quantitative RT-PCR. In contrast to the gastric epithelial cells not exposed to H. pylori, gastric epithelial cells—MKN28, MKN45, and MKN74—upregulated TSLP expression after H. pylori colonization (Fig. 1A). TSLP expression enhanced by H. pylori colonization was detectable when we used low epithelial cell/bacterium ratios (e.g., 1 cell per 70 to 100 bacteria) (Fig. 1B); it reached a maximal level at 1 cell per 150 to 200 bacteria and did not increase at ratios of 1 cell per 300 bacteria or higher. Because 150 to 200 bacteria can actually adhere to gastric epithelial cell membranes (6), the condition of epithelial cells expressing TSLP by H. pylori might be similar to mucosal lesions of H. pylori-infected gastritis patients. Next, we investigated the time course of H. pylori-induced TSLP expression (Fig. 1C). Upregulation of TSLP expression was induced after 12 h of H. pylori colonization and was sustained after 24 h of colonization.
FIG. 1.
TSLP expression in gastric epithelial cells after H. pylori colonization. (A) Various human gastric epithelial cell lines were cultured for 24 h with (closed bars) or without (open bars) H. pylori at 1 cell per 150 bacteria. (B) MKN28 and MKN45 cells were cultured for 24 h with H. pylori at the indicated cell/bacterium ratios. (C) MKN28 and MKN45 cells were cultured for the indicated times with H. pylori at 1 cell per 150 bacteria. Expression levels of mRNA encoding TSLP were measured using real-time quantitative RT-PCR. The data represent the means of three independent experiments. The error bars represent standard deviations (SD). *, P < 0.05.
Direct contact of H. pylori triggers human gastric epithelial cells to produce TSLP.
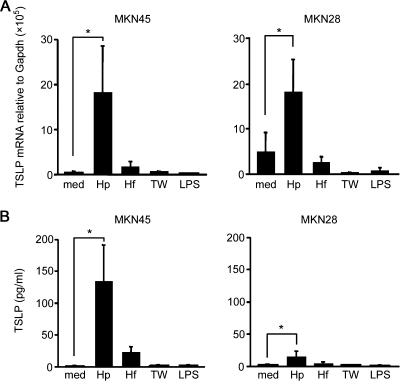
H. felis is a gastric Helicobacter that colonizes the stomachs of laboratory mice, dogs, and cats and can induce active chronic gastritis that mimics the pathological features observed in H. pyroli-induced gastritis in humans (4, 9, 19, 20, 29). Next, we examined whether H. felis colonization can induce expression of TSLP in gastric epithelial cells. MKN28 and MKN45 cells were cultured for 24 h with Helicobacter bacteria, and the expression levels of mRNA encoding TSLP were measured using real-time quantitative RT-PCR. TSLP cytokine production in the culture supernatant was assessed by protein ELISA. In contrast to the exposure to H. pylori, gastric epithelial cells did not upregulate TSLP expression after H. felis colonization (Fig. 2A and B). It seemed likely that one mechanism by which H. pylori, but not H. felis, could mediate induction of TSLP expression was by the secretion of specific proinflammatory factors. However, when gastric epithelial cells were separated from H. pylori by culture in a Transwell, induction of TSLP expression was not observed (Fig. 2A and B). Indeed, one of the proinflammatory factors, the Toll-like receptor 4 ligand LPS (from Escherichia coli), also failed to induce TSLP expression in gastric epithelial cells (Fig. 2A and B). Taken together, these data suggest that direct contact of H. pylori with human gastric epithelial cells is essential for induction of TSLP expression in these cells.
FIG. 2.
TSLP expression in gastric epithelial cells, depending on direct contact with H. pylori. MKN45 and MKN28 cells were cultured for 24 h in the presence of 1 μg/ml LPS, H. felis (Hf), or H. pylori at 1 cell per 150 bacteria with (TW) or without (Hp) using Transwell, or medium alone (med). The expression levels of TSLP mRNA were measured using real-time RT-PCR (A) or those of TSLP protein in the culture supernatant were measured by protein ELISA (B). The data represent the means of three independent experiments. The error bars represent SD. *, P < 0.05.
Direct contact of H. pylori triggers human gastric epithelial cells to produce MIP-3α.
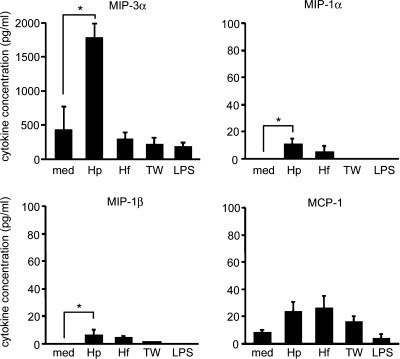
Chemokine production by epithelial cells plays a critical role in the migration of immune cells in inflamed mucosal lesions, and H. pylori infection induces upregulation of MIP-3α gene expression in gastric epithelial cells in humans and mice (26, 49). Next, we examined whether H. pylori colonization can induce production of chemokines attracting myeloid lineage cells, such as MCP-1 (also called CCL-2), MIP-1α (CCL3), MIP-1β (CCL4), and MIP-3α (CCL20), together with TSLP, in gastric epithelial cells. MKN45 cells were cultured for 24 h with Helicobacter bacteria, and chemokine production in the culture supernatant was assessed by protein ELISA. In contrast to MCP-1, MIP-1α, or MIP-1β, production of MIP-3α was strongly upregulated after colonization by H. pylori, but not H. felis, in gastric epithelial cells (Fig. 3). In addition, when gastric epithelial cells were separated from H. pylori by culture in a Transwell, no induction of MIP-3α production was observed (Fig. 3). Moreover, LPS (from Escherichia coli) failed to induce MIP-3α production in gastric epithelial cells (Fig. 3). Taken together, these data suggest that direct contact of H. pylori with human gastric epithelial cells is also essential for induction of MIP-3α production in these cells.
FIG. 3.
Chemokine production by gastric epithelial cells, depending on direct contact with H. pylori. The cells were cultured as described in the legend to Fig. 2. MIP-3α, MIP-1α, MIP-1β, and MCP-1 were measured in the culture supernatant by protein ELISA. The data represent the means of three independent experiments. The error bars represent SD. *, P < 0.05.
TSLP-containing supernatants from H. pylori-colonized gastric epithelial cells enhanced surface CD80 expression in DCs.
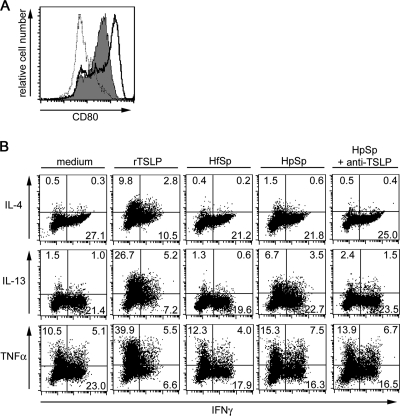
Human CD11c+ blood immature DCs respond to the stimulation of TSLP, and TSLP stimulation enhances CD80 expression in DCs (23, 44). To examine whether TSLP-containing supernatants from the H. pylori-infected gastric epithelial cells enhanced cell surface expression of CD80 in DCs, we isolated blood myeloid DCs and incubated them with supernatants from the H. pylori-infected gastric epithelial cells. After 24 h of incubation, we analyzed the surface expression of CD80 on DCs by flow cytometry. Recombinant TSLP protein induced upregulation of surface CD80 expression in DCs (Fig. 4A). Notably, after DCs were incubated with supernatants from H. pylori-infected epithelial cells, the conditioned cells also enhanced surface CD80 expression (Fig. 4A).
FIG. 4.
CD80 expression on myeloid DCs and the cytokine-producing capacity of CD4+ T cells are expanded by activated DCs. (A) Purified CD11c+ myeloid DCs were cultured with recombinant TSLP (solid line, open histogram) or supernatants from MKN45 cells with (filled histogram) or without (dotted line, open histogram) exposure to H. pylori at 1 cell per 150 bacteria. After 24 h of incubation, the surface expression of CD80 on DCs was determined by flow cytometry. The data represent one of three experiments. (B) Purified DCs were cultured with rTSLP or supernatants from MKN45 cells exposed to H. felis (HfSp), H. pylori (HpSp) at 1 cell per 150 bacteria, or medium alone (medium). For neutralization of TSLP, supernatants were incubated with anti-human TSLP (20 μg/ml) (HpSp + anti-TSLP). After 24 h of incubation, DCs were cocultured with allogeneic naïve CD4+ T cells at a 1:5 ratio of DCs to T cells. After 7 days of coculture, T cells were restimulated for 6 h with PMA plus ionomycin, and production of indicated T-cell-derived cytokines was determined by intracellular cytokine staining. Shown are dot blot profiles of the indicated cytokine-producing cells. The numbers indicate the percentages of cells in each quadrant. The data represent one of three independent experiments.
DCs conditioned by TSLP-containing supernatants prime naïve CD4 T cells to differentiate into effectors producing both Th1 and Th2 cytokines.
TSLP-activated DCs induce differentiation of inflammatory Th2 cells, and DCs activated with TSLP and CD40 ligand induce the differentiation of effectors producing both Th1 and Th2 cytokines (23, 42, 44). Next, we conditioned DCs with TSLP-containing supernatants from the H. pylori-colonized gastric epithelial cells and cocultured these DCs with allogeneic naïve CD4+ T cells at a 1:5 ratio of DCs to T cells. After 7 days of coculture, we evaluated the cytokine production capacity using intracellular cytokine staining of primed T cells restimulated with PMA plus ionomycin. This staining demonstrated that DCs conditioned with supernatants from gastric epithelial cells without Helicobacter colonization (medium) primed CD4+ T cells to produce IFN-γ and TNF-α, but not IL-4 or IL-13 (Fig. 4B). In contrast, DCs activated with recombinant TSLP protein (rTSLP) primed CD4+ T cells to produce IL-4, IL-13, and TNF-α as expected. Although DCs incubated with supernatants from H. felis-colonized gastric epithelial cells (HfSp) did not change the Th1 cytokine production profile of cocultured CD4+ T cells, DCs incubated with supernatants from H. pylori-colonized gastric epithelial cells (HpSp) did change it to a mixed Th1 and Th2 profile in which cocultured T cells produced IL-4 and IL-13, together with IFN-γ and TNF-α. After incubation of the TSLP-containing supernatants with neutralizing antibodies to human TSLP, however, the conditioned DCs (HpSp plus anti-TSLP) did not prime T cells to produce high levels of IL-4 and IL-13. These data suggest that DCs conditioned with supernatants from the H. pylori-colonized gastric epithelial cells promote naïve CD4+ T cells to differentiate into effectors producing both Th1 and Th2 cytokines and that TSLP in the supernatants is responsible for the conditioning of DCs to prime CD4+ T cells to produce Th2 cytokines.
H. pylori-colonized gastric epithelial cells sequentially upregulate TSLP and B-cell-activating factor BAFF.
BAFF (also called BLyS) is a powerful regulator of B-cell biology (2, 17, 33, 39). Human TSLP-secreting tonsillar epithelial cells produce BAFF to induce class switching by stimulating B cells (48). Next, we tested whether H. pylori colonization on gastric epithelial cells can upregulate BAFF gene expression in gastric epithelial cells. Such cells were cultured with H. pylori, and the expression levels of mRNA encoding BAFF were measured using real-time quantitative RT-PCR. In contrast to gastric epithelial cells not exposed to H. pylori, gastric epithelial cells that were exposed upregulated BAFF expression (Fig. 5). Although induction of TSLP expression peaked at 12 h after H. pylori colonization, induction of BAFF expression was detected after 12 h of H. pylori colonization and increased even after 36 h. These findings suggest that H. pylori colonization induces upregulation of TSLP and subsequently of BAFF expression in gastric epithelial cells.
FIG. 5.
TSLP and BAFF expression in gastric epithelial cells after H. pylori colonization. MKN28 cells were cultured for the indicated times with (closed bars) or without (open bars) H. pylori at 1 cell per 150 bacteria. The expression levels of mRNAs encoding TSLP and BAFF were measured using real-time quantitative RT-PCR. The data represent the means of three independent experiments. The error bars represent SD. *, P < 0.05.
Expression of TSLP is induced in mucosal lesions from H. pylori-infected gastritis patients.
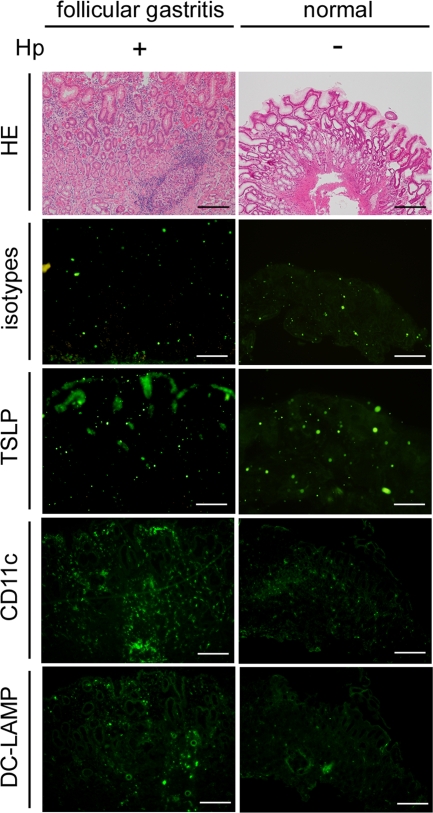
Finally, we evaluated the expression of TSLP in mucosal lesions from patients with H. pylori-infected follicular gastritis in which B-cell activation, including Th2 responses, was apparently involved. Frozen sections of mucosal lesions from follicular gastritis were stained with anti-TSLP antibodies. As shown in Fig. 6, immunoglobulin isotype control antibodies did not produce any positive staining, and there was no detectable immunostaining for TSLP in normal gastric mucosa. However, we found anti-TSLP staining of epithelial cells in the inflamed gastric mucosa from patients with H. pylori-infected follicular gastritis. TSLP expression was associated with the presence of CD11c+ DCs within the inflamed gastric mucosa and was also associated with the presence of DC-lysosome-associated membrane protein (DC-LAMP, which is a DC activation marker)-positive cells. These data suggest that expression of TSLP is enhanced in mucosal lesions from H. pylori-induced follicular gastritis patients, but not in normal gastric mucosa, and also that TSLP expressed by gastric epithelial cells may play an important role in DC-mediated T-cell activation of a process related to Th2 inflammation in H. pylori-induced chronic gastritis.
FIG. 6.
Immunohistological staining of gastric mucosa. H. pylori (Hp)-infected inflamed mucosa containing a lymphoid follicle from a patient with follicular gastritis (left column) and non-H. pylori-infected normal mucosa (right column) were stained with hematoxylin and eosin (HE), immunoglobulin isotype control antibodies (isotypes), anti-human TSLP (TSLP), anti-CD11c (CD11c), or anti-DC-LAMP (DC-LAMP). All scale bars, 100 μm.
DISCUSSION
In the present study, we demonstrated that H. pylori triggered human gastric epithelial cells to produce TSLP, together with the DC-attracting chemokine MIP-3α and B-cell activating factor BAFF. DCs conditioned by H. pylori-infected epithelial cells expressed high levels of costimulatory molecules and primed naïve CD4 T cells to differentiate into effectors producing both Th1 and Th2 cytokines.
H. pylori-induced atrophic gastritis is characterized by marked infiltration of CD4+ T cells that produce IFN-γ (12, 14). Development of H. pylori-induced atrophic gastritis is severely impaired in mice lacking CD4+ T cells or IFN-γ production (7, 32). In addition, development of Th1 cell-mediated atrophic gastritis is also severely impaired in Helicobacter-infected Peyer's patch-null mice, which normally develop well-organized lymphoid organs, except for Peyer's patches. In these mice, the marked colonization of bacteria in the gastric mucosa can be detected (18, 25).
Although the gastric mucosa originally does not have a lymphoid apparatus, H. pylori infection triggers the development of MALT-like structures consisting of lymphoid aggregates and organized B-cell follicles in patients with H. pylori-induced gastritis (10, 11, 34). High-level expression of B-cell-activating chemokine 1 (BCA-1) (also called CXCL13) and its receptor, CXC chemokine receptor (CXCR) 5, is observed in lymphoid aggregates and in the mantle zone of secondary lymphoid follicles in Helicobacter-induced follicular gastritis, suggesting that the chemokine-chemokine receptor interaction triggers the recruitment of lymphocytes (24). However, the mechanisms underlying the triggering of B-cell activation, including Th2 responses apparently involved in H. pylori-induced chronic gastritis, are not fully understood.
Chronic inflammatory processes in both autoimmunity and infection are characterized by the infiltration of a variety of immune cells, such as T cells, macrophages, and DCs, but also B cells and plasma cells. These cellular elements often organize the de novo formation of B-cell follicles and T-cell areas (1). In mouse models of H. pylori-induced follicular gastritis, the inflamed gastric mucosa contains B-cell follicles with germinal centers, T-cell areas, and high endothelial venules (28, 31). On the other hand, the constitutive expression of BAFF in secondary lymphoid tissues is essential for sustaining the long-term survival of mature B cells in vivo (17, 33). Human TSLP-secreting tonsillar epithelial cells produce BAFF to induce class switching by stimulating B cells (48). In addition, a recent human study suggested an association between circulating levels of BAFF in ectopic germinal centers of the salivary gland in primary Sjögren's syndrome (16, 37). Taken together, these results suggest that TSLP, together with BAFF, produced by H. pylori-infected gastric mucosa leads to inflammatory Th2 responses and maintenance of B-cell activation.
TSLP is involved in a variety of immune responses in the mucosal immune system in humans and mice. Epithelial cells in the tonsils and intestines release TSLP (13, 30, 38, 43, 48, 50). Human TSLP-secreting tonsillar epithelial cells produce BAFF to induce class switching by stimulating B cells (48). TSLP-conditioned DCs induce homeostatic noninflammatory Th2 responses and produce a proliferation-inducing ligand (APRIL) (13, 30, 43, 50). This results in enhancement of IgA2 class switching by intestinal epithelial cells under physiological conditions. In addition, TSLP-mediated signaling plays critical roles in host-protective Th2 cytokine-dependent immunity to the intestinal nematode pathogen Trichuris (40, 50). TSLP is involved in the regulation of Th1-type inflammation in a mouse model of colitis (40). We showed that H. pylori, one of the major pathogens in the human gastrointestinal tract, triggered gastric epithelial cells to produce TSLP, implying that TSLP may be crucial in a variety of immune responses in the gastrointestinal tract, including the stomach.
In mice, TSLP appears to suppress Th1 responses by acting on DCs in the mucosal immune system. In infection of mice by Trichuris, neutralization of TSLP or deletion of the TSLP receptor (TSLPR) in normally resistant mice resulted in defective expression of Th2 cytokines and persistent intestinal infection (40). In the intestinal inflammation in these mice, expression of inflammatory cytokines, such as IFN-γ, IL-17, and IL-12/23p40, was abundant. Neutralization of IFN-γ rescued the Th2 response and restored antiworm immunity in TSLP-deficient mice. In humans, although TSLP-activated DCs promote CD4+ T cells to differentiate into inflammatory Th2 cells, TSLP primes DCs to produce large amounts of IL-12 following CD40 ligand stimulation. DCs activated with TSLP and CD40 ligand induce the differentiation of naïve CD4+ T cells into effectors producing both Th1 and Th2 cytokines (23, 42, 44). These data suggest that, in the human system, induction of Th1 responses mediated by IL-12 is not suppressed under TSLP-induced Th2 inflammation and that IL-12-mediated negative regulation of Th2 responses is not effective in TSLP-induced Th2 inflammation. In this study, DCs conditioned by Helicobacter-infected epithelial cells primed naïve CD4 T cells to differentiate into effectors producing both Th1 and Th2 cytokines. Taken together, although Th1 cytokine-producing CD4+ T cells markedly infiltrate inflamed mucosa in Helicobacter-induced chronic gastritis, these Th1 cytokines may not suppress Th2 cytokine production by TSLP-conditioned DCs.
Several recent studies have shown that microorganism-derived stimulation and/or proinflammatory cytokines upregulate expression of both TSLP and MIP-3α in human epithelial cells, and these upregulations are directly controlled by NF-κB (15, 21, 22, 36). In addition, although it is unclear whether BAFF expression is under the direct control of NF-κB, a recent study demonstrated that Toll-like receptor 3 ligand poly(I·C) induced upregulation of TSLP and subsequently of BAFF production in human tonsillar epithelial cells (48). In this study, we demonstrated that H. pylori triggered human gastric epithelial cells to produce TSLP, together with MIP-3α and BAFF. Because H. pylori products induce NF-κB activation in gastric epithelial cells (8, 27, 41, 47), H. pylori-induced NF-κB activation in gastric epithelial cells may directly and/or indirectly upregulate the expression of TSLP, MIP-3α, and BAFF in these cells.
In conclusion, we have demonstrated that H. pylori triggered gastric epithelial cells to produce TSLP, MIP-3α, and BAFF and that DCs conditioned by Helicobacter-infected epithelial cells triggered differentiation of T cells with a mixed Th1 and Th2 profile. These results, and the finding that TSLP was expressed by the epithelial cells of human follicular gastritis, suggest that H. pylori can directly trigger epithelial cells to produce TSLP and that TSLP-mediated DC activation may be involved in Th2 responses triggering B-cell activation in H. pylori-induced gastritis.
Acknowledgments
We thank Dovie Wylie for assistance in preparation of the manuscript.
This work is supported by Grants-in-Aid for Scientific Research 18012029, 18015028, 18209027, 18590679, and 20390207 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; a Grant-in-Aid for Research from the Japanese Society of Gastroenterology; and Grants-in-Aid from the Naito Foundation, the Novartis Foundation for the Promotion of Science, the Uehara Memorial Foundation, the Takeda Science Foundation, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, the Astellas Foundation for Research on Metabolic Disorders, and the Yakult Bioscience Research Foundation.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 19 October 2009.
REFERENCES
- 1.Aloisi, F., and R. Pujol-Borrell. 2006. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 6:205-217. [DOI] [PubMed] [Google Scholar]
- 2.Brink, R. 2006. Regulation of B cell self-tolerance by BAFF. Semin. Immunol. 18:276-283. [DOI] [PubMed] [Google Scholar]
- 3.Chiba, T., H. Seno, H. Marusawa, Y. Wakatsuki, and K. Okazaki. 2006. Host factors are important in determining clinical outcomes of Helicobacter pylori infection. J. Gastroenterol. 41:1-9. [DOI] [PubMed] [Google Scholar]
- 4.Dick, E., A. Lee, G. Watson, and J. O'Rourke. 1989. Use of the mouse for the isolation and investigation of stomach-associated, spiral-helical shaped bacteria from man and other animals. J. Med. Microbiol. 29:55-62. [DOI] [PubMed] [Google Scholar]
- 5.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston, 1994. Am. J. Surg. Pathol. 10:1161-1181. [DOI] [PubMed] [Google Scholar]
- 6.Dunn, B. E., M. Altmann, and G. P. Campbell. 1991. Adherence of Helicobacter pylori to gastric carcinoma cells: analysis by flow cytometry. Rev. Infect. Dis. 13(Suppl. 8):S657-S664. [DOI] [PubMed] [Google Scholar]
- 7.Eaton, K. A., M. Mefford, and T. Thevenot. 2001. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J. Immunol. 166:7456-7461. [DOI] [PubMed] [Google Scholar]
- 8.Farinha, P., and R. D. Gascoyne. 2005. Helicobacter pylori and MALT lymphoma. Gastroenterology 128:1579-1605. [DOI] [PubMed] [Google Scholar]
- 9.Fox, J. G., M. Blanco, J. C. Murphy, N. S. Taylor, A. Lee, Z. Kabok, and J. Pappo. 1993. Local and systemic immune responses in murine Helicobacter felis active chronic gastritis. Infect. Immun. 61:2309-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genta, R. M., and H. W. Hamner. 1994. The significance of lymphoid follicles in the interpretation of gastric biopsy specimens. Arch. Pathol. Lab. Med. 118:740-743. [PubMed] [Google Scholar]
- 11.Genta, R. M., H. W. Hamner, and D. Y. Graham. 1993. Gastric lymphoid follicles in Helicobacter pylori infection: frequency, distribution, and response to triple therapy. Hum. Pathol. 24:577-583. [DOI] [PubMed] [Google Scholar]
- 12.Harris, P. R., L. E. Smythies, P. D. Smith, and A. Dubois. 2000. Inflammatory cytokine mRNA expression during early and persistent Helicobacter pylori infection in nonhuman primates. J. Infect. Dis. 181:783-786. [DOI] [PubMed] [Google Scholar]
- 13.He, B., W. Xu, P. A. Santini, A. D. Polydorides, A. Chiu, J. Estrella, M. Shan, A. Chadburn, V. Villanacci, A. Plebani, D. M. Knowles, M. Rescigno, and A. Cerutti. 2007. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26:812-826. [DOI] [PubMed] [Google Scholar]
- 14.Itoh, T., Y. Wakatsuki, M. Yoshida, T. Usui, Y. Matsunaga, S. Kaneko, T. Chiba, and T. Kita. 1999. The vast majority of gastric T cells are polarized to produce T helper 1 type cytokines upon antigenic stimulation despite the absence of Helicobacter pylori infection. J. Gastroenterol. 34:560-570. [DOI] [PubMed] [Google Scholar]
- 15.Izadpanah, A., M. B. Dwinell, L. Eckmann, N. M. Varki, and M. F. Kagnoff. 2001. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G710-G719. [DOI] [PubMed] [Google Scholar]
- 16.Jonsson, M. V., P. Szodoray, S. Jellestad, R. Jonsson, and K. Skarstein. 2005. Association between circulating levels of the novel TNF family members APRIL and BAFF and lymphoid organization in primary Sjögren's syndrome. J. Clin. Immunol. 25:189-201. [DOI] [PubMed] [Google Scholar]
- 17.Kalled, S. L. 2006. Impact of the BAFF/BR3 axis on B cell survival, germinal center maintenance and antibody production. Semin. Immunol. 18:290-296. [DOI] [PubMed] [Google Scholar]
- 18.Kiriya, K., N. Watanabe, A. Nishio, K. Okazaki, M. Kido, K. Saga, J. Tanaka, T. Akamatsu, S. Ohashi, M. Asada, T. Fukui, and T. Chiba. 2007. Essential role of Peyer's patches in the development of Helicobacter-induced gastritis. Int. Immunol. 19:435-446. [DOI] [PubMed] [Google Scholar]
- 19.Lee, A., J. G. Fox, G. Otto, and J. Murphy. 1990. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology 99:1315-1323. [DOI] [PubMed] [Google Scholar]
- 20.Lee, A., S. Krakowka, J. G. Fox, G. Otto, K. A. Eaton, and J. C. Murphy. 1992. Role of Helicobacter felis in chronic canine gastritis. Vet. Pathol. 29:487-494. [DOI] [PubMed] [Google Scholar]
- 21.Lee, H. C., and S. F. Ziegler. 2007. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFκB. Proc. Natl. Acad. Sci. USA 104:914-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, H. C., M. B. Headley, M. Iseki, K. Ikuta, and S. F. Ziegler. Inhibition of NF-κB-mediated TSLP expression by retinoid X receptor. J. Immunol. 181:5189-5193. [DOI] [PMC free article] [PubMed]
- 23.Liu, Y. J., V. V. Soumelis, N. Watanabe, T. Ito, Y. H. Wang, R. de Waal Malefyt, M. Omori, B. Zhou, and S. F. Ziegler. 2007. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 25:193-219. [DOI] [PubMed] [Google Scholar]
- 24.Mazzucchelli, L., A. Blaser, A. Kappeler, P. Schärli, J. A. Laissue, M. Baggiolini, and M. Uguccioni. 1999. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J. Clin. Invest. 104:R49-R54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagai, S., H. Mimuro, T. Yamada, T. Baba, K. Moro, T. Nochi, H. Kiyono, T. Suzuki, C. Sasakawa, and S. Koyasu. 2007. Role of Peyer's patches in the induction of Helicobacter pylori-induced gastritis. Proc. Natl. Acad. Sci. USA 104:8971-8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishi, T., K. Okazaki, K. Kawasaki, T. Fukui, H. Tamaki, M. Matsuura, M. Asada, T. Watanabe, K. Uchida, N. Watanabe, H. Nakase, M. Ohana, H. Hiai, and T. Chiba. 2003. Involvement of myeloid dendritic cells in the development of gastric secondary lymphoid follicles in Helicobacter pylori-infected neonatally thymectomized BALB/c mice. Infect. Immunol. 71:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Keeffe, J., and A. P. Moran. 2008. Conventional, regulatory, and unconventional T cells in the immunologic response to Helicobacter pylori. Helicobacter 13:1-19. [DOI] [PubMed] [Google Scholar]
- 28.Oshima, C., K. Okazaki, Y. Matsushima, M. Sawada, T. Chiba, K. Takahashi, H. Hiai, T. Katakai, S. Kasakura, and T. Masuda. 2000. Induction of follicular gastritis following postthymectomy autoimmune gastritis in Helicobacter pylori-infected BALB/c mice. Infect. Immun. 68:100-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otto, G., S. H. Hazell, J. G. Fox, C. R. Howlett, J. C. Murphy, J. O'Rourke, and A. Lee. 1994. Animal and public health implications of gastric colonization of cats by Helicobacter-like organisms. J. Clin. Microbiol. 32:1043-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rimoldi, M., M. Chieppa, V. Salucci, F. Avogadri, A. Sonzogni, G. M. Sampietro, A. Nespoli, G. Viale, P. Allavena, and M. Rescigno. 2005. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 6:507-514. [DOI] [PubMed] [Google Scholar]
- 31.Shomer, N. H., J. G. Fox, A. E. Juedes, and N. H. Ruddle. 2003. Helicobacter-induced chronic active lymphoid aggregates have characteristics of tertiary lymphoid tissue. Infect. Immun. 71:3572-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smythies, L. E., K. B. Waites, J. R. Lindsey, P. R. Harris, P. Ghiara, and P. D. Smith. 2000. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-γ, gene-deficient mice. J. Immunol. 165:1022-1029. [DOI] [PubMed] [Google Scholar]
- 33.Stadanlick, J. E., and M. P. Cancro. 2008. BAFF and the plasticity of peripheral B cell tolerance. Curr. Opin. Immunol. 20:158-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stolte, M., and S. Eidt. 1989. Lymphoid follicles in antral mucosa: immune response to Campylobacter pylori? J. Clin. Pathol. 42:1269-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suerbaum, S., and P. Michetti. 2002. Helicobacter pylori infection. N. Engl. J. Med. 347:1175-1186. [DOI] [PubMed] [Google Scholar]
- 36.Sugita, S., T. Kohno, K. Yamamoto, Y. Imaizumi, H. Nakajima, T. Ishimaru, and T. Matsuyama. 2002. Induction of macrophage-inflammatory protein-3alpha gene expression by TNF-dependent NF-kappaB activation. J. Immunol. 168:5621-5628. [DOI] [PubMed] [Google Scholar]
- 37.Szodoray, P., P. Alex, M. V. Jonsson, N. Knowlton, I. Dozmorov, B. Nakken, N. Delaleu, R. Jonsson, and M. Centola. 2005. Distinct profiles of Sjögren's syndrome patients with ectopic salivary gland germinal centers revealed by serum cytokines and BAFF. Clin. Immunol. 117:168-176. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka, J., K. Saga, M. Kido, H. Nishiura, T. Akamatsu, T. Chiba, and N. Watanabe. 2009. Proinflammatory Th2 cytokines induce production of thymic stromal lymphopoietin in human colonic epithelial cells. Dig. Dis. Sci. doi: 10.1007/s10620-009-0979-x. [DOI] [PMC free article] [PubMed]
- 39.Tangye, S. G., V. L. Bryant, A. K. Cuss, and K. L. Good. 2006. BAFF, APRIL and human B cell disorders. Semin. Immunol. 18:305-317. [DOI] [PubMed] [Google Scholar]
- 40.Taylor, B. C., C. Zaph, A. E. Troy, Y. Du, K. J. Guild, M. R. Comeau, and D. Artis. 2009. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 206:655-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Mémet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. 5:1166-1174. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe, N., S. Hanabuchi, M. A. Marloie-Provost, S. Antonenko, Y. J. Liu, and V. Soumelis. 2005. Human TSLP promotes CD40-ligand-induced IL-12 production by myeloid dendritic cells but maintains their Th2 priming potential. Blood 105:4749-4751. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe, N., S. Hanabuchi, V. Soumelis, W. Yuan, S. Ho, R. de Waal Malefyt, and Y. J. Liu. 2004. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat. Immunol. 5:426-434. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe, N., V. Soumelis, and Y. J. Liu. 2005. Human thymic stromal lymphopoietin triggers dendritic cell-mediated allergic inflammation and CD4+ T cell homeostatic expansion. Adv. Exp. Med. Biol. 560:69-75. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe, N., Y. H. Wang, H. K. Lee, T. Ito, Y. H. Wang, W. Cao, and Y. J. Liu. 2005. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 436:1181-1185. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe, T., M. Tada, H. Nagai, S. Sasaki, and M. Nakao. 1998. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology 115:642-648. [DOI] [PubMed] [Google Scholar]
- 47.Wilson, K. T., and J. E. Crabtree. 2007. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. 133:288-308. [DOI] [PubMed] [Google Scholar]
- 48.Xu, W., B. He, A. Chiu, A. Chadburn, M. Shan, M. Buldys, A. Ding, D. M. Knowles, P. A. Santini, and A. Cerutti. 2007. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat. Immunol. 8:294-303. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida, A., H. Isomoto, J. Hisatsune, M. Nakayama, Y. Nakashima, K. Matsushima, Y. Mizuta, T. Hayashi, Y. Yamaoka, T. Azuma, J. Moss, T. Hirayama, and S. Kohno. 2009. Enhanced expression of CCL20 in human Helicobacter pylori-associated gastritis. Clin. Immunol. 130:290-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaph, C., A. E. Troy, B. C. Taylor, L. D. Berman-Booty, K. J. Guild, Y. Du, E. A. Yost, A. D. Gruber, M. J. May, F. R. Greten, L. Eckmann, M. Karin, and D. Artis. 2007. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature 446:552-556. [DOI] [PubMed] [Google Scholar]