Abstract
Interferon gamma induces striking phenotypic alterations in confluent cultures of human vascular endothelial cells (HEC), including cell shape change from polygonal to elongated and cytoskeletal actin rearrangement from dense peripheral bands to longitudinal bundles of stress fibers. Since many transmembrane proteins, including class I major histocompatibility complex (MHC) proteins, interact with cytoskeletal actin, an interferon-gamma-induced anisotropic arrangement of stress fibers might cause anisotropic lateral diffusion of HEC class I MHC proteins. To test this hypothesis, we adapted the fluorescence photobleaching recovery technique to allow measurement of anisotropic diffusion of fluorescently labeled molecules on two-dimensional surfaces. A highly eccentric elliptical Gaussian laser beam was used to photobleach the sample and to monitor fluorescence recovery. In this technique, named "line fluorescence photobleaching recovery," lateral diffusion is measured along that axis of the sample that is perpendicular to the major axis of the elliptical beam. The lateral diffusion coefficient and fractional mobility are obtained by fitting the experimental data to a theoretical recovery curve, the form of which is determined by the solution to a modified version of the diffusion equation in which a tensor is used to describe diffusion in two orthogonal directions. Fluorescein-conjugated murine monoclonal antibodies were used to label class I MHC proteins on interferon-gamma-treated HEC and human dermal fibroblasts. These two cultured human cell types were found to be similar in their elongated shape and anisotropic stress fiber organization. Class I MHC protein lateral mobility was compared to that of fluorescein-labeled phosphatidyl-ethanolamine, a membrane phospholipid probe. Class I MHC proteins diffused anisotropically on human dermal fibroblasts, whereas fluorescein-labeled phosphatidylethanolamine diffused isotropically on this cell type. In contrast, both class I MHC proteins and fluorescein-labeled phosphatidylethanolamine diffused isotropically on interferon-gamma-treated HEC. These data suggest that neither elongated shape nor anisotropic stress fiber arrangement is sufficient to induce anisotropic diffusion of proteins on the HEC plasma membrane.
Full text
PDF


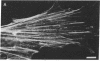
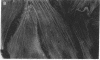
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizenbud B. M., Gershon N. D. Diffusion of molecules on biological membranes of nonplanar form. II. Diffusion anisotropy. Biophys J. 1985 Oct;48(4):543–546. doi: 10.1016/S0006-3495(85)83811-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash J. F., Louvard D., Singer S. J. Antibody-induced linkages of plasma membrane proteins to intracellular actomyosin-containing filaments in cultured fibroblasts. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5584–5588. doi: 10.1073/pnas.74.12.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bierer B. E., Herrmann S. H., Brown C. S., Burakoff S. J., Golan D. E. Lateral mobility of class I histocompatibility antigens in B lymphoblastoid cell membranes: modulation by cross-linking and effect of cell density. J Cell Biol. 1987 Sep;105(3):1147–1152. doi: 10.1083/jcb.105.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Singer S. J. Transmembrane interactions and the mechanism of capping of surface receptors by their specific ligands. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5031–5035. doi: 10.1073/pnas.74.11.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Gall W. E., Edelman G. M. Lateral diffusion of surface molecules in animal cells and tissues. Science. 1981 Aug 21;213(4510):903–905. doi: 10.1126/science.7196087. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Golan D. E., Brown C. S., Cianci C. M., Furlong S. T., Caulfield J. P. Schistosomula of Schistosoma mansoni use lysophosphatidylcholine to lyse adherent human red blood cells and immobilize red cell membrane components. J Cell Biol. 1986 Sep;103(3):819–828. doi: 10.1083/jcb.103.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henis Y. I., Elson E. L. Inhibition of the mobility of mouse lymphocyte surface immunoglobulins by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1072–1076. doi: 10.1073/pnas.78.2.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., O'Dell D., August J. T. Lateral diffusion of an 80,000-dalton glycoprotein in the plasma membrane of murine fibroblasts: relationships to cell structure and function. J Cell Biol. 1984 Nov;99(5):1624–1633. doi: 10.1083/jcb.99.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitza H. G., McGregor G., Jacobson K. A. Direct measurement of lateral transport in membranes by using time-resolved spatial photometry. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4122–4126. doi: 10.1073/pnas.82.12.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E. Fluorescence redistribution after photobleaching. A new multipoint analysis of membrane translational dynamics. Biophys J. 1979 Nov;28(2):281–291. doi: 10.1016/S0006-3495(79)85176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T. Stabilizing infrastructure of cell membranes. Annu Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over surface components. Biochim Biophys Acta. 1976 Apr 13;457(1):57–108. doi: 10.1016/0304-4157(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Owicki J. C., McConnell H. M. Lateral diffusion in inhomogeneous membranes. Model membranes containing cholesterol. Biophys J. 1980 Jun;30(3):383–397. doi: 10.1016/S0006-3495(80)85103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty H. R., Smith L. M., Fearon D. T., McConnell H. M. Lateral distribution and diffusion of the C3b receptor of complement, HLA antigens, and lipid probes in peripheral blood leukocytes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6587–6591. doi: 10.1073/pnas.77.11.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Collins T., Gimbrone M. A., Jr, Cotran R. S., Gitlin J. D., Fiers W., Clayberger C., Krensky A. M., Burakoff S. J., Reiss C. S. Lymphocytes recognize human vascular endothelial and dermal fibroblast Ia antigens induced by recombinant immune interferon. Nature. 1983 Oct 20;305(5936):726–729. doi: 10.1038/305726a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Guild B. C., Strominger J. L., Veatch W. R. Purification of HLA-A2 antigen, fluorescent labeling of its intracellular region, and demonstration of an interaction between fluorescently labeled HLA-A2 antigen and lymphoblastoid cell cytoskeleton proteins in vitro. Biochemistry. 1981 Sep 15;20(19):5625–5633. doi: 10.1021/bi00522a042. [DOI] [PubMed] [Google Scholar]
- Scahill S. J., Devos R., Van der Heyden J., Fiers W. Expression and characterization of the product of a human immune interferon cDNA gene in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4654–4658. doi: 10.1073/pnas.80.15.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Axelrod D., Koppel D. E., Webb W. W., Elson E. L. Lateral transport of a lipid probe and labeled proteins on a cell membrane. Science. 1977 Jan 21;195(4275):307–309. doi: 10.1126/science.556653. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Elson E. L., Webb W. W., Yahara I., Rutishauser U., Edelman G. M. Receptor diffusion on cell surfaces modulated by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1110–1114. doi: 10.1073/pnas.74.3.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. A., Clark W. R., McConnell H. M. Anisotropic molecular motion on cell surfaces. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5641–5644. doi: 10.1073/pnas.76.11.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolpen A. H., Guinan E. C., Fiers W., Pober J. S. Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am J Pathol. 1986 Apr;123(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Wier M. L., Edidin M. Effects of cell density and extracellular matrix on the lateral diffusion of major histocompatibility antigens in cultured fibroblasts. J Cell Biol. 1986 Jul;103(1):215–222. doi: 10.1083/jcb.103.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D. E., Hagopian S. S., Lewis R. G., Voglmayr J. K., Fairbanks G. Lateral regionalization and diffusion of a maturation-dependent antigen in the ram sperm plasma membrane. J Cell Biol. 1986 May;102(5):1826–1831. doi: 10.1083/jcb.102.5.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu E. S., Tank D. W., Webb W. W. Unconstrained lateral diffusion of concanavalin A receptors on bulbous lymphocytes. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4962–4966. doi: 10.1073/pnas.79.16.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yguerabide J., Schmidt J. A., Yguerabide E. E. Lateral mobility in membranes as detected by fluorescence recovery after photobleaching. Biophys J. 1982 Oct;40(1):69–75. doi: 10.1016/S0006-3495(82)84459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]