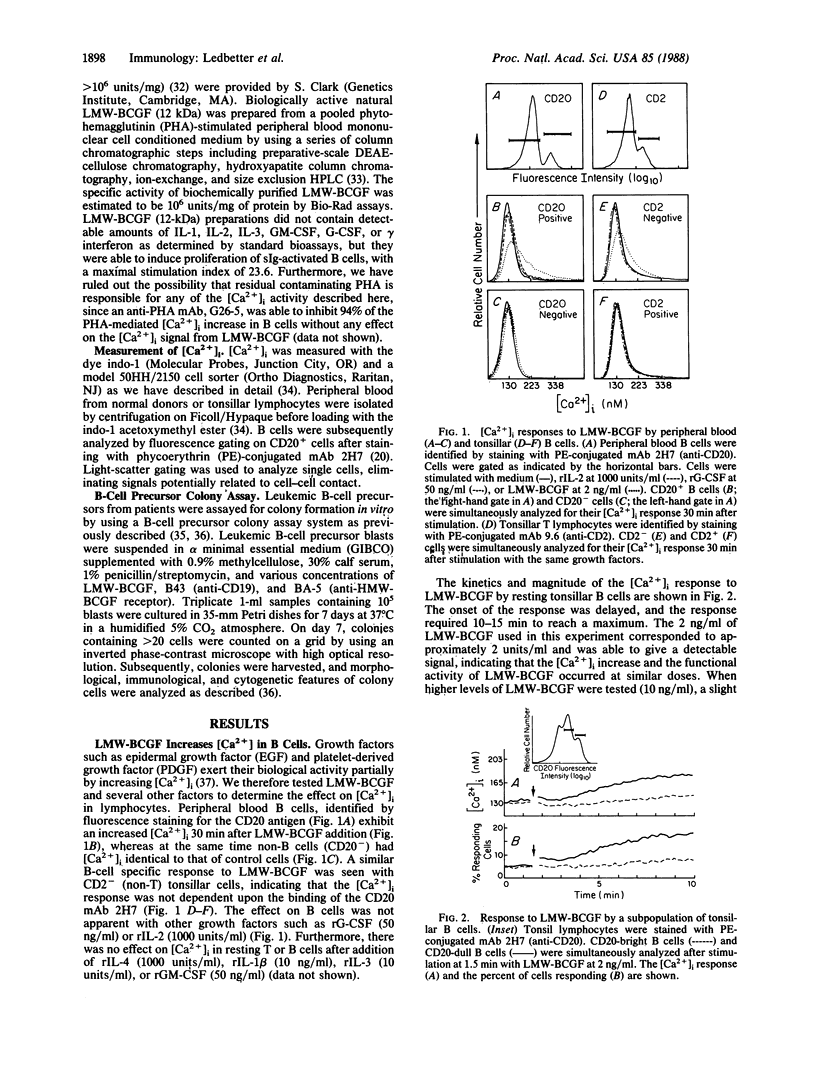
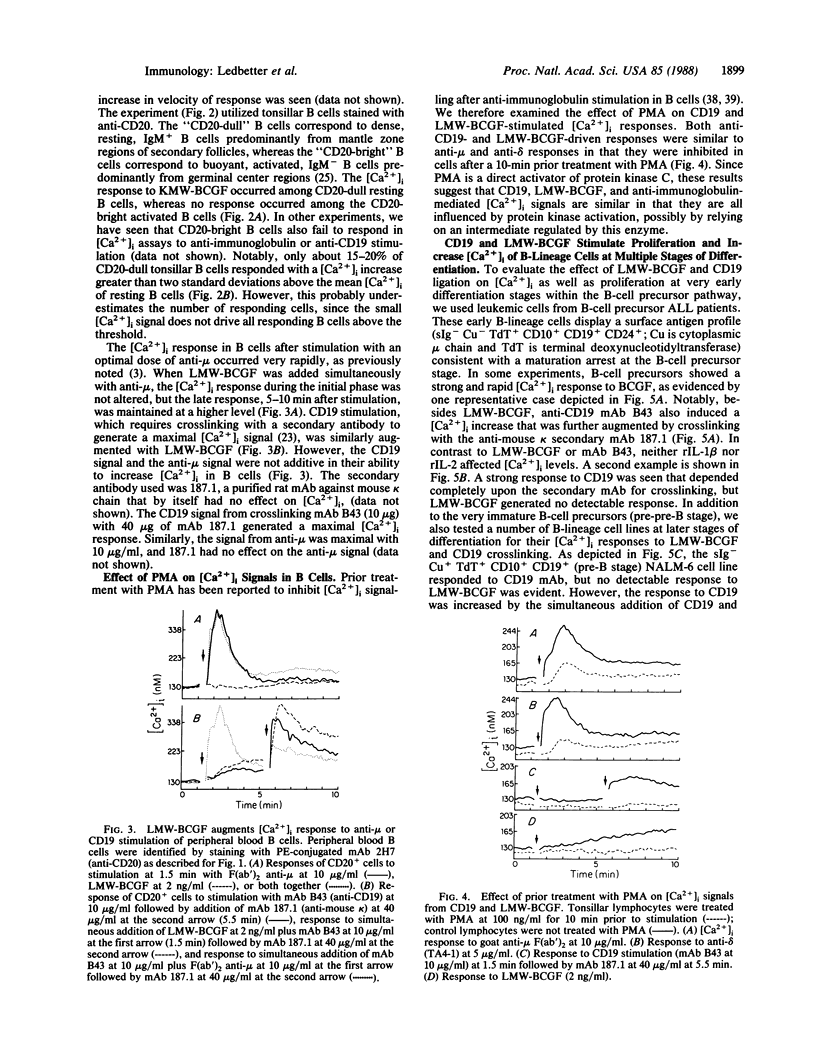
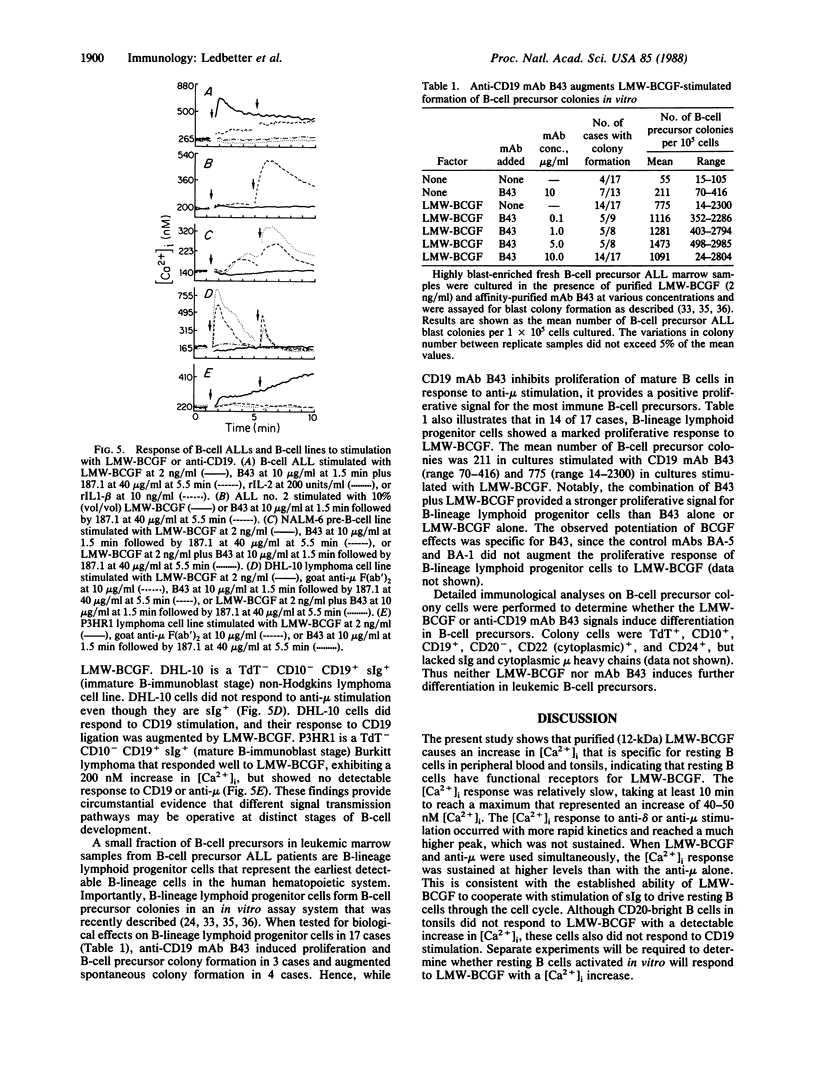
Abstract
Increases in cytoplasmic free calcium ([Ca2+]i) can be induced in resting B cells either by a low molecular weight (12-kDa) B-cell growth factor (LMW-BCGF) or by crosslinking the B-cell antigen CD19 with monoclonal antibody (mAb). LMW-BCGF causes a slow [Ca2+]i increase in peripheral blood and tonsillar B cells but has no effect on [Ca2+]i in resting T cells. B-cell [Ca2+]i responses mediated by anti-surface immunoglobulin (sIg) or anti-CD19 are potentiated by LMW-BCGF, but anti-sIg and anti-CD19 do not show additive [Ca2+]i responses. LMW-BCGF- and anti-CD19-induced [Ca2+]i signals are similar to the sIgM or sIgD-mediated signals in that they are inhibited by prior treatment with phorbol 12-myristate 13-acetate. However, LMW-BCGF- and CD19-mediated signals do not depend on the expression of sIg, since they were also observed on sIg-B-cell precursor acute lymphoblastic leukemia (ALL) cells. Both anti-CD19 and LMW-BCGF stimulated in vitro colony formation by ALL cells and showed additive effects when used together. [Ca2+]i responses to LMW-BCGF or CD19 cross-linking were also evident on certain pre-B-cell and lymphoma B-cell lines.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrus J. L., Jr, Jurgensen C. H., Brown E. J., Fauci A. S. Purification to homogeneity of a high molecular weight human B cell growth factor; demonstration of specific binding to activated B cells; and development of a monoclonal antibody to the factor. J Exp Med. 1985 Oct 1;162(4):1319–1335. doi: 10.1084/jem.162.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Klaus G. G. Crosslinking of surface immunoglobulin and Fc receptors on B lymphocytes inhibits stimulation of inositol phospholipid breakdown via the antigen receptors. J Exp Med. 1985 Dec 1;162(6):1825–1836. doi: 10.1084/jem.162.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Meade C. J., Turner G. A., Klaus G. G. B lymphocyte receptors and polyphosphoinositide degradation. Cell. 1985 Jul;41(3):999–1006. doi: 10.1016/s0092-8674(85)80080-5. [DOI] [PubMed] [Google Scholar]
- Cambier J. C., Monroe J. G. B cell activation. V. Differentiation signaling of B cell membrane depolarization, increased I-A expression, G0 to G1 transition, and thymidine uptake by anti-IgM and anti-IgD antibodies. J Immunol. 1984 Aug;133(2):576–581. [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4494–4498. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall K. M., Cambier J. C. B cell activation. VIII. Membrane immunoglobulins transduce signals via activation of phosphatidylinositol hydrolysis. J Immunol. 1984 Dec;133(6):3382–3386. [PubMed] [Google Scholar]
- DeFranco A. L., Raveche E. S., Paul W. E. Separate control of B lymphocyte early activation and proliferation in response to anti-IgM antibodies. J Immunol. 1985 Jul;135(1):87–94. [PubMed] [Google Scholar]
- Defrance T., Aubry J. P., Vanbervliet B., Banchereau J. Human interferon-gamma acts as a B cell growth factor in the anti-IgM antibody co-stimulatory assay but has no direct B cell differentiation activity. J Immunol. 1986 Dec 15;137(12):3861–3867. [PubMed] [Google Scholar]
- Defranco A. L., Raveche E. S., Asofsky R., Paul W. E. Frequency of B lymphocytes responsive to anti-immunoglobulin. J Exp Med. 1982 May 1;155(5):1523–1536. doi: 10.1084/jem.155.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. R., DeFranco A. L. Phorbol esters and dioctanoylglycerol block anti-IgM-stimulated phosphoinositide hydrolysis in the murine B cell lymphoma WEHI-231. J Immunol. 1987 Feb 1;138(3):868–876. [PubMed] [Google Scholar]
- Gordon J., Rowe M., Walker L., Guy G. Ligation of the CD23,p45 (BLAST-2,EBVCS) antigen triggers the cell-cycle progression of activated B lymphocytes. Eur J Immunol. 1986 Sep;16(9):1075–1080. doi: 10.1002/eji.1830160908. [DOI] [PubMed] [Google Scholar]
- Howard M., Mizel S. B., Lachman L., Ansel J., Johnson B., Paul W. E. Role of interleukin 1 in anti-immunoglobulin-induced B cell proliferation. J Exp Med. 1983 May 1;157(5):1529–1543. doi: 10.1084/jem.157.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Clark E. A. Surface phenotype and function of tonsillar germinal center and mantle zone B cell subsets. Hum Immunol. 1986 Jan;15(1):30–43. doi: 10.1016/0198-8859(86)90315-0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Grosmaire L. S., Rabinovitch P. S. Crosslinking of surface antigens causes mobilization of intracellular ionized calcium in T lymphocytes. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1384–1388. doi: 10.1073/pnas.84.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Shu G., Gallagher M., Clark E. A. Augmentation of normal and malignant B cell proliferation by monoclonal antibody to the B cell-specific antigen BP50 (CDW40). J Immunol. 1987 Feb 1;138(3):788–794. [PubMed] [Google Scholar]
- Lipsky P. E., Thompson P. A., Rosenwasser L. J., Dinarello C. A. The role of interleukin 1 in human B cell activation: inhibition of B cell proliferation and the generation of immunoglobulin-secreting cells by an antibody against human leukocytic pyrogen. J Immunol. 1983 Jun;130(6):2708–2714. [PubMed] [Google Scholar]
- Maizel A. L., Morgan J. W., Mehta S. R., Kouttab N. M., Bator J. M., Sahasrabuddhe C. G. Long-term growth of human B cells and their use in a microassay for B-cell growth factor. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5047–5051. doi: 10.1073/pnas.80.16.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Mehta S. R., Conrad D., Sandler R., Morgan J., Montagna R., Maizel A. L. Purification of human B cell growth factor. J Immunol. 1985 Nov;135(5):3298–3302. [PubMed] [Google Scholar]
- Melchers F., Erdei A., Schulz T., Dierich M. P. Growth control of activated, synchronized murine B cells by the C3d fragment of human complement. Nature. 1985 Sep 19;317(6034):264–267. doi: 10.1038/317264a0. [DOI] [PubMed] [Google Scholar]
- Mizuguchi J., Beaven M. A., Li J. H., Paul W. E. Phorbol myristate acetate inhibits anti-IgM-mediated signaling in resting B cells. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4474–4478. doi: 10.1073/pnas.83.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Aerts R. J., Tertoolen L. G., de Laat S. W. The epidermal growth factor-induced calcium signal in A431 cells. J Biol Chem. 1986 Jan 5;261(1):279–284. [PubMed] [Google Scholar]
- Nakagawa T., Hirano T., Nakagawa N., Yoshizaki K., Kishimoto T. Effect of recombinant IL 2 and gamma-IFN on proliferation and differentiation of human B cells. J Immunol. 1985 Feb;134(2):959–966. [PubMed] [Google Scholar]
- Pezzutto A., Dörken B., Moldenhauer G., Clark E. A. Amplification of human B cell activation by a monoclonal antibody to the B cell-specific antigen CD22, Bp 130/140. J Immunol. 1987 Jan 1;138(1):98–103. [PubMed] [Google Scholar]
- Pezzutto A., Dörken B., Rabinovitch P. S., Ledbetter J. A., Moldenhauer G., Clark E. A. CD19 monoclonal antibody HD37 inhibits anti-immunoglobulin-induced B cell activation and proliferation. J Immunol. 1987 May 1;138(9):2793–2799. [PubMed] [Google Scholar]
- Pozzan T., Arslan P., Tsien R. Y., Rink T. J. Anti-immunoglobulin, cytoplasmic free calcium, and capping in B lymphocytes. J Cell Biol. 1982 Aug;94(2):335–340. doi: 10.1083/jcb.94.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch P. S., June C. H., Grossmann A., Ledbetter J. A. Heterogeneity among T cells in intracellular free calcium responses after mitogen stimulation with PHA or anti-CD3. Simultaneous use of indo-1 and immunofluorescence with flow cytometry. J Immunol. 1986 Aug 1;137(3):952–961. [PubMed] [Google Scholar]
- Sieff C. A., Emerson S. G., Donahue R. E., Nathan D. G., Wang E. A., Wong G. G., Clark S. C. Human recombinant granulocyte-macrophage colony-stimulating factor: a multilineage hematopoietin. Science. 1985 Dec 6;230(4730):1171–1173. doi: 10.1126/science.3877981. [DOI] [PubMed] [Google Scholar]
- Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Fauci A. S., Heerema N. A., Song C. W., Mehta S. R., Gajl-Peczalska K., Chandan M., Ambrus J. L. B-cell growth factor receptor expression and B-cell growth factor response of leukemic B cell precursors and B lineage lymphoid progenitor cells. Blood. 1987 Oct;70(4):1020–1034. [PubMed] [Google Scholar]
- Uckun F. M., Gajl-Peczalska K. J., Kersey J. H., Houston L. L., Vallera D. A. Use of a novel colony assay to evaluate the cytotoxicity of an immunotoxin containing pokeweed antiviral protein against blast progenitor cells freshly obtained from patients with common B-lineage acute lymphoblastic leukemia. J Exp Med. 1986 Feb 1;163(2):347–368. doi: 10.1084/jem.163.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Jaszcz W., Ambrus J. L., Fauci A. S., Gajl-Peczalska K., Song C. W., Wick M. R., Myers D. E., Waddick K., Ledbetter J. A. Detailed studies on expression and function of CD19 surface determinant by using B43 monoclonal antibody and the clinical potential of anti-CD19 immunotoxins. Blood. 1988 Jan;71(1):13–29. [PubMed] [Google Scholar]
- Uckun F. M., Kersey J. H., Gajl-Peczalska K. J., Heerema N. A., Provisor A. J., Haag D., Gilchrist G., Song C. W., Arthur D. C., Roloff J. Heterogeneity of cultured leukemic lymphoid progenitor cells from B cell precursor acute lymphoblastic leukemia (ALL) patients. J Clin Invest. 1987 Sep;80(3):639–646. doi: 10.1172/JCI113116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]
- Yelton D. E., Desaymard C., Scharff M. D. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1981;1(1):5–11. doi: 10.1089/hyb.1.1981.1.5. [DOI] [PubMed] [Google Scholar]
- Yokota T., Otsuka T., Mosmann T., Banchereau J., DeFrance T., Blanchard D., De Vries J. E., Lee F., Arai K. Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5894–5898. doi: 10.1073/pnas.83.16.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubler R. H., Lowenthal J. W., Erard F., Hashimoto N., Devos R., MacDonald H. R. Activated B cells express receptors for, and proliferate in response to, pure interleukin 2. J Exp Med. 1984 Oct 1;160(4):1170–1183. doi: 10.1084/jem.160.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]


