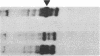
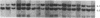Abstract
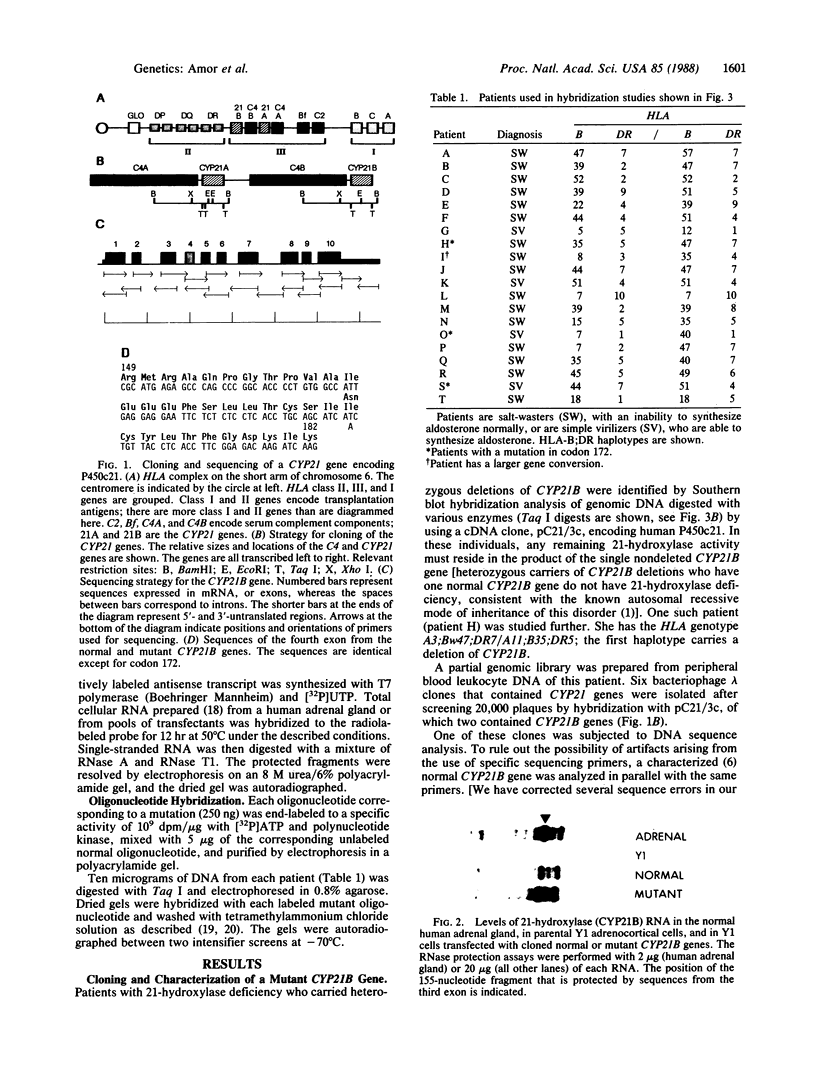
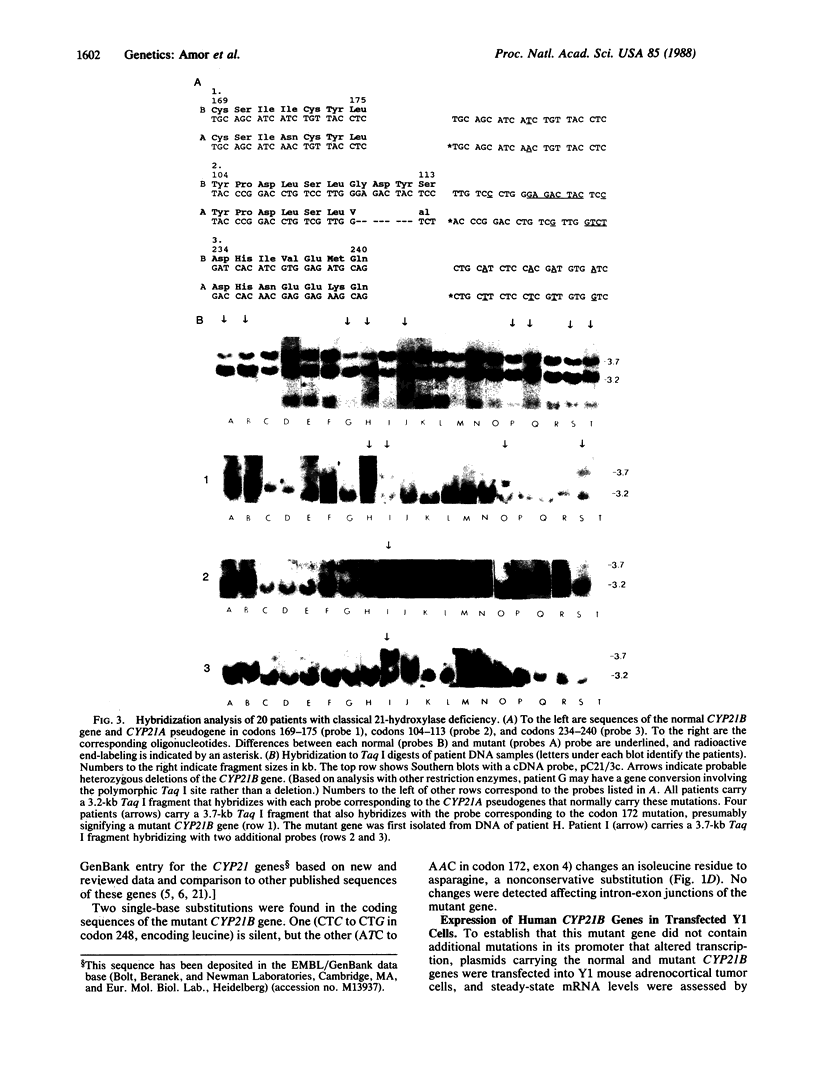
Steroid 21-hydroxylase deficiency is the most common cause of congenital adrenal hyperplasia. It results from a deficiency in a specific cytochrome P450, P450c21 (P450XXIA). The gene encoding this protein (CYP21B) and a closely linked pseudogene (CYP21A) are located in the HLA complex on chromosome 6p. Many mutant alleles are associated with deletions of CYP21B; we report the cloning and characterization of a nondeleted mutant CYP21B gene. This mutant gene is expressed on transfection into mouse Y1 adrenal cells, producing mRNA levels similar to those seen after transfection of the normal CYP21B gene. In codon 172 of the mutant gene, the normal codon ATC, encoding isoleucine, has been changed to AAC, encoding asparagine. This mutation is normally present in the CYP21A pseudogene, so that it may have been transferred to the mutant CYP21B gene by gene conversion. Hybridization of oligonucleotide probes corresponding to this and two other mutations normally present in CYP21A demonstrated that 4 out of 20 patients carried the codon 172 mutation; in one of these patients, the mutation was present as part of a larger gene conversion involving at least exons 3-6. Gene conversion may be a frequent cause of 21-hydroxylase deficiency alleles due to the presence of six chi-like sequences (GCTGGGG) in the CYP21 genes and the close proximity of the CYP21A pseudogene, which has several potentially deleterious mutations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw K. D., Waterman M. R., Couch R. T., Simpson E. R., Zuber M. X. Characterization of complementary deoxyribonucleic acid for human adrenocortical 17 alpha-hydroxylase: a probe for analysis of 17 alpha-hydroxylase deficiency. Mol Endocrinol. 1987 May;1(5):348–354. doi: 10.1210/mend-1-5-348. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Campbell R. D., Porter R. R. Mapping of steroid 21-hydroxylase genes adjacent to complement component C4 genes in HLA, the major histocompatibility complex in man. Proc Natl Acad Sci U S A. 1985 Jan;82(2):521–525. doi: 10.1073/pnas.82.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. D., Galbraith L. J., Seidman J. G., White P. C., Parker K. L. Nucleotide sequence analysis of murine 21-hydroxylase genes: mutations affecting gene expression. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9601–9605. doi: 10.1073/pnas.83.24.9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- DiLella A. G., Woo S. L. Hybridization of genomic DNA to oligonucleotide probes in the presence of tetramethylammonium chloride. Methods Enzymol. 1987;152:447–451. doi: 10.1016/0076-6879(87)52052-3. [DOI] [PubMed] [Google Scholar]
- Evans M. I., Chrousos G. P., Mann D. W., Larsen J. W., Jr, Green I., McCluskey J., Loriaux D. L., Fletcher J. C., Koons G., Overpeck J. Pharmacologic suppression of the fetal adrenal gland in utero. Attempted prevention of abnormal external genital masculinization in suspected congenital adrenal hyperplasia. JAMA. 1985 Feb 15;253(7):1015–1020. [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Nebert D. W., Hardwick J. P., Kasper C. B. Complete cDNA and protein sequence of a pregnenolone 16 alpha-carbonitrile-induced cytochrome P-450. A representative of a new gene family. J Biol Chem. 1985 Jun 25;260(12):7435–7441. [PubMed] [Google Scholar]
- Higashi Y., Yoshioka H., Yamane M., Gotoh O., Fujii-Kuriyama Y. Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and a genuine gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2841–2845. doi: 10.1073/pnas.83.9.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal A. K., Gonzalez F. J., Nebert D. W. Human dioxin-inducible cytochrome P1-450: complementary DNA and amino acid sequence. Science. 1985 Apr 5;228(4695):80–83. doi: 10.1126/science.3838385. [DOI] [PubMed] [Google Scholar]
- Krawinkel U., Zoebelein G., Brüggemann M., Radbruch A., Rajewsky K. Recombination between antibody heavy chain variable-region genes: evidence for gene conversion. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4997–5001. doi: 10.1073/pnas.80.16.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y., Sogawa K., Suwa Y., Muramatsu M., Fujii-Kuriyama Y. Gene structure of a phenobarbital-inducible cytochrome P-450 in rat liver. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3958–3962. doi: 10.1073/pnas.80.13.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B., Levin W. The P450 gene superfamily: recommended nomenclature. DNA. 1987 Feb;6(1):1–11. doi: 10.1089/dna.1987.6.1. [DOI] [PubMed] [Google Scholar]
- Parker K. L., Chaplin D. D., Wong M., Seidman J. G., Smith J. A., Schimmer B. P. Expression of murine 21-hydroxylase in mouse adrenal glands and in transfected Y1 adrenocortical tumor cells. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7860–7864. doi: 10.1073/pnas.82.23.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues N. R., Dunham I., Yu C. Y., Carroll M. C., Porter R. R., Campbell R. D. Molecular characterization of the HLA-linked steroid 21-hydroxylase B gene from an individual with congenital adrenal hyperplasia. EMBO J. 1987 Jun;6(6):1653–1661. doi: 10.1002/j.1460-2075.1987.tb02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsby G., Carroll M. C., Porter R. R., Grant D. B., Hjelm M. Deletion of the steroid 21-hydroxylase and complement C4 genes in congenital adrenal hyperplasia. J Med Genet. 1986 Jun;23(3):204–209. doi: 10.1136/jmg.23.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Kunes S. M., Schultz D. W., Taylor A., Triman K. L. Structure of chi hotspots of generalized recombination. Cell. 1981 May;24(2):429–436. doi: 10.1016/0092-8674(81)90333-0. [DOI] [PubMed] [Google Scholar]
- Stoner E., Dimartino-Nardi J., Kuhnle U., Levine L. S., Oberfield S. E., New M. I. Is salt-wasting in congenital adrenal hyperplasia due to the same gene as the fasciculata defect? Clin Endocrinol (Oxf) 1986 Jan;24(1):9–20. doi: 10.1111/j.1365-2265.1986.tb03249.x. [DOI] [PubMed] [Google Scholar]
- Werkmeister J. W., New M. I., Dupont B., White P. C. Frequent deletion and duplication of the steroid 21-hydroxylase genes. Am J Hum Genet. 1986 Oct;39(4):461–469. [PMC free article] [PubMed] [Google Scholar]
- White P. C., Grossberger D., Onufer B. J., Chaplin D. D., New M. I., Dupont B., Strominger J. L. Two genes encoding steroid 21-hydroxylase are located near the genes encoding the fourth component of complement in man. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1089–1093. doi: 10.1073/pnas.82.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Cloning and expression of cDNA encoding a bovine adrenal cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1986–1990. doi: 10.1073/pnas.81.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Congenital adrenal hyperplasia (2). N Engl J Med. 1987 Jun 18;316(25):1580–1586. doi: 10.1056/NEJM198706183162506. [DOI] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7505–7509. doi: 10.1073/pnas.81.23.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Structure of human steroid 21-hydroxylase genes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5111–5115. doi: 10.1073/pnas.83.14.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Saunders T. L., Bach F. H. Polymorphism of human Ia antigens generated by reciprocal intergenic exchange between two DR beta loci. Nature. 1986 Dec 18;324(6098):676–679. doi: 10.1038/324676a0. [DOI] [PubMed] [Google Scholar]
- Wyman A. R., White R. A highly polymorphic locus in human DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6754–6758. doi: 10.1073/pnas.77.11.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H., Morohashi K., Sogawa K., Yamane M., Kominami S., Takemori S., Okada Y., Omura T., Fujii-Kuriyama Y. Structural analysis of cloned cDNA for mRNA of microsomal cytochrome P-450(C21) which catalyzes steroid 21-hydroxylation in bovine adrenal cortex. J Biol Chem. 1986 Mar 25;261(9):4106–4109. [PubMed] [Google Scholar]