Abstract
RNA editing in higher plant organelles results in the conversion of specific cytidine residues to uridine residues in RNA. The recognition of a specific target C site by the editing machinery involves trans-acting factors that bind to the RNA upstream of the C to be edited. In the last few years, analysis of mutants affected in chloroplast biogenesis has identified several pentatricopeptide repeat (PPR) proteins from the PLS subfamily that are essential for the editing of particular RNA transcripts. We selected other genes from the same subfamily and used a reverse genetics approach to identify six new chloroplast editing factors in Arabidopsis thaliana (OTP80, OTP81, OTP82, OTP84, OTP85, and OTP86). These six factors account for nine editing sites not previously assigned to an editing factor and, together with the nine PPR editing proteins previously described, explain more than half of the 34 editing events in Arabidopsis chloroplasts. OTP80, OTP81, OTP85, and OTP86 target only one editing site each, OTP82 two sites, and OTP84 three sites in different transcripts. An analysis of the target sites requiring the five editing factors involved in editing of multiple sites (CRR22, CRR28, CLB19, OTP82, and OTP84) suggests that editing factors can generally distinguish pyrimidines from purines and, at some positions, must be able to recognize specific bases.
INTRODUCTION
The term RNA editing covers a multitude of processes that lead to posttranscriptional sequence alterations in RNAs. In plants, RNA editing generally indicates highly specific cytidine-to-uridine conversions that are observed in both mitochondrial and plastid transcripts. Although similar editing events occur in other organisms (see review by Simpson and Emeson, 1996), it is clear that this form of RNA editing arose in an immediate ancestor of land plants and is not homologous to editing processes found in other phyla and may not be mechanistically related. It is likely that >500 sites are specifically altered in Arabidopsis thaliana mitochondrial transcripts (Giegé and Brennicke, 1999; Bentolila et al., 2008; Zehrmann et al., 2008), and at least 34 sites are known to be changed in Arabidopsis plastid transcripts (Chateigner-Boutin and Small, 2007). RNA editing typically affects the transcripts of protein-coding genes but has also been found to modify noncoding transcribed regions, structural RNAs, and intron sequences. RNA editing is essential for correct gene expression: proteins translated from edited transcripts are usually different from the ones deduced from the gene sequence and usually present higher similarity to the corresponding nonplant homologs (reviewed in Shikanai, 2006).
A major question concerning the editing process is the manner by which the hundreds of editing sites are specifically targeted. In vivo experiments using transgenic chloroplasts have shown that mRNA editing sites are recognized via cis-acting elements that are generally located within ∼30 nucleotides of the editing site (Chaudhuri et al., 1995; Bock et al., 1996, 1997; Chaudhuri and Maliga, 1996; Reed et al., 2001). This has been confirmed in vitro using synthetic RNA substrates to explore editing specificity in both chloroplast (Miyamoto et al., 2002; Hegeman et al., 2005; Hayes et al., 2006) and mitochondrial extracts (Neuwirt et al., 2005; Takenaka et al., 2004, 2008; Verbitskiy et al., 2008). In other systems with such pervasive RNA editing, target sites are recognized by guide RNAs complementary to the RNA strand to be edited (Simpson and Emeson, 1996). However, there is good biochemical evidence that the trans-factors that bind to the cis-elements neighboring editing sites in plant organelles are proteins (Chaudhuri et al., 1995; Bock and Koop, 1997; Hirose and Sugiura, 2001). UV cross-linking experiments in an in vitro tobacco (Nicotiana tabacum) editing system reveals that proteins with distinct molecular masses of 25, 56, 70, 91, and 93 kD specifically bind to the cis-acting elements required for editing in psbL, psbE, petB, rpoB, and rpoA, respectively (Hirose and Sugiura, 2001; Miyamoto et al., 2002, 2004; Kobayashi et al., 2008). The same protein of 95 kD specifically binds cis-acting elements of two editing sites, ndhB-9 and ndhF-2, showing that the same trans-factor can recognize more than one site (Kobayashi et al., 2008). However, none of these biochemically investigated factors has been identified.
A lack of editing of many of the sites identified in plant organelles would be expected to lead to striking phenotypes due to misexpression of proteins involved in photosynthesis or respiration. Investigation of Arabidopsis mutants with such phenotypes has identified a number of nuclear genes suspected to encode RNA-editing specificity factors (Kotera et al., 2005; Okuda et al., 2007, 2009; Chateigner-Boutin et al., 2008; Cai et al., 2009; Kim et al., 2009; Robbins et al., 2009; Yu et al., 2009; Zehrmann et al., 2009; Zhou et al., 2009). In each case, loss of one of these factors leads to a specific loss of editing of one or at most a few sites. Proof that any of these genetically identified factors directly targets its editing site is limited to a study of the protein CRR4. This factor is required for editing of the initiation codon of plastid ndhD transcripts and binds specifically within the region −25/+10 spanning the editing site (Okuda et al., 2006). CRR4, like of all of the editing factors described to date, is a pentatricopeptide repeat (PPR) protein (Schmitz-Linneweber and Small, 2008), characterized by tandem arrays of the 35–amino acid motif (Small and Peeters, 2000) for which this family of proteins is named. PPR proteins form a large family of >450 RNA binding proteins in Arabidopsis, the majority of which are thought to be targeted to mitochondria or chloroplasts (Lurin et al., 2004). More precisely, the reported organelle editing factors are all members of the plant-specific E and DYW subclasses of the PPR family (Table 1), characterized by distinctive C-terminal domains (Lurin et al., 2004; O'Toole et al., 2008). The E (extended) domain is a degenerate motif with some similarities to PPR motifs, while the DYW domain, named for its typical Asp-Tyr-Trp C-terminal tripeptide, is much more highly conserved, and its presence correlates phylogenetically with plant organelle RNA editing (Salone et al., 2007; Rudinger et al., 2008).
Table 1.
Candidate and Known Genes Affecting Cchloroplast RNA Editing
| Arabidopsis Locus | Subclass | Predotar | TargetP | Gene Name | Editing Defect (Genome Position) | AA Change | T-DNA Insertion Lines | T-DNA Location |
|---|---|---|---|---|---|---|---|---|
| At2g02980 | DYW | pC | C | OTP85 | ndhD (116,494) | S>L | SAIL_544_B03 | CDS |
| At2g29760 | DYW | C | C | OTP81 | rps12 intron (69,553) | SALK_092402 | 5′UTR | |
| At3g03580 | DYW | pC | C | None | GABI_895H11 | CDS | ||
| At3g08820 | DYW | C | N | None | SALK_023916 | CDS | ||
| At3g57430 | DYW | ER | C | OTP84 | ndhF (112,349), psbZ (35,800)ndhB (94,999) | S>L, S>LP>L | SAIL_568_C04 | CDS |
| SALK_120902 | CDS | |||||||
| At3g62890 | DYW | N | C | None | SAIL_1249_D04 | CDS | ||
| SALK_044324 | CDS | |||||||
| At3g63370 | DYW | pC | C | OTP86 | rps14 (37161) | S>L | SALK_102445 | CDS |
| At4g35130 | DYW | C | C | None | SALK_118555 | CDS | ||
| At1g74600 | E | C | C | None | GABI_073C06 | CDS | ||
| At1g77170 | E | pM | C | None | SAIL_1291_C04 | CDS | ||
| At3g22150 | E | C | C | None | SALK_040629 | CDS | ||
| At3g29230 | E | pC | M | None | SAIL_205_G08 | CDS | ||
| At4g04370 | E | pC | N | None | SALK_025427 | CDS | ||
| At4g25270 | E | pC | C | None | SALK_090845 | CDS | ||
| At5g59200 | E | C | C | OTP80 | rpl23 (86,056) | S>L | SALK_060533SALK_111721 | CDSCDS |
| At1g08070 | DYW | pC | C | OTP82 | ndhG (118,858), ndhB (95,644) | S>F, S>L | SAIL_851_G04 | CDS |
| SALK_027812 | CDS | |||||||
| At1g11290 | DYW | N | C | CRR22 | ndhB (96,419), ndhD (116,281) rpoB (25,779) | Okuda et al. (2009) | ||
| At1g15510 | DYW | C | C | AtECB2 | accD (57,868) | Yu et al. (2009) | ||
| At1g59720 | DYW | N | C | CRR28 | ndhB (96,698), ndhD (116,290) | Okuda et al. (2009) | ||
| At3g22690 | DYW | pC | C | YS1 | rpoB (25,992) | Zhou et al. (2009) | ||
| At5g13270 | DYW | C | C | RARE1 | accD (57,868) | Robbins et al. (2009) | ||
| At5g48910 | DYW | N | C | LPA66 | psbF (63,985) | Cai et al. (2009) | ||
| At2g45350 | E | pC | N | CRR4 | ndhD (117,166) | Kotera et al. (2005) | ||
| At1g05750 | E+ | C | C | CLB19 | rpoA (78,691), clpP (69,942) | Chateigner-Boutin et al. (2008) | ||
| At5g55740 |
E+ |
C |
C |
CRR21 |
ndhD (116,785) |
Okuda et al. (2007) |
pC, potentially plastid; C, plastid; pM, potentially mitochondrial; M, mitochondrial; ER, secretory pathway; N, none; AA, amino acid; UTR, untranslated region; CDS, coding sequence. The new editing factors revealed by this study are indicated in bold.
Most of the editing factors described so far were identified through forward genetic screens, generally via the distinctive phenotype caused by loss of editing. However, the similarity among all of them suggested to us that a reverse genetics approach concentrating on genes encoding similar proteins might identify new editing specificity factors. Using a high-resolution melting of amplicons technique for screening for editing defects (Chateigner-Boutin and Small, 2007), we identified six novel Arabidopsis mutants affected in editing of plastid mRNAs. An analysis of these mutants and comparison with the previously described mutants allows us to make some progress toward understanding the recognition of editing sites in plant organelles.
RESULTS
Identification of PPR Genes Required for RNA Editing in Plastids
All previously identified factors required for editing of specific sites are proteins of the E or DYW subclasses of the PPR family. As the number of editing sites in chloroplasts is much lower than that in mitochondria and therefore easier to screen, we chose to focus on E and DYW members predicted to be targeted to plastids by TargetP (Emanuelsson et al., 2000) or Predotar (Small et al., 2004). Sixteen candidate genes were selected because of the availability of T-DNA insertion mutants for each gene (Table 1). For each mutant, the location of the T-DNA insertion was verified by PCR and sequencing, and homozygous mutant lines identified (see Methods for the insertion sites). To test if a T-DNA insertion in these genes leads to an RNA editing defect, the status of the 34 editing sites in Arabidopsis plastids was systematically examined in each mutant using a high-resolution melting screen (Chateigner-Boutin and Small, 2007).
Among these 16 genes, the disruption of six of them leads to a defect in RNA editing in plastids. We respectively named these genes OTP80, OTP81, OTP82, OTP84, OTP85, and OTP86 (for Organelle Transcript Processing). OTP80 encodes a PPR protein from the E subclass, whereas OTP81, 82, 84, 85, and 86 encode members of the DYW subclass. OTP82 was independently identified in another screen and will be described elsewhere. Disruption of the other 10 genes did not lead to an observable defect in RNA editing of any of the 34 sites we tested.
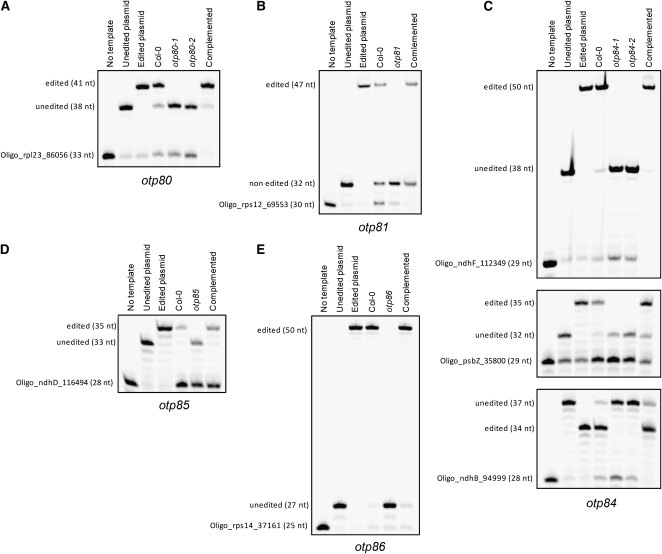
In the mutants otp80, otp81, otp85, and otp86, a single editing site was affected, but in otp84, three different editing sites appeared to remain unedited (Table 1; see Supplemental Figure 1 online). All of the editing defects were confirmed by a more sensitive and quantitative poisoned primer extension assay (Figure 1). In otp81, editing in the rps12 intron (at genome position 69,553) is reduced to 2% (Figure 1B). We consider the residual editing to indicate that this is a weak allele, since the T-DNA is inserted into the 5′-untranslated region of OTP81 (see Methods). The mutants otp80, otp85, and otp86 are totally impaired in the editing of rpl23 (86,056), ndhD (116494), and rps14 (37,161), respectively (Figures 1A to 1D and 1E). In otp84, three defects in RNA editing were observed: ndhB (94,999), ndhF (112349), and psbZ (35,800) (Figure 1C). All three sites remain completely unedited in the mutant.
Figure 1.
Editing Defects in otp80, otp81, otp84, otp85, and otp86 Mutants.
Poisoned primer extension assays were conducted on the editing sites rpl23 (nucleotide 86,056) for otp80 (A), rps12 intron (69,553) for otp81 (B), ndhF (112,349), psbZ (35,800), and ndhB (94,999) for otp84 (C), top to bottom; ndhD (116494) for otp85 (D), and rps14 (37,161) for otp86 (E). The editing sites are specified relative to the nucleotide sequence of the complete Arabidopsis chloroplast genome.
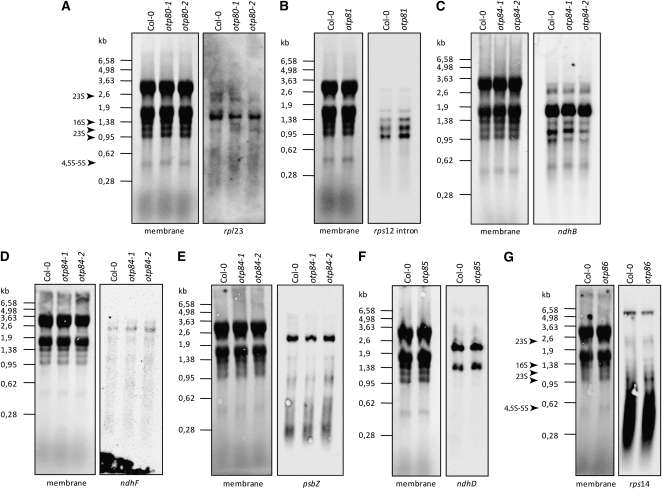
To investigate whether the editing defects we observed in these mutants were secondarily caused by altered RNA processing or modified RNA stability, the transcripts affected were analyzed by RNA gel blots (Figure 2). The hybridization patterns reveal no striking differences in the pattern and level of the transcripts whose editing is impaired in the mutants (rpl23, rps12 intron, ndhB, ndhF, psbZ, ndhD, and rps14). Furthermore, none of the other editing sites in the same transcripts is affected in any of these mutants (see Supplemental Figure 1 online).
Figure 2.
Transcript Profiles of Genes with the Editing Defects in otp80, otp81, otp84, otp85, and otp86 Mutants.
otp80 (A), otp81 (B), otp84 ([C] to [E]), otp85 (F), and otp86 (G) mutants. Fifteen micrograms of RNA from 15-d-old seedlings was loaded on formaldehyde agarose gels and transferred onto a membrane. Hybridizations were performed under high-stringency conditions using antisense RNA probes for the genes specified for each blot. The sizes of RNA markers are shown in kilobases. rRNA stained with methylene blue on the membranes is shown as a loading control (membrane). The arrows indicate the plastid rRNAs in otp80 and otp85 mutants.
Complementation experiments were performed for all the otp mutants. The introduction of a wild-type copy of the corresponding OTP gene into each mutant restores the editing at the defective sites to wild-type levels (Figure 1). These results support a direct and specific role of these OTP factors in RNA editing of these sites.
OTP80, OTP81, OTP84, OTP85, and OTP86 Are Localized in Plastids
The chloroplast editing defects in these mutants suggest that the PPR proteins encoded by these genes reside in plastids. To verify this, the N-terminal 100 amino acids of the proteins (72 amino acids in the case of OTP85) were fused with green fluorescent protein (GFP) driven by expression from the cauliflower mosaic virus 35S promoter. The plasmids containing the chimeric genes were transiently expressed in wild-type Arabidopsis cells by bombardment. Analysis of GFP fluorescence in transformed cells revealed that the fluorescence colocalized with a chloroplast marker: the fusion of red fluorescent protein (RFP) with the small subunit of Arabidopsis ribulose biphosphate carboxylase (Carrie et al., 2009) (see Supplemental Figure 2 online). Thus, OTP80, OTP81, OTP84, OTP85, and OTP86 are localized in plastids consistent with their role in editing plastid transcripts.
Requirement of RNA Editing at the Sites Affected by OTP Factors
The site that is not edited in otp81 lies in the intron of rps12, and this altered sequence might affect splicing of rps12 transcripts. However, RNA gel blot hybridization shows no difference in the pattern of rps12 transcripts between otp81 and the wild type (Figure 2B). Thus, editing of the rps12 intron is not necessary for the stabilization of the precursor transcripts, nor correct splicing, and there is no reason to think that the amount or function of Rps12 is deleteriously affected in the otp81 mutant.
The conversion of cytidine to uridine at the sites affected in the other otp mutants is not silent and changes the nature of the amino acids in the proteins encoded by these transcripts (Table 1). The alteration of editing at these sites might be expected to have an impact on the function of these proteins. Nevertheless, otp80, otp84, otp85, and otp86 show normal growth under standard conditions (see Supplemental Figure 3 online).
The mutants otp80 and otp86 are both affected in editing of transcripts encoding ribosomal proteins and thus could be expected to show translation defects. Translation in Arabidopsis plastids is thought to be essential (reviewed in Schmitz-Linneweber and Small, 2008). However, methylene blue staining of total RNA from otp80 and otp86 did not reveal any major defects in the integrity or quantity of the chloroplast rRNAs (indicated by arrows in Figures 2A and 2G). This suggests that these editing events are not essential for normal ribosome accumulation in plastids, in agreement with their normal growth phenotype, despite the presumed effects on the amino acid sequences of Rps14 and Rpl23 (Table 1).
The mutant otp85 lacks editing of ndhD (nucleotide 116494). RNA editing at this site changes a Ser codon to a Leu codon. The mutant otp84 is impaired in the editing of the three editing sites ndhB (94,999), ndhF (112,349), and psbZ (35,800). RNA editing at ndhB (94,999) converts a Pro codon to a Leu codon, while editing at ndhF (112349) and psbZ (35,800) convert Ser codons to Leu codons (Table 1). NdhB, NdhD, and NdhF are subunits of the chloroplast NAD(P)H dehydrogenase (NDH) complex involved in photosystem I cyclic electron flow (Shikanai, 2007). The NDH complex catalyzes electron donation to plastoquinone from the stromal electron pool. PsbZ is a core subunit of photosystem II (Swiatek et al., 2001). The editing defects in ndhB, ndhF, and psbZ could affect the function of the encoded proteins, which should alter photosynthetic parameters.
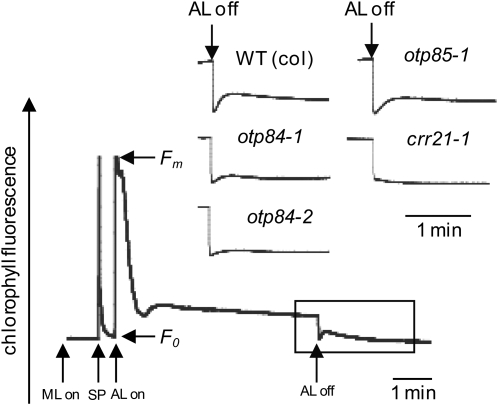
NDH complex activity can be monitored as a transient increase in chlorophyll fluorescence reflecting plastoquinone reduction after turning off actinic light (Shikanai et al., 1998). Figure 3 shows a typical chlorophyll fluorescence trace from wild-type Arabidopsis and in crr21, a mutant lacking NDH activity (Okuda et al., 2007). In otp84, the postillumination increase of chlorophyll fluorescence is modified but, unlike crr21, is not completely abolished. This indicates that NDH activity is diminished, but not absent, in the mutant. This also suggests that Leu residues at positions 494 in NdhB and/or 97 in NdhF are required for optimal NDH stability or activity. The postillumination increase of chlorophyll fluorescence in otp85 is not modified (Figure 4), indicating that a Leu at position 225 in NdhD is not essential for NDH activity.
Figure 3.
Monitoring of NDH Activity Using Chlorophyll Fluorescence Analysis for otp84 and otp85 Mutants.
The curve shows a typical trace of chlorophyll fluorescence in the wild type and a mutant totally impaired in NDH activity (crr21) (Okuda et al., 2007) compared with traces from otp85 and two independent otp84 mutants (otp84-1 and otp84-2). Leaves were exposed to actinic light (AL) (50 μmol of photons m−2 s−1) for 5 min. AL was turned off, and the subsequent change in chlorophyll fluorescence level was monitored. The transient increase in chlorophyll fluorescence is due to the plastoquinone reduction based on NDH activity. Insets are magnified traces from the boxed area. The fluorescence levels were normalized by the maximum fluorescence at closed photosystem II centers in the dark (Fm) levels. ML, measuring light; SP, a saturating pulse of white light.
Figure 4.
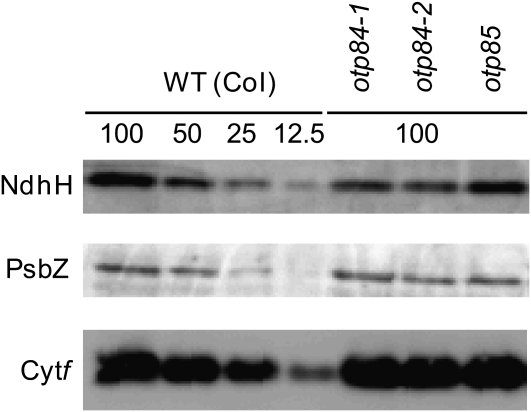
Protein Blot Analysis of the NDH Complex and the Major Photosynthetic Complexes.
Immunodetection of an NDH subunit, NdhH; a subunit of photosystem II, PsbZ; and a subunit of the Cytb6f complex, cytochrome f. The proteins were extracted from thylakoid membrane fractions. Lanes were loaded with protein samples corresponding to 0.5 μg chlorophyll for Cytf, 1 μg chlorophyll for PsbZ, and 5 μg chlorophyll for NdhH (100%) and the series of dilutions indicated as a percentage of the original sample.
RNA editing defects result in amino acid changes that may directly alter protein function or act by destabilizing the protein or by affecting its ability to form complexes with other proteins. To assess whether the subunits affected in otp84 and otp85 stably accumulate in vivo, protein blots were analyzed using antibodies against NdhH and PsbZ (Figure 4). The NDH complex is unstable without NdhB and NdhD (Peng et al., 2008), and an antibody against NdhH can be used to monitor accumulation of the complex. In otp84, the level of NdhH is reduced to 50 to 25% of the wild type. This result suggests that Leu residues at positions 494 in NdhB and/or 97 in NdhF are important for normal accumulation of the NDH complex. The decrease in the amount of the NDH complex largely explains the observed decrease in activity, although we cannot exclude that the specific activity of the complex is also decreased. In otp85, the accumulation of the NDH complex is not affected.
In addition to ndhB (94,999) and ndhF (112349), otp84 is impaired in editing of psbZ (35,800), leading to retention of a Ser (rather than Leu) codon at position 17 in PsbZ, a core subunit of photosystem II (Swiatek et al., 2001). Immunodetection of PsbZ on blots of otp84 extracts (Figure 4) reveals no defect in the accumulation of the protein in the mutant. Consistent with the normal growth of otp84 (see Supplemental Figure 3 online), there were no alterations to the rate of electron transport through photosystem II nor to nonphotochemical quenching of chlorophyll fluorescence, which reflects ΔpH formation (see Supplemental Figure 4 online). These results suggest that a Leu at position 17 in PsbZ is not important for its function under standard growth conditions.
Multiple Editing Sites and Recognition Specificity
A major outstanding question is how editing factors recognize their target sites and of particular interest are those factors that appear to recognize more than one site. Biochemical evidence suggests that most of the sequence recognition is concentrated within the 15 nucleotides immediately upstream of the editing site (Hirose and Sugiura, 2001; Miyamoto et al., 2002, 2004; Kobayashi et al., 2008). However, as remarked in previous work (Chateigner-Boutin et al., 2008; Okuda et al., 2009), editing sites requiring the same factor do not show extensive sequence identity in this crucial region, raising doubts as to how these putative specificity factors might operate. To investigate these questions, we undertook a systematic bioinformatic analysis of all plastid editing sites in Arabidopsis and the factors that recognize them.
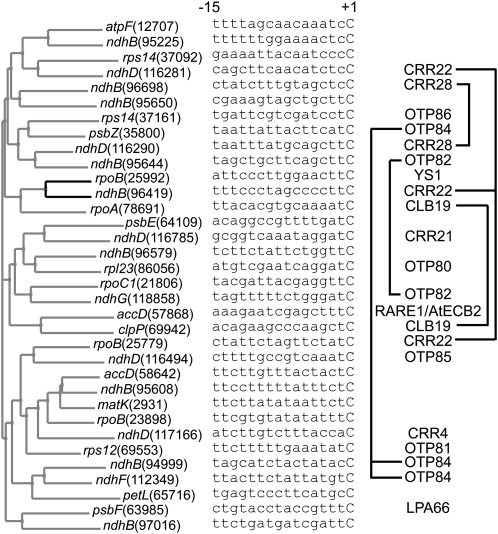
Confirmation that editing sites requiring the same factor are not particularly similar to each other is shown in Figure 5. Only ndhB (94,999) and ndhF (112349) (requiring OTP84) pair together, and even this grouping is only weakly supported. The only strongly supported grouping in this tree is between rpoB(25,992) and ndhB(96,419), but these require different editing factors (YS1 and CRR22, respectively). At first sight, these data raise doubts that the identified editing factors can work as commonly assumed (i.e., by binding to the region immediately upstream of the target C). We reasoned that we had sufficient data to test this assumption and therefore examined whether the apparently dissimilar target sites requiring the same factors nevertheless contain sufficient conserved nucleotides to unambiguously define a binding site consensus. If an unambiguous consensus could be found, then this would be consistent with the protein in question acting as a specificity factor by binding to this consensus sequence.
Figure 5.
Assessment of Sequence Similarities among Editing Sites.
For the 34 known editing sites in the Arabidopsis chloroplast genome (Chateigner-Boutin and Small, 2007), the 15 nucleotides upstream of the editing site were aligned with respect to the editing site (the final C in each sequence) and a distance tree was constructed. Only the grouping of rpoB (25,992) with ndhB (96,419) has bootstrap support of >50%, and this is indicated by the black lines in the tree. The right panel indicates the PPR proteins involved in the recognition of each editing site.
For each set of editing sites requiring a single factor, we calculated six possible consensus sequences using the following assumptions: (1) that PPR proteins can distinguish all four nucleotides; (2) that PPR proteins can distinguish purines (A and G) from pyrimidines (C and U); (3) that PPR proteins can distinguish the number of hydrogen bonding groups in each base (i.e., can distinguish A or U from G or C) or various combinations of these criteria (Table 2). Each consensus was then matched against both strands of the entire Arabidopsis chloroplast genome and the number of matches recorded. Finally, matches within duplicated or untranscribed sequences were discounted. The results indicate that with the exception of the sites recognized by CRR22, in all other cases there is sufficient conservation of sequence to unambiguously identify the edited sites from all other transcribed sequences within plastids, based on the assumptions (1) and (2) listed above.
Table 2.
Analyses of the Editing Sites Requiring the Same PPR Proteins
| PPR | Consensus | Hits | Filtered Hits | Location | |
|---|---|---|---|---|---|
| OTP82 | a | UAG-U–U—G–UC | 15 | ||
| b | YRRYY-YYYYRR–YC | 27 | |||
| c | UAGYU-YUYYRG–UC | 3 | 2 | ndhB, ndhG | |
| d | WWS-W–W—SSWWC | 260 | |||
| e | UAG-U–U—GSWUC | 4 | |||
| f | UAGYU-YUYYRGSWUC | 3 | 2 | ndhB, ndhG | |
| CRR22 | a | ——-A——-C | 17,078 | ||
| b | Y–YYYYRRY-YY-YC | 59 | |||
| c | Y–YYYYARY-YY-YC | 35 | 12 | psbK, ycf2, ycf1, matK, 3′UTR atpH, rpoB, 3′UTR rps4, ndhB, ndhD, ndhG, ndhA, ycf1 | |
| d | -W—–W—–W-C | 15,439 | |||
| e | -W—–A—–W-C | 7,646 | |||
| f | YW-YYYYARY-YYWYC | 23 | 8 | psbK, ycf1, 3′UTR atpH, rpoB, ndhB, ndhD, ndhG, ndhA | |
| OTP84 | a | U——UA-U—-C | 444 | ||
| b | Y-RY–YYRYY-YRYC | 28 | |||
| c | U-RY–YUAYU-YRYC | 7 | 3 | psbZ, ndhB, ndhF | |
| d | WW–WW-WW-WW—C | 1,732 | |||
| e | UW–WW-UA-UW—C | 88 | |||
| f | UWRYWWYUAYUWYRYC | 5 | 3 | psbZ, ndhB, ndhF | |
| CLB19 | a | –A-A-G–CAA–UC | 16 | ||
| b | -YR-R-RY-YRRR-YC | 74 | |||
| c | -YA-A-GY-CAAR-UC | 4 | 2 | clpP, rpoA | |
| d | W-WSW-S-SSWW–WC | 39 | |||
| e | W-ASA-G-SCAA–UC | 2 | |||
| f | WYASA-GYSCAAR-UC | 2 | 2 | clpP, rpoA | |
| CRR28 | a | –AU-U-UG-AGCU-C | 16 | ||
| b | Y-RYYY-YRYRRYYYC | 6 | |||
| c | Y-AUYU-UG-AGCU-C | 3 | 2 | ndhB, ndhD | |
| d | -WWW-WWWS-WSSW-C | 79 | |||
| e | -WAU-UWUG-AGCU-C | 3 | |||
| f |
YWAUYUWUG-AGCU-C |
3 |
2 |
ndhB, ndhD |
Consensus sequences were derived as follows from the 15 nucleotides immediately upstream of the edited C (and including the editing site itself): (a) full conservation of nucleotides (A, U, G, and C); (b) conservation of purines (A or G = R) or pyrimidines (U or C = Y); (c) combination of a and b; (d) conservation of number of hydrogen bonding groups (A or U = W, G or C = S); (e) combination of a and d; (f) combination of a, b, and d. The “Hits” column indicates the number of times each consensus is found within both strands of the Arabidopsis plastid genome. “Filtered Hits” removes duplicate matches within the inverted repeats, matches to noncoding strands, and matches to intergenic regions that do not form stably accumulated transcripts. The editing sites known to be recognized by each factor are highlighted in bold. UTR, untranslated region.
DISCUSSION
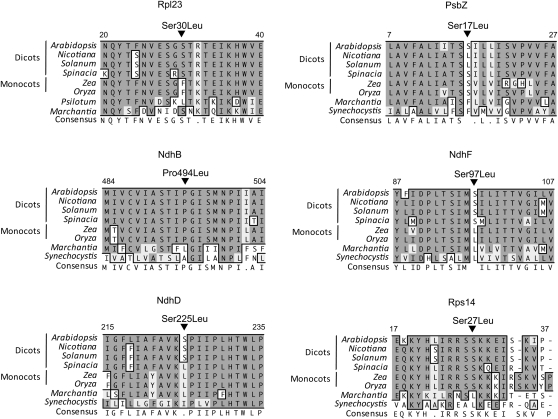
A reverse genetic screen of T-DNA insertion mutants allowed us to identify six new PPR proteins required for RNA editing of nine sites in plastid RNAs. Unlike the majority of the previously characterized editing mutants (Okuda et al., 2006, 2007, 2009; Chateigner-Boutin et al., 2008; Kim et al., 2009; Yu et al., 2009; Zhou et al., 2009), which were identified via visible growth or fluorescence phenotypes, these mutants were identified directly via a screen for unedited RNAs. It is striking that of the eight mutants discovered in this way, including RARE1 (Robbins et al., 2009) and MEF1 (Zehrmann et al., 2009), none have growth or physiological defects, apart from the partial decrease in NDH activity observed for otp84. This rather suggests that many more RNA editing events may be phenotypically silent than previously expected, at least under optimal growth conditions. This observation has implications for the evolutionary stability of editing events (Tillich et al., 2009), as it implies that selection pressure on both the editing sites and the factors that recognize them may be weaker than often thought. It should be noted, however, that as editing almost always restores codons for conserved amino acids (Figure 6), it is likely that under some conditions it would be possible to observe deleterious effects of a lack of editing of these apparently silent sites.
Figure 6.
Partial Sequence Alignments of Rpl23, PsbZ, NdhB, NdhF, Rps14, and NdhD around the Amino Acids Affected by RNA Editing.
Arabidopsis Rpl23, PsbZ, NdhB, NdhF, Rps14, and NdhD proteins were aligned with their homologs from other species. The alignment was performed using ClustalW (Thompson et al., 1994). Amino acids that are fully or semiconserved are shaded black or gray, respectively. Numbers indicate amino acid positions in the protein. The arrows above the sequences indicate the positions of edited codons.
More than half of the candidate genes we examined could not be linked to RNA editing in plastids. This might be for a number of different reasons, including genetic redundancy, errors in targeting predictions (such that the protein functions in mitochondria, not plastids), incomplete surveying of editing sites (it is still possible that there are more than the 34 known editing sites in Arabidopsis plastid RNAs), or because the protein has functions unrelated to editing. As an example of the last case, the DYW protein CRR2 is required for RNA processing (accumulation of monocistronic ndhB mRNA), not RNA editing (Hashimoto et al., 2003).
Although prediction of editing factors is not yet infallible, nevertheless 22 of the 34 known editing sites in plastids have been assigned corresponding PPR trans-factors, and we expect that the remaining editing events are also likely to require a PPR protein from the plant-specific PLS subfamily. A total of 44 of these proteins are predicted to be targeted to plastids (Lurin et al., 2004), which is more than sufficient to account for all the known editing sites in plastids. In mitochondria, the number of editing sites is considerably higher than the number of PLS subfamily PPR proteins predicted to be targeted to mitochondria: 82 PPR proteins (Lurin et al., 2004) for >500 editing sites (Giegé and Brennicke, 1999; Bentolila et al., 2008; Zehrmann et al., 2008). This suggests that in mitochondria, a single PPR editing factor should, on average, cover more sites than in plastids. Indeed, in plastids, the majority of editing factors are required for a single site, with a maximum of three in the cases of CRR22 (Okuda et al., 2009) and OTP84. The first mitochondrial editing factors to be found, OGR1 and MEF-1, are required for at least seven and three editing sites in rice (Oryza sativa) and Arabidopsis mitochondria, respectively (Kim et al., 2009; Zehrmann et al., 2009).
The sequences surrounding the editing sites identified in plant organelles do not show any unequivocal consensus, apart the from the one or two bases immediately surrounding the site (reviewed in Mulligan et al., 1999 and reexamined in Cummings and Myers, 2004). This probably represents preferences of the enzyme catalyzing the editing reaction rather than a target for postulated specificity factor(s). The key question of how specific C residues are recognized for editing from all other C residues in organelle transcripts remained unanswered for many years. Experiments with transgenic plastids and in vitro RNA editing assays using organelle extracts delimited the primary region recognized by putative trans-factors to the 20 or so nucleotides immediately upstream of the editing site, with, in most cases, the most important recognition elements situated from −5 to −15 with respect to the edited C (reviewed in Shikanai, 2006). Binding to this region was confirmed for CRR4, the first editing factor to be genetically identified (Okuda et al., 2006), and it has been assumed that the other PPR proteins identified as editing factors function in a similar way (Okuda et al., 2007, 2009; Chateigner-Boutin et al., 2008; Cai et al., 2009; Kim et al., 2009; Robbins et al., 2009; Yu et al., 2009; Zehrmann et al., 2009; Zhou et al., 2009). However, no proof of specific binding has been shown for any of these subsequent editing factors, including the six new ones identified in the screen described here.
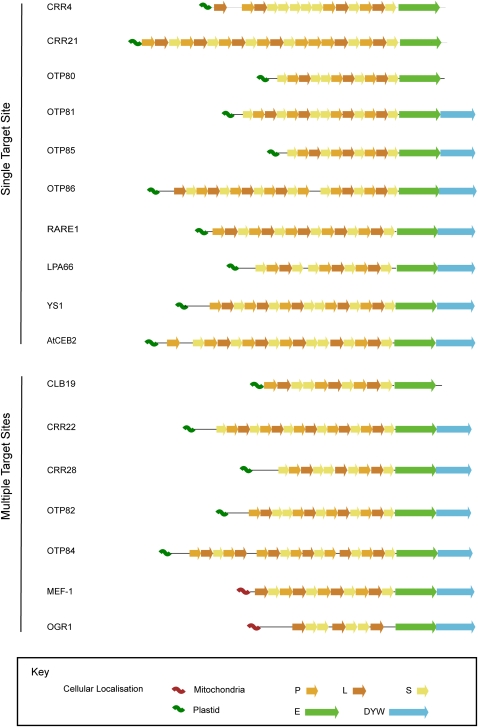
Some confirmation of specific binding comes from comparing the factors genetically identified in Arabidopsis to those biochemically identified in tobacco. Cross-linking experiments trapping putative protein trans-factors bound to the cis-acting elements of editing sites (Kobayashi et al., 2008) identified a 91-kD protein binding the rpoB-1 site which, based on size, could correspond to the tobacco ortholog of YS1, a PPR protein required for the editing of the same site in Arabidopsis plastids (Zhou et al., 2009) and whose predicted molecular mass (discounting the plastid targeting sequence) is 90.3 kD. An even more compelling match is seen between the 95-kD protein binding the two RNA editing sites ndhB-9 and ndhF-1 in tobacco (Kobayashi et al., 2008) and OTP84, which we show to recognize the equivalent Arabidopsis editing sites ndhB (94,999) and ndhF (112349). The predicted molecular mass of OTP84 (discounting the plastid targeting sequence) is 94.8 kD. All the observations so far are entirely consistent with editing site specificity being determined by binding of the relevant PPR protein to the sequence upstream of the target C, in accordance with the general view of how PPR proteins function in RNA metabolism (Delannoy et al., 2007). It is worth noting that there is no significant difference in length, number of motifs, or any other aspect of the protein sequence or structure that we can find to distinguish PPR proteins recognizing multiple target sites from those that recognize single sites (Figure 7).
Figure 7.
Schematic Structure of Plant PPR Proteins Involved in Editing of Plastid or Mitochondrial Transcripts.
PPR proteins are grouped according to their number of target sites (single or multiple). All the identified PPR motifs are indicated (as defined in Lurin et al., 2004), as well as the C-terminal E and DYW domains and the N-terminal organelle targeting sequences. P represents canonical 35–amino acid PPR motifs; L (long) and S (short) represent variant PPR motifs with generally 36 and 31 amino acids, respectively.
Only a few PPR proteins have been shown unambiguously to bind to multiple target sites, of which the best-studied are the maize (Zea mays) proteins CRP1 (Schmitz-Linneweber et al., 2005) and PPR10 (Pfalz et al., 2009). In these two cases the multiple target sites have almost identical sequences. This is not true of the multiple editing sites targeted by single factors. In our study, a distance tree relating the Arabidopsis plastid editing sites (Figure 5) does not group the editing sites known to be recognized by a single PPR factor except for the ndhB (94,999) and ndhF (112349) sites mentioned above. Previously proposed clusters based on apparent sequence similarity (Chateigner-Boutin and Hanson, 2002; Chateigner-Boutin and Hanson, 2003) do not exactly correspond to groups of sites recognized by single factors. This prompted us to test whether the commonly held assumption that these editing factors bind the biochemically defined cis-elements is consistent with the data.
We reasoned that an unambiguous consensus between sites requiring the same editing factor would be consistent with the hypothesis that the factor is involved in target site recognition. We addressed this by generating various consensus sequences (Table 2) from the multiple target sites, using the results from published in vitro experiments (Hirose and Sugiura, 2001; Miyamoto et al., 2002, 2004; Kobayashi et al., 2008) to delimit the region of interest from −15 to the editing site. We found that it was possible to define an unambiguous 15-nucleotide consensus for each of the editing factors required for editing of multiple sites, except in the case of CRR22. We conclude that the data are consistent with the hypothesis that these PPR editing factors bind to multiple target sites and consistent with the biochemical evidence that recognition involves the nucleotides upstream of the editing site. The one exception is CRR22, which will be discussed in more detail later.
In the cases where a consensus can be found, the pattern of nucleotide similarity is informative regarding what sequence features might be being recognized. RNA nucleotides tend to interact base-specifically with proteins in one of two ways: via stacking interactions with the aromatic rings or via hydrogen bonding. Sequence specificity can be achieved by positive base-specific bonding interactions or by negative steric hindrance (Auweter et al., 2006). Based on these considerations, we considered three (mutually compatible) hypotheses: that editing factors can distinguish all four RNA nucleotides, that they can distinguish purines (A and G) from pyrimidines (C and U), or that they can distinguish bases by Watson-Crick hydrogen bonding patterns (G and C versus A and U). None of these consensuses alone provided sufficient specificity to unambiguously define the edited sites within the plastid transcriptome, but combining conserved nucleotide positions with conserved purines or pyrimidines is sufficient (in the cases where a consensus could be achieved). We conclude that to achieve the required specificity, editing factors must be able to distinguish purine nucleotides from pyrimidine nucleotides, and at least at some positions, be able to uniquely distinguish one of the four nucleotides. The relatively poor performance of the consensus based on A/U versus C/G suggests that this distinction is not important in determining binding specificity.
This analysis is consistent with previous biochemical data. Kobayashi et al. (2008) conducted experiments using RNAs competing with the factor required for editing ndhB-9 and ndhF-1 (as explained above, this is almost certainly the tobacco ortholog of OTP84). Competitor RNAs were constructed by scanning mutagenesis in which each successive five-nucleotide block in the region from −15 to −1 was substituted with its complementary nucleotide sequence. These mutations therefore switched the purine/pyrimidine nature of the residues in the upstream cis-elements. For both sites, mutations introduced into the −15 to −6 region abolished competition for the binding of the editing factor, whereas mutations in the −5 to −1 region had a weaker effect. Consistent with this, mutagenesis of the ndhF RNA substrate showed that purine/pyrimidine exchanges in the −15 to −1 upstream region abolish in vitro editing of the ndhF-1 site (Sasaki et al., 2006).
Throughout the analysis discussed above, the exception is CRR22. This PPR editing factor is required for editing the three sites ndhB (96,419), ndhD (116,281), and rpoB (25,779) (Okuda et al., 2009), but the sequence conservation between these three sites is not sufficient to explain how these three sites can be distinguished from other equally similar sequences scattered throughout the plastid transcriptome (Table 2). One possibility might be that CRR22 recognizes sequences outside the region studied here, but the sequence similarity around these sites is no greater over a more extended region. Another possibility is that different arrays of PPR motifs within CRR22 recognize different target sites, thus allowing a single protein to bind to several unrelated targets. However, CRR22 is not longer than other editing factors (Figure 7), which might be expected if it contained two or more independent binding domains. We therefore need to consider whether CRR22 truly acts as a specificity factor at all three sites it is required for or whether there might be an alternative explanation for its involvement in editing at one or more of these sites. Recently, two different PPR editing factors, RARE1 and ECB2, were demonstrated to be required for editing of a single site, accD (57,868), in Arabidopsis chloroplasts (Robbins et al., 2009; Yu et al., 2009). As all the other evidence suggests that a single PPR protein is sufficient to define editing specificity, the implication of two proteins in the editing of accD (57,868) implies that one of them may have a different role, as might be the case for CRR22. The most attractive hypothesis would be that this different role is to recruit the editing enzyme or even to catalyze the editing reaction itself. Like CRR22, both RARE1 and AtECB2 belong to the DYW subfamily, and one model for RNA editing proposes that the C-terminal DYW domain carries the cytidine deaminase editing activity (Salone et al., 2007).
METHODS
All the primers used in this study are listed in Supplemental Table 1 online.
Plant Material
Arabidopsis thaliana ecotype Columbia (Col-0) was used in this study. The T-DNA insertion mutant lines were obtained from the ABRC Stock Center. Accession numbers are provided in Table 1.
Genetic Analysis
Total cellular DNA was isolated as described by Edwards et al. (1991). Plants were genotyped for homozygous lines by PCR, and the insertion position was confirmed by sequencing with a T-DNA left border primer. otp80-1 (SALK_111721, Col-0, insertion site +141), otp80-2 (SALK_060533, Col-0, insertion site +221), otp81 (SALK_092402, Col-0, insertion site −15), otp84-1 (SAIL_568_C04, Col-0, insertion site +932), otp84-2 (SALK_120902, Col-0, insertion site +642), otp85 (SAIL_544_B03, Col-0, insertion site +1328), and otp86 (SALK_102445, Col-0, insertion site +698).
Analysis of Targeting via GFP Fusions
The first 300 bp of the coding sequences of the PPR genes were amplified using Phusion DNA polymerase (Finnzymes) with primers listed in Supplemental Table 1 containing the attB sites for Gateway cloning according to the manufacturer's instructions (Invitrogen). The GFP vector used and the chloroplast targeting marker encoding RFP fused to the small subunit of Arabidopsis ribulose biphosphate carboxylase were kindly provided by James Whelan (University of Western Australia) (Carrie et al., 2009). Biolistic transformations of GFP and RFP constructs were performed on Arabidopsis cell culture (Carrie et al., 2007). The GFP construct and the chloroplast RFP marker (5 μg each) were coprecipitated onto gold particles and transformed using the biolistic PDS-1000/He system (Bio-Rad). Particles were bombarded onto 2 mL of Arabidopsis cell suspension resting on filter paper on osmoticum plates (2.17 g/L Murashige and Skoog modified basal salt mixture, 30 g/L sucrose, 0.5 mg/L naphthalene acetic acid, 0.05 mg/L kinetin, and 36.44 g/L mannitol). After bombardment, the cells were placed in the dark at 22°C for 24 h. Observation of transient GFP and RFP expression was performed using an Olympus BX61 fluorescence microscope with excitation wavelengths of 460/480 nm (GFP) and 535/555 nm (RFP) and emission wavelengths of 495 to 540 nm (GFP) and 570 to 625 nm (RFP). Subsequent images were captured using Cell imaging software as previously described (Carrie et al., 2007; Murcha et al., 2007).
Genetic Complementation
The 1870-, 3138-, 2673-, 2278-, and 2883-bp fragments containing the respective coding sequence of OTP80, OTP81, OTP84, OTP85, and OTP86 were amplified by PCR on total cellular DNA. These constructs were cloned into pGWB1 (OTP80, OTP81, and OTP85) or pGWB2 (OTP84 and OTP86) binary vectors and introduced into otp mutants via Agrobacterium tumefaciens GV3101. Transformants were obtained by selection on Murashige and Skoog agar plates containing 25μg/mL hygromycin and confirmed by PCR.
Analysis of RNA Editing
High-resolution melting analysis of amplicons was performed as previously described (Chateigner-Boutin and Small, 2007) using the primers listed by Okuda et al. (2009). Poisoned primer extension of RT-PCR products was performed as described by Chateigner-Boutin and Small (2007). RT-PCR products were obtained with primers surrounding the editing sites and serve as templates for the extension reaction from a labeled 6-carboxyfluorescein primer that anneals next to the target editing site. The extension is stopped by the incorporation of ddGTP or ddCTP at the location of the editing site for unedited molecules producing a short unedited product. The extension is stopped at the next G/C for the edited molecules producing a longer edited product. For the ndhB (94,999) site, the extension is stopped by the incorporation of ddATP leading to a short edited product and a long unedited product.
RNA Preparation and Analysis
Total RNA from leaves of 15-d-old plantlets was isolated using TRIzol reagent (Invitrogen) as recommended by the manufacturer. Fifteen micrograms of RNA was fractionated on 1.2% (w/v) formaldehyde agarose gels and transferred onto Hybond N+ nylon membranes (GE Healthcare). RNA integrity, loading, and transfer were checked by staining the membrane with methylene blue.
RNA probes were internally labeled with biotinylated cytidine by transcription of PCR products cloned in pGEM-T Easy vector (Promega). The primers used for the PCR are listed in Supplemental Table 1 online. Clones with inserts in antisense orientation were amplified by PCR using the forward primer and M13/pUC reverse. The PCR products served as a template for in vitro transcription with SP6 polymerase following the manufacturer's instructions (Maxiscript Ambion).
To prepare the ndhF RNA probe, the ndhF sequence was amplified using an antisense primer linked to the T7 promoter. The PCR product served as template for in vitro transcription with T7 polymerase (Maxiscript Ambion).
Prehybridization of the membrane was performed for 1 h in hybridization buffer (5× SSC, 50% [v/v] formamide, 0.5% SDS, and 100 μg/mL heparin) at 68°C. Hybridization with RNA probes was performed in the same buffer overnight at 68°C, followed by three 15-min washes at room temperature in 1× SSC/0.5% SDS and two washes at 60°C in 0.1× SSC/0.1% SDS for 20 min and 1 h, respectively. Signal detection was performed using the Chemiluminescent Nucleic Acid Detection Module (Pierce) and read in an ImageQuant-RT ECL (Amersham).
Chlorophyll Fluorescence Analysis
Chlorophyll fluorescence was measured using a MINI-PAM portable chlorophyll fluorometer (Waltz). The transient increase in chlorophyll fluorescence after turning off actinic light was monitored as previously described (Shikanai et al., 1998).
Immunoblot Analysis
Chloroplasts were isolated from the leaves of 4-week-old plants as previously described (Okuda et al., 2007). Samples were normalized by measuring chlorophyll concentration. The protein samples were separated by 12.5% SDS-PAGE. After electrophoresis, the proteins were transferred onto a Hybond-P membrane (GE Healthcare) and incubated with specific antibodies. The signals were detected using an ECL Advance Western Blotting Detection Kit (for NdhH; GE Healthcare) or an ECL Plus Western Blotting Detection Kit (for the others; GE Healthcare). The signals were visualized by a LAS1000 chemiluminescence analyzer (Fuji Film).
Bioinformatic Analysis
The 15 nucleotides sequences upstream of editing sites were aligned and clustered with ClustalW 1.83 (Thompson et al., 1994) using the default parameters. Consensuses for editing sites recognized by the same factor were calculated by hand and searched against the Arabidopsis plastid genome sequence using fuzznuc from the EMBOSS package (Rice et al., 2000).
Accession Numbers
The genes described in this article correspond to the following Arabidopsis Genome Initiative codes: At5g59200 (OTP80), At2g29760 (OTP81), At3g57430 (OTP84), At2g02980 (OTP85), and At3g63370 (OTP86). Accession information for T-DNA insertion lines is provided in Table 1. Editing sites are specified relative to the nucleotide sequence of the complete Arabidopsis chloroplast genome (GenBank accession number AP000423).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. High-Resolution Melting Screen of otp80, otp81, otp84, otp85, and otp86 Mutants.
Supplemental. Figure 2. Analysis of Subcellular Localization of the OTP80, OTP81, OTP84, OTP85, and OTP86 Proteins.
Supplemental Figure 3. Phenotype of otp80, otp81, otp84, otp85, otp86, and Wild-Type Arabidopsis Plants.
Supplemental Figure 4. In Vivo Analysis of Electron Transport Activity Using Light Intensity Dependence of Electron Transport Rate.
Supplemental Table 1. Oligonucleotides Used in This Study.
Supplemental Data Set 1. Text File of the Alignment Used for the Analysis Shown in Figure 5.
Supplementary Material
Acknowledgments
This work is funded through the Australian Research Council Centres of Excellence scheme (CE0561495), an International Science Linkages grant (CG120098) from the Australian Government Department of Innovation, Industry, Science, and Research, and scholarships from the Region of Alsace and the University of Western Australia. K.H. is the holder of a Lavoisier Fellowship, and I.S. is a West Australian State Premier's Fellow. T.S. was supported by Grants-in-Aid (17GS0316 and 16085206) and by Genomics for Agricultural Innovation (GPN0008).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Ian Small (iansmall@cyllene.uwa.edu.au).
Online version contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Auweter, S.D., Oberstrass, F.C., and Allain, F.H. (2006). Sequence-specific binding of single-stranded RNA: Is there a code for recognition? Nucleic Acids Res. 34 4943–4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentolila, S., Elliott, L.E., and Hanson, M.R. (2008). Genetic architecture of mitochondrial editing in Arabidopsis thaliana. Genetics 178 1693–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock, R., Hermann, M., and Fuchs, M. (1997). Identification of critical nucleotide positions for plastid RNA editing site recognition. RNA 3 1194–1200. [PMC free article] [PubMed] [Google Scholar]
- Bock, R., Hermann, M., and Kössel, H. (1996). In vivo dissection of cis-acting determinants for plastid RNA editing. EMBO J. 15 5052–5059. [PMC free article] [PubMed] [Google Scholar]
- Bock, R., and Koop, H.U. (1997). Extraplastidic site-specific factors mediate RNA editing in chloroplasts. EMBO J. 16 3282–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, W., Ji, D., Peng, L., Guo, J., Ma, J., Zou, M., Lu, C., and Zhang, L. (2009). LPA66 is required for editing psbF chloroplast transcripts in Arabidopsis. Plant Physiol. 150 1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrie, C., Kühn, K., Murcha, M.W., Duncan, O., Small, I.D., O'Toole, N., and Whelan, J. (2009). Approaches to defining dual-targeted proteins in Arabidopsis. Plant J. 57 1128–1139. [DOI] [PubMed] [Google Scholar]
- Carrie, C., Murcha, M.W., Millar, A.H., Smith, S.M., and Whelan, J. (2007). Nine 3-ketoacyl-CoA thiolases (KATs) and acetoacetyl-CoA thiolases (ACATs) encoded by five genes in Arabidopsis thaliana are targeted either to peroxisomes or cytosol but not to mitochondria. Plant Mol. Biol. 63 97–108. [DOI] [PubMed] [Google Scholar]
- Chateigner-Boutin, A.L., and Hanson, M.R. (2002). Cross-competition in transgenic chloroplasts expressing single editing sites reveals shared cis elements. Mol. Cell. Biol. 22 8448–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chateigner-Boutin, A.L., and Hanson, M.R. (2003). Developmental co-variation of RNA editing extent of plastid editing sites exhibiting similar cis-elements. Nucleic Acids Res. 31 2586–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chateigner-Boutin, A.L., Ramos-Vega, M., Guevara-García, A., Andrés, C., de la Luz Gutiérrez-Nava, M., Cantero, A., Delannoy, E., Jiménez, L.F., Lurin, C., Small, I., and León, P. (2008). CLB19, a pentatricopeptide repeat protein required for editing of rpoA and clpP chloroplast transcripts. Plant J. 56 590–602. [DOI] [PubMed] [Google Scholar]
- Chateigner-Boutin, A.L., and Small, I. (2007). A rapid high-throughput method for the detection and quantification of RNA editing based on high-resolution melting of amplicons. Nucleic Acids Res. 35 e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri, S., Carrer, H., and Maliga, P. (1995). Site-specific factor involved in the editing of the psbL mRNA in tobacco plastids. EMBO J. 14 2951–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri, S., and Maliga, P. (1996). Sequences directing C to U editing of the plastid psbL mRNA are located within a 22 nucleotide segment spanning the editing site. EMBO J. 15 5958–5964. [PMC free article] [PubMed] [Google Scholar]
- Cummings, M.P., and Myers, D.S. (2004). Simple statistical models predict C-to-U edited sites in plant mitochondrial RNA. BMC Bioinformatics 5 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delannoy, E., Stanley, W.A., Bond, C.S., and Small, I.D. (2007). Pentatricopeptide repeat (PPR) proteins as sequence-specificity factors in post-transcriptional processes in organelles. Biochem. Soc. Trans. 35 1643–1647. [DOI] [PubMed] [Google Scholar]
- Edwards, K., Johnstone, C., and Thompson, C. (1991). A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 19 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson, O., Nielsen, H., Brunak, S., and von Heijne, G. (2000). Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 300 1005–1016. [DOI] [PubMed] [Google Scholar]
- Giegé, P., and Brennicke, A. (1999). RNA editing in Arabidopsis mitochondria effects 441 C to U changes in ORFs. Proc. Natl. Acad. Sci. USA 96 15324–15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, M., Endo, T., Peltier, G., Tasaka, M., and Shikanai, T. (2003). A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis. Plant J. 36 541–549. [DOI] [PubMed] [Google Scholar]
- Hayes, M.L., Reed, M.L., Hegeman, C.E., and Hanson, M.R. (2006). Sequence elements critical for efficient RNA editing of a tobacco chloroplast transcript in vivo and in vitro. Nucleic Acids Res. 34 3742–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman, C.E., Hayes, M.L., and Hanson, M.R. (2005). Substrate and cofactor requirements for RNA editing of chloroplast transcripts in Arabidopsis in vitro. Plant J. 42 124–132. [DOI] [PubMed] [Google Scholar]
- Hirose, T., and Sugiura, M. (2001). Involvement of a site-specific trans-acting factor and a common RNA-binding protein in the editing of chloroplast mRNAs: Development of a chloroplast in vitro RNA editing system. EMBO J. 20 1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.R., Yang, J.I., Moon, S., Ryu, C.H., An, K., Kim, K.M., Yim, J., and An, G. (2009). Rice OGR1 encodes a pentatricopeptide repeat-DYW protein and is essential for RNA editing in mitochondria. Plant J. 59 738–749. [DOI] [PubMed] [Google Scholar]
- Kobayashi, Y., Matsuo, M., Sakamoto, K., Wakasugi, T., Yamada, K., and Obokata, J. (2008). Two RNA editing sites with cis-acting elements of moderate sequence identity are recognized by an identical site-recognition protein in tobacco chloroplasts. Nucleic Acids Res. 36 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotera, E., Tasaka, M., and Shikanai, T. (2005). A pentatricopeptide repeat protein is essential for RNA editing in chloroplasts. Nature 433 326–330. [DOI] [PubMed] [Google Scholar]
- Lurin, C., et al. (2004). Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16 2089–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, T., Obokata, J., and Sugiura, M. (2002). Recognition of RNA editing sites is directed by unique proteins in chloroplasts: Biochemical identification of cis-acting elements and trans-acting factors involved in RNA editing in tobacco and pea chloroplasts. Mol. Cell. Biol. 22 6726–6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, T., Obokata, J., and Sugiura, M. (2004). A site-specific factor interacts directly with its cognate RNA editing site in chloroplast transcripts. Proc. Natl. Acad. Sci. USA 101 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan, R.M., Williams, M.A., and Shanahan, M.T. (1999). RNA editing site recognition in higher plant mitochondria. J. Hered. 90 338–344. [DOI] [PubMed] [Google Scholar]
- Murcha, M.W., Elhafez, D., Lister, R., Tonti-Filippini, J., Baumgartner, M., Philippar, K., Carrie, C., Mokranjac, D., Soll, J., and Whelan, J. (2007). Characterization of the preprotein and amino acid transporter gene family in Arabidopsis. Plant Physiol. 143 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwirt, J., Takenaka, M., van der Merwe, J.A., and Brennicke, A. (2005). An in vitro RNA editing system from cauliflower mitochondria: editing site recognition parameters can vary in different plant species. RNA 11 1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda, K., Chateigner-Boutin, A.L., Nakamura, T., Delannoy, E., Sugita, M., Myouga, F., Motohashi, R., Shinozaki, K., Small, I., and Shikanai, T. (2009). Pentatricopeptide repeat proteins with the DYW motif have distinct molecular functions in RNA editing and RNA cleavage in Arabidopsis chloroplasts. Plant Cell 21 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda, K., Myouga, F., Motohashi, R., Shinozaki, K., and Shikanai, T. (2007). Conserved domain structure of pentatricopeptide repeat proteins involved in chloroplast RNA editing. Proc. Natl. Acad. Sci. USA 104 8178–8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda, K., Nakamura, T., Sugita, M., Shimizu, T., and Shikanai, T. (2006). A pentatricopeptide repeat protein is a site recognition factor in chloroplast RNA editing. J. Biol. Chem. 281 37661–37667. [DOI] [PubMed] [Google Scholar]
- O'Toole, N., Hattori, M., Andres, C., Iida, K., Lurin, C., Schmitz-Linneweber, C., Sugita, M., and Small, I. (2008). On the expansion of the pentatricopeptide repeat gene family in plants. Mol. Biol. Evol. 25 1120–1128. [DOI] [PubMed] [Google Scholar]
- Peng, L., Shimizu, H., and Shikanai, T. (2008). The chloroplast NAD(P)H dehydrogenase complex interacts with photosystem I in Arabidopsis. J. Biol. Chem. 283 34873–34879. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pfalz, J., Bayraktar, O.A., Prikryl, J., and Barkan, A. (2009). Site-specific binding of a PPR protein defines and stabilizes 5′ and 3′ mRNA termini in chloroplasts. EMBO J. 28 2042–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, M.L., Peeters, N.M., and Hanson, M.R. (2001). A single alteration 20 nt 5′ to an editing target inhibits chloroplast RNA editing in vivo. Nucleic Acids Res. 29 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, P., Longden, I., and Bleasby, A. (2000). EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. 16 276–277. [DOI] [PubMed] [Google Scholar]
- Robbins, J.C., Heller, W.P., and Hanson, M.R. (2009). A comparative genomics approach identifies a PPR-DYW protein that is essential for C-to-U editing of the Arabidopsis chloroplast accD transcript. RNA 15 1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudinger, M., Polsakiewicz, M., and Knoop, V. (2008). Organellar RNA editing and plant-specific extensions of pentatricopeptide repeat proteins in jungermanniid but not in marchantiid liverworts. Mol. Biol. Evol. 25 1405–1414. [DOI] [PubMed] [Google Scholar]
- Salone, V., Rudinger, M., Polsakiewicz, M., Hoffmann, B., Groth-Malonek, M., Szurek, B., Small, I., Knoop, V., and Lurin, C. (2007). A hypothesis on the identification of the editing enzyme in plant organelles. FEBS Lett. 581 4132–4138. [DOI] [PubMed] [Google Scholar]
- Sasaki, T., Yukawa, Y., Wakasugi, T., Yamada, K., and Sugiura, M. (2006). A simple in vitro RNA editing assay for chloroplast transcripts using fluorescent dideoxynucleotides: Distinct types of sequence elements required for editing of ndh transcripts. Plant J. 47 802–810. [DOI] [PubMed] [Google Scholar]
- Schmitz-Linneweber, C., and Small, I. (2008). Pentatricopeptide repeat proteins: A socket set for organelle gene expression. Trends Plant Sci. 13 663–670. [DOI] [PubMed] [Google Scholar]
- Schmitz-Linneweber, C., Williams-Carrier, R., and Barkan, A. (2005). RNA immunoprecipitation and microarray analysis show a chloroplast pentatricopeptide repeat protein to be associated with the 5′ region of mRNAs whose translation it activates. Plant Cell 17 2791–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai, T. (2006). RNA editing in plant organelles: Machinery, physiological function and evolution. Cell. Mol. Life Sci. 63 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai, T. (2007). Cyclic electron transport around photosystem I: Genetic approaches. Annu. Rev. Plant Biol. 58 199–217. [DOI] [PubMed] [Google Scholar]
- Shikanai, T., Endo, T., Hashimoto, T., Yamada, Y., Asada, K., and Yokota, A. (1998). Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around photosystem I. Proc. Natl. Acad. Sci. USA 95 9705–9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, L., and Emeson, R.B. (1996). RNA editing. Annu. Rev. Neurosci. 19 27–52. [DOI] [PubMed] [Google Scholar]
- Small, I., Peeters, N., Legeai, F., and Lurin, C. (2004). Predotar: A tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 4 1581–1590. [DOI] [PubMed] [Google Scholar]
- Small, I.D., and Peeters, N. (2000). The PPR motif - A TPR-related motif prevalent in plant organellar proteins. Trends Biochem. Sci. 25 46–47. [DOI] [PubMed] [Google Scholar]
- Swiatek, M., Kuras, R., Sokolenko, A., Higgs, D., Olive, J., Cinque, G., Müller, B., Eichacker, L.A., Stern, D.B., Bassi, R., Herrmann, R.G., and Wollman, F.A. (2001). The chloroplast gene ycf9 encodes a photosystem II (PSII) core subunit, PsbZ, that participates in PSII supramolecular architecture. Plant Cell 13 1347–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka, M., Neuwirt, J., and Brennicke, A. (2004). Complex cis-elements determine an RNA editing site in pea mitochondria. Nucleic Acids Res. 32 4137–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka, M., Verbitskiy, D., van der Merwe, J.A., Zehrmann, A., and Brennicke, A. (2008). The process of RNA editing in plant mitochondria. Mitochondrion 8 35–46. [DOI] [PubMed] [Google Scholar]
- Thompson, J.D., Higgins, D.G., and Gibson, T.J. (1994). CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillich, M., Le Sy, V., Schulerowitz, K., von Haeseler, A., Maier, U.G., and Schmitz-Linneweber, C. (2009). Loss of matK RNA editing in seed plant chloroplasts. BMC Evol. Biol. 9 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbitskiy, D., van der Merwe, J.A., Zehrmann, A., Brennicke, A., and Takenaka, M. (2008). Multiple specificity recognition motifs enhance plant mitochondrial RNA editing in vitro. J. Biol. Chem. 283 24374–24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Q.B., Jiang, Y., Chong, K., and Yang, Z.N. (2009). AtECB2, a pentatricopeptide repeat protein, is required for chloroplast transcript accD RNA editing and early chloroplast biogenesis in Arabidopsis thaliana. Plant J. 59: 1011–1023. [DOI] [PubMed]
- Zehrmann, A., van der Merwe, J.A., Verbitskiy, D., Brennicke, A., and Takenaka, M. (2008). Seven large variations in the extent of RNA editing in plant mitochondria between three ecotypes of Arabidopsis thaliana. Mitochondrion 8 319–327. [DOI] [PubMed] [Google Scholar]
- Zehrmann, A., Verbitskiy, D., van der Merwe, J.A., Brennicke, A., and Takenaka, M. (2009). A DYW domain-containing pentatricopeptide repeat protein is required for RNA editing at multiple sites in mitochondria of Arabidopsis thaliana. Plant Cell 21 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, W., Cheng, Y., Yap, A., Chateigner-Boutin, A.L., Delannoy, E., Hammani, K., Small, I., and Huang, J. (2009). The Arabidopsis gene YS1 encoding a DYW protein is required for editing of rpoB transcripts and the rapid development of chloroplasts during early growth. Plant J. 58 82–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.