Abstract
In this paper, we demonstrate that two characteristic properties of mammalian brains emerge when scaling-up modular, cortical structures. Firstly, the glia-to-neuron ratio is not constant across brains of different sizes: large mammalian brains have more glia per neuron than smaller brains. Our analyses suggest that if one assumes that glia number is proportional to wiring, a particular quantitative relationship emerges between brain size and glia-to-neuron ratio that fits the empirical data. Secondly, many authors have reported that the number of neurons underlying one mm2 of mammalian cortex is remarkably constant, across both areas and species. Here, we will show that such a constancy emerges when enlarging modular, cortical brain structures. Our analyses thus corroborate recent studies on the mammalian brain as a scalable architecture, providing a possible mechanism to explain some of the principles, constancies and rules that hold across brains of different size.
Keywords: Comparative neuroanatomy, Glia-to-neuron index, Neuron number
1 Introduction
Brains function by virtue of their connectivity. In contrast with human mass communication networks, point-to-point communications between nerve cells cannot use shared lines. Instead, a physical structure must be present for each connection. Imagine that each phone were connected with a separate wire to each of a thousand other phones. Whereas this might be a feasible design for a small town, in a country as large as America the landscape would be dominated by huge, thick cable structures, easily overwhelming the highway system in size. Similarly, large brains face a severe connectivity problem. In this paper, we look at the consequences of brain connectivity ‘designs’ that have been successful in the evolution of large-brained animals, in particular of mammals.
In an earlier analysis (Murre and Sturdy 1995), we calculated the volume of connective structures given the number of neurons, their connective graph, and selected packing strategy. A surprising finding was that for brain structures consisting of many densely connected neurons, it is most efficient to place the neuron bodies in a cortex with the connecting structures running underneath. This ‘potato peel’ approach leads to a smaller wiring volume than the packing strategy whereby the same number of neurons would be intermingled with the connecting structures (a ‘spaghetti with meatballs model’). This was surprising to us because we had expected it to be the other way around. After all, intermingling seems to offer opportunities for optimal component placement (Cherniak 1994, 1995).
Another conclusion of this research was that introducing patchy or modular connectivity, with occasional long-range connections that have many end-point connections to a patch or module, was much more efficient than random (but sparse) connectivity, especially for large brains. The efficiency of this structure was only surpassed by nearest neighbor connection schemes where neurons connect only to the direct neighborhood. These latter structures, however, are suitable only for local processing. For many processing functions, especially for associative memory, a significant proportion of long-range connections is required. Other authors have come to similar conclusions about the brain’s wiring (e.g., Cherniak et al. 1999; Mitchison 1991, 1992; Young 1992). Mitchison (1991), for instance, showed that dividing one cortical area into two (as with the stripes and blobs in the cortex) may reduce the volume taken up by their connections, especially in larger brains. Patchy modularity, thus, may help to reduce wiring length, particularly in larger brains.
Modularity causes a brain to differentiate without necessarily limiting it to mainly local processing. Such differentiation may have many functional advantages, leading to better learning and generalization capacity (Murre 1992). Computer simulations of neural networks that were evaluated on their capacity to carry out a simple visual recognition task also tended to evolve neural network architectures with different streams (Happel and Murre 1994), even if no penalties for wiring length were given. Indeed, recent detailed neural networks of the visual system show a high degree of regularity in wiring based on patchy modular connectivity (e.g., Deco and Rolls 2002; Li 1999; Roelfsema et al. 2002).
On the basis of these findings, we might conjecture that patchy modular brains with a cortical structure are more expandable in evolution compared to brains that are less differentiated. Patchy modularity and other limitations on connectivity often tend to enhance processing efficiency, provided they do not limit the brain to local processing only (Murre 1992). A cortical, modular structure could thus at the same time expand in size and processing efficiency. The mammalian brain in particular fits these characteristics, causing it to be “eminently scalable” (Striedter 2005, p. 284). Indeed, recent neuroanatomical analyses hint to the scalability of mammalian brains (Clark et al. 2001).
Mammalian brains have (patchy) modular connectivity and cortices. On the basis of these considerations, we expect them to be at the same time expandable and powerful, capable of complex adaptive processing tasks. In this paper, we use the same theoretical framework as Murre and Sturdy (1995) and demonstrate that two characteristic properties of mammalian brains emerge when scaling up brain structures of that size: (1) The glial index, the quotient of the number of glia and neurons per unit volume, is not constant across different brains. Large brains have more glia per neuron (Friede 1954; Hawkins and Olszewski 1957; Tower and Young 1973). Our analyses suggest that if one assumes that glia number is proportional to wiring length (resulting in a constant glia density, Tower 1967), a particular quantitative relationship emerges between mammalian brain size and glia-to-neuron ratio that fits the empirical data well. (2) Many authors have reported that the number of neurons underneath a mm2 of mammalian cortex is remarkably constant, across both species and areas (e.g., Rockel et al. 1980, see also Bok 1959; Beaulieu and Colonnier 1985; Braitenberg and Schüz 1991; Hendry et al. 1987; Shankle et al. 1998). In this paper, we will confirm this constancy using neuroanatomical data from across the literature, and show that when scaling-up modular, cortical brain structures, as an emergent (theoretical) property we predict exactly such a constancy. Our analyses thus corroborate recent studies in suggesting a scalable architecture in the mammalian brain, proposing a mechanism to explain constancies or rules that hold across mammalian brains of different size.
2 Model
2.1 Glia volume in a cortical modular structure
We do not intend to model brain anatomy in full detail. The model should rather be seen as a first step toward a better understanding of some general principles underlying brain design, deliberately ignoring some structures while taking hints from highly simplified ones. Our model uses the main principles and assumptions described in Murre and Sturdy (1995). We assume that the entire volume of the brain is given by the sum of the volumes of all wiring (all cell processes, including dendrites and axons, see also Braitenberg 2001; Murre and Sturdy 1995) and the volume of glia. We also assume that the fiber’s cross-sectional area, or radius r , remains constant (Braitenberg 2001; Mitchison 1992). We use a structure in which neurons are positioned equidistantly in two dimensions on the surface of a sphere, with connecting structures running through its internal volume (Murre and Sturdy 1995). This structure approximates to the gray matter of the brain (cortex, neurons), with white matter (connections) filling the interior (albeit we do not model gray matter volume explicitly, see also Sect. 3.1). For larger, convoluted brains, convolutions are modeled as occurring entirely in the gray matter and the surface of the white matter is modeled as the surface of the sphere. Further, we consider a modular connectivity design, in which the network is subdivided into many modules. Within a module, connectivity may be dense. Connectivity is much sparser at the module-level, where any given neuron is connected to only a subset of the available modules. There is considerable consensus that this connectivity pattern is the most plausible for the mammalian brain (see e.g., Mountcastle 1978; Szentágothai 1975 or Ruppin et al. 1993; Wen and Chklovskii 2005; for similar connectivity schemes).
We use a snaking fiber structure (Fig. 1, see also Murre and Sturdy 1995), in which a neurons ‘snakes’ along all its targets, like for instance, the parallel fibers in the cerebellum which snake along purkinje cells. Neurons often show elaborate arborization, but arborization in itself does not reduce total fiber length. Specifically, with neurons placed in a rectangular grid and bifurcations coinciding with neurons, any branching structure can be replaced by a snaking structure that has the same total fiber length (Murre and Sturdy 1995). Compared to the branching neuron, derivations based upon a snaking model are more straightforward, which is why we will use them here.
Fig. 1.
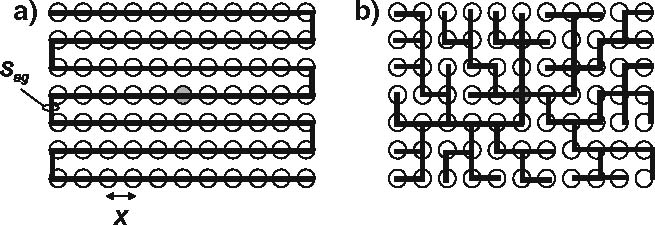
Two connection strategies. A pre-synaptic neuron connects to b post-synaptic targets (white circles). a A snaking connection strategy. b A branching connection strategy. Total fiber length is equal in a and b. Longest path-length in a scales O(b), longest path-length in b is O(b 1/2)
Following Tower (1967), we assume that the density of glia is constant, regardless of neuron number or fiber length. This is similar to adding a glia volume that is proportionate to the length of the fibers, so that the total system becomes one of naked fibers with increased thickness only. Its total cross-sectional area s ag can be decomposed as follows:
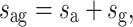 |
1 |
where s a is the cross-sectional surface taken up by the neuron’s processes (dendrites and axons), and s g is the cross-sectional surface taken up by glia.
We assume that any given pre-synaptic neuron n is only connected to a subset m of the available modules; thus the total number of modules in the volume may, but need not, exceed m. Also, target modules need not be the same for every pre-synaptic neuron. In a modular structure, wiring volume can be decomposed into global fiber volume A g, which consists of long-range connections to the target modules, and local fiber volume A l ,which consists of short-range branches within the target modules. We will derive global fiber volume first. Let us assume for simplicity that the target modules are distributed regularly throughout the cortical sheet (see also Murre and Sturdy 1995). If the modules are spread regularly, they will be spaced nearly equidistantly. With m target modules distributed regularly throughout the cortical sheet, on average n/m neurons lie in between two targets, and axon length in between targets is l = (n/m)1/2 neurons. With interneuron distance x, 1 l is (n/m)1/2 x meters. All n neurons send a fiber to m modules, so that the total fiber volume for the long-range connections A g is:
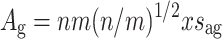 |
2 |
.
We assume that within a target module a neuron connects to b post-synaptic neurons lying in the neighborhood of its long-range fiber. With distance x in between every two neurons, local branching volume in one target module will be bxs ag. With m target modules this becomes mbxs ag. Each of n pre-synaptic neurons makes local connections within target modules, so total fiber volume within target modules A l is nmbxs ag. Note that this equation allows for different numbers of local targets across modules, as well as targets within the pre-synaptic module, as long as the total number of local connections mb remains the same.
Combining local and global connection volume gives the following expression for V = A g + A l :
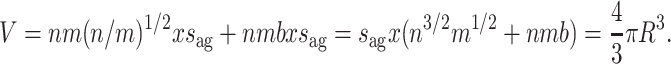 |
3 |
The surface of this spherical connectivity volume V is occupied by n neurons that are located equidistantly, so that each of the neurons occupies a small square of 4π R 2/n on the white matter surface. By taking the square root of this, we obtain an expression for x:
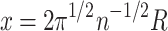 |
4 |
.
Substituting the derived value for x in Eq. (3) gives an expression for R, and results in a volume of:
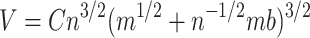 |
5 |
, where C is a constant:
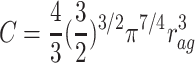 |
6 |
.
The expression for glia volume proper is the same, except for the constant (cf. Eq. 1):
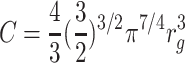 |
7 |
.
For large n, glia volume scales as O(n 3/2). As glia cells are not markedly larger in larger species (Friede 1963), this implies that the glia-to-neuron ratio is larger in bigger brains.
2.2 Surface area of a cortical modular structure
Let us now see what happens to the surface of this modular, cortical structure when we enlarge its volume. The model does not explicitly distinguish between gray and white matter volume, but it still allows for a derivation of the relation neuron number∼gray matter surface area. We will first consider the gray matter surface of small, non-convoluted brains for which gray matter∼brain volume2/3 (Hofman 1985). Let us assume that the number of neurons increases with factor α. In that case, the sphere’s new, bigger volume is:
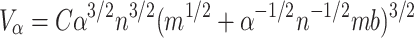 |
8 |
.
Thus, when b is sufficiently small and with large n, the connectivity volume increases with α 3/2:
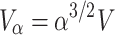 |
9 |
, where V is the volume with n neurons. The radius R of this enlarged sphere is:
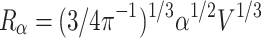 |
10 |
, and its cortical surface is:
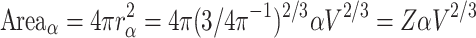 |
11 |
. The radius R of the smaller sphere with n neurons is:
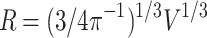 |
12 |
, with gray matter surface:
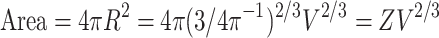 |
13 |
. So that the ratio of the two surfaces is:
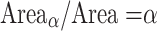 |
14 |
.
This implies that the surface of a modular, cortical brain enlarges with factor α if the number of neurons increases with α. These analyses predict that interneuron distance on the gray matter surface is identical across both area and brain size, which implies that the number of neurons underlying one unit of cortical surface is constant, across both area and species.
A somewhat different relationship holds for larger, convoluted brains. Here, gray matter surface area scales as the 8/9 power of brain volume (Hofman 1985, 1989; Prothero 1997):
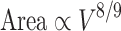 |
15 |
so that
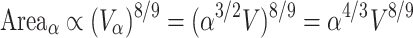 |
16 |
Although this analysis does not predict a linear relationship between neuron number and gray matter surface, it does show that the cortical surface of the larger, convoluted brain is at least large enough to accommodate the newly added neurons.
3 Results
3.1 Glia-to-neuron index
Our analyses have quantified a relationship between the number of glia and neurons, which we can compare to real neuroanatomical data. In order to do this, we assume a constant glia density (Tower 1967) of 45% glia per unit volume (Cherniak 1990), which is similar to setting glia cross-sectional area at 45% of the total cross-sectional area. As a first approximation, we assume that glia density is the same for global and local connections. Later, we will show what happens when glia density is different for the two. Using different numbers of neurons, we arrive at the total volume taken up by glia cells for brains of different sizes. Dividing by single cell volume gives total glia number, from which the glia-to-neuron index can be derived. We use m = 8 target modules, b = 1,250 within-module connections, and r = 0.1µm (Murre and Sturdy 1995). This estimate of m is likely at the high end of the range. However, the glia-to-neuron relationship suggested by the model does not depend highly on the precise value of m, and we merely choose to illustrate one value here. The value of b results after dividing a total of 10,000 connections (Arbib 1972; Carpenter 1984; Palm 1982) over the 8 target modules.
The results of our exploration are presented in Fig. 2 (solid line). The fit between the empirical index and the index as predicted by the model is encouraging, explaining with the model 89% of the variance in the data. Unfortunately, we could not find empirical data differentiating between the types of glia cells, preventing us from drawing any conclusions on the relation between a particular kind of glia cell and neuron number. For illustrational purposes, we also plotted the glia-to-neuron index using a smaller value of m(m = 4), and, consequently, higher b(b = 2, 500) (dashed line). Note that the model does not depend highly on the value of its parameters: glia volume scales as the 3/2 power of the number of neurons (i.e., g∼n 3/2, see Sect. 2.1), automatically resulting in more glia per neuron for larger brains, as has been found experimentally (Friede 1954; Hawkins and Olszewski 1957; Tower and Young 1973). In a similar vein, changing, for example, the fraction of glia cells per unit volume does not qualitatively change our results, provided it is constant across brain size. Such a constant percentage of glial cells per unit volume has been observed in neuroanatomy (Tower 1967; Cherniak 1990).
Fig. 2.
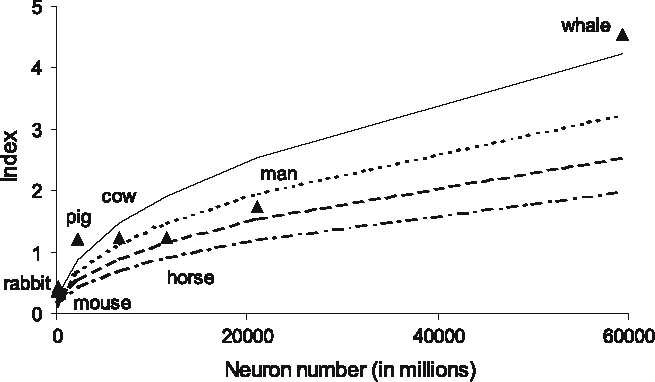
The empirical glia-to-neuron index (triangles) and the index with the modular topology in a cortical model (solid, dotted, dash-dotted and dashed lines). Index data from Friede (1954) and Hawkins and Olszewski (1957). Neuron number for the mouse is from Braitenberg and Schüz (1991), and human cortical neuron number is from Pakkenberg and Gundersen (1997). All other neuron numbers were derived by multiplying cortical surface area (Blinkov and Glezer 1968; Mayhew et al. 1990; Nieuwenhuys et al. 1998) with 90,000 neurons/ mm2 (Average of Fig. 3). We used cortical surface area of the ox as an approximation of the surface area of the cow. Surface area of the whale is a mean of three whale cortices. Solid line: m = 8, b = 1,250, dashed, dotted and dash-dotted line: m = 4, b = 2,500. Dashed and solid line: glia density is 45% per unit volume for both local and global connections, dotted line: glia density is 50% per unit volume for global connections, and 45% for local connections, dash-dotted line: glia density is 40% per unit volume for global connections, and 45% per unit volume for local connections. In all cases r 2 = 0.89
A potential concern may be that we have generalized glia density across gray and white matter. Glia density could be different for the two, and this could affect the derived glia-to-neuron ratio, as the fraction of white matter is not constant across brains of different size. Our model does not differentiate explicitly between gray and white matter. One could argue, however, that the model’s global connections mostly determine white matter volume, while the model’ps local connections would dominate in gray matter. Thus, to further refine the model’s predictions, we also use different glia densities for global and local connections. We could not find any specifications regarding glia density in white matter in the literature and chose to illustrate several densities in Fig. 2 (dotted line: 50% glia per unit white matter volume, dash-dotted line: 40% glia per unit white matter volume, both lines: 45% glia per unit gray matter volume).Changing the percentage glia for local connections has a negligible effect on the glia-to-neuron ratio and is not illustrated here. Notice that for different glia densities, the model’s prediction approximates the empirical data rather well (Fig. 2).
3.2 A constant neuron number underlying 1 mm2 cortical surface
A second prediction of our model is that the interneuron distance across cortical modular brains of different sizes should be constant, at least for non-convoluted brains. Many authors have reported on such a constancy across different species (e.g., Rockel et al. 1980, see also Bok 1959; Beaulieu and Colonnier 1985; Braitenberg and Schüz 1991; Hendry et al. 1987; Shankle et al. 1998), though others have come to different conclusions (Beaulieu and Colonnier 1989; Haug 1987; Skoglund et al. 1996). Figure 3 summarizes neuronal numbers underneath a unit volume of pial surface across different species and areas as reported in the literature.2 The counting techniques used in different studies diverge widely, and it is therefore difficult to directly compare results across studies. Nonetheless, although absolute brain volume may vary a factor thousand with each other (e.g., the total number of cortical neurons in the mouse is approximately 16 million neurons, whereas the total number of cortical neurons in the human is around 21 billion, Braitenberg and Schüz 1991; Pakkenberg and Gundersen 1997), there appears to be much less variation in the number of neurons underlying one squared millimeter of cortical surface. Our prediction thus appears to be supported by the empirical data.
Fig. 3.
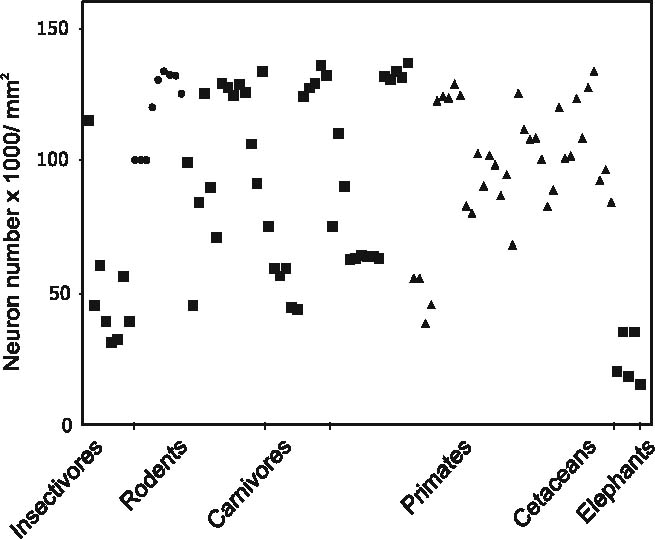
Neuron number in the gray matter of cortex underlying one square millimeter across species and areas. Shown are all data known to us (across authors, areas and species). Triangles are data from the human, circles are from the mouse. For comparison: the total number of cortical neurons in the mouse is approximately 16 million neurons (Braitenberg and Schüz 1991), whereas the total number of cortical neurons in the human is around 21 billion (Pakkenberg and Gundersen 1997): a 1,000-fold difference. Data from Beaulieu (1993), Beaulieu and Colonnier (1985), Beaulieu and Colonnier (1989), Braitenberg and Schüz (1991), Finlay and Slattery (1983), Haug (1987), Hendry et al. (1987), Rockel et al. (1980), Shankle et al. (1998), Skoglund et al. (1996), Stolzenburg et al. (1989)
4 Discussion
Simplified and unrealistic as these derivations may be, they do produce some predictions that come strikingly close to empirical values. We predicted (albeit in hindsight) that mammalian brains would have a constant number of neurons underneath a unit of pial surface and across a large range of sizes, and we predicted a certain relationship between brain size and glia-to-neuron ratio. These characteristics arose when scaling-up modular, cortical brains. For both predictions there is considerable evidence. A bold conclusion from our theoretical exercise might be that the mammalian neocortex was able to expand greatly in evolution because it was modular and had a cortical structure. Patchy modularity and other limitations on connectivity often tend to improve processing efficiency, provided they do not constrain the brain to local processing only. Mammalian neocortex could thus at the same time enlarge its size and its processing efficiency (Striedter 2005).
There is mounting consensus that as smaller brains evolve into larger ones, new structures may emerge as duplications of existing structures, without essential changes in tissue (Kaas 1982, 2005; Rakic 1995). Our analyses show that such newly emerged structures can easily be accommodated on the surface of the bigger brain. Alternatively, new structures may have emerged as differentiated parts of old structures. For example, a layered brain part may have evolved from a brain without layers (Kaas 2005). Primate primary visual cortex may reflect such a rule, with the stria gennarii representing a layer of white matter within the gray matter of the area (e.g., Mitchison 1992). Indeed, this area has twice the number of neurons underneath a squared millimeter of surface than the rest of the cortex (Rockel et al. 1980).
Many reasons for the remarkable constancy underlying one unit of cortical surface may be adduced. For example, one might posit a ‘window constraint’, arguing that each neuron’s connecting structures need access to the white matter and that the window between white and gray matter has room for only a limited number of connecting ‘wires’. New neurons piled above such a window will be starved for external connections and can therefore have only local processing functions. The need for such neurons will be limited, which is why there would be a theoretical upper-limit on the number of neurons underneath a unit volume of pial surface (Murre and Sturdy 1995). Though such explanations have their merit, the constancy also emerges from scaling-up modular, cortical brain structures. Some words of caution seem merited here: firstly, rather than a causal relationship between connectivity volume and the number of neurons underlying one squared millimeter of cortical surface, our model shows that the surface of an enlarged sphere is large enough to accommodate the newly added neurons, without essential changes in the structure of neural tissue. This is consistent with the idea that new structures emerge as duplications of existing structures, but does not rule out alternative explanations (e.g., Murre and Sturdy 1995). For example, it is conceivable that several processes took place during evolution: optimization of the number of wires to the white matter (the original Murre and Sturdy (1995) explanation), together with a duplication of existing (optimal) structures (as suggested here). Secondly, biology dictates that gray matter surface area scales as the 8/9 power of brain volume in larger (convoluted) brains (Hofman 1985, 1989; Prothero 1997), which is, according to our model, larger than necessary to accommodate the newly added neurons. It would be interesting to see whether different restrictions on connectivity and/or tissue or functional constraints could resolve this discrepancy for convoluted brains.
Many explanations for a higher glia-to-neuron index in larger brains have been proposed. Friede (1954), for instance, related the index to qualitative parameters on the phylogenetic stage of animals, while Hawkins and Olszewski (1957) concluded that not phylogenetic stage but size of the brain is the main determinant of the glial index. The glia-to-neuron ratio has also been correlated with the length of axons, as longer axons would need more glia for their support (Friede 1963; Friede and Van Houten 1962). Our analyses corroborate the latter conclusion. Because glia add a volume proportionate to the length of axons (see Sect. 2.1), the higher glia-to-neuron ratio in larger brains is mainly due to the fact that axons are longer in larger brains.
Previous studies have related the brain’s design to a minimization of conduction delays (Wen and Chklovskii 2005), provided scaling laws (Changizi and Shimojo 2005; Karbowski 2003) and related these to connectivity (Changizi 2001; Braitenberg 2001), or have shown that modular connectivity (Mitchison 1991; Murre and Sturdy 1995) and a segregation in white and gray matter (Murre and Sturdy 1995, Ruppin et al. 1993) results in less wiring. Here we have extended these studies by taking into account glia volume and by showing that expanding modular, cortical structures results in a larger glia-to-neuron ratio in larger brains, as well as a constant neuron number underlying 1 mm2 of cortical surface across brain size. For both predictions exists considerable evidence (e.g., Braitenberg and Schüz 1991; Friede 1954; Rockel et al. 1980; Tower and Young 1973).
We conclude that modular, cortical brains form a scalable architecture (Clark et al. 2001). A larger glia-to-neuron index in larger brains (e.g., Friede 1954) and a constant neuron number underlying 1 mm2 of pial surface (e.g. Rockel et al. 1980) simply arise when scaling-up the mammalian brain.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Technically, interneuron distance x reflects the projected interneuron distance on the white matter surface, rather than the true interneuron distance on the cortical sheet. This becomes apparent when we derive an explicit expression for x in Eq. (4), where white matter surface (rather than gray matter surface) scales as the 2/3 power of connectivity volume. For non-convoluted brains, the projected interneuron distance equals the true interneuron distance on the cortical sheet. Note that, had we instead used distance on a flattened cortical surface together with the empirical scaling law surface∼volume8/9 for convoluted brains (Hofman 1985, 1989; Prothero 1997), we would have arrived at an incorrect expression for the volume of the convoluted brain, namely one that consisted of a flattened cortex with loosely packed and/or too long connections running underneath. While Eq. (4) is valid for the derivation of white matter volume in convoluted brains, it results in an underestimation of gray matter volume, for which a derivation using the true interneuron distance on the cortical sheet would have been more correct. However, we expect the effect to be small because local connections (which are arguably dominant in gray matter, see Sect. 3) have a negligible effect on total volume.
Area 17 has a highly laminar structure in primates, with the stria gennarii representing a layer of white matter within the gray matter of the area (e.g., Mitchison 1992). The make-up of this area appears to have followed a different evolutionary principle (see Discussion), which is why we have excluded this area from our analyses.
Contributor Information
Janneke F. M. Jehee, Phone: +1-615-3222835, FAX: +1-615-3438449, Email: janneke.jehee@vanderbilt.edu
Jaap M. J. Murre, Email: jaap@murre.com
References
- Arbib MA. The metaphorical brain: an introduction to cybernetics as artificial intelligence and brain theory. New York: Wiley; 1972. [Google Scholar]
- Beaulieu C. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain Res. 1993;609:284–292. doi: 10.1016/0006-8993(93)90884-P. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Colonnier M. A comparison of the number of neurons in individual laminae of cortical areas 17, 18 and posteromedial suprasylvian (PMLS) area in the cat. Brain Res. 1985;339:166–170. doi: 10.1016/0006-8993(85)90639-0. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Colonnier M. Number of neurons in individual laminae of areas 3B, 4γ, and 6aα of the cat cerebral cortex: a comparison with major visual areas. J Comparat Neurol. 1989;279:228–234. doi: 10.1002/cne.902790206. [DOI] [PubMed] [Google Scholar]
- Blinkov SM, Glezer II. The human brain in figures and tables: a quantitative handbook. New York: Plenum Press; 1968. [Google Scholar]
- Bok ST. Histonomy of the cerebral cortex. Amsterdam: Elsevier; 1959. [Google Scholar]
- Braitenberg V. Brain size and number of neurons: an exercise in synthetic neuroanatomy. J Comput Neurosci. 2001;10:71–77. doi: 10.1023/A:1008920127052. [DOI] [PubMed] [Google Scholar]
- Braitenberg V, Schüz A. Anatomy of the cortex: statistics and geometry. New York: Springer; 1991. [Google Scholar]
- Carpenter RHS. Neurophysiology. London: Edward Arnold; 1984. [Google Scholar]
- Changizi MA. Principles underlying mammalian neocortical scaling. Biol Cybern. 2001;84:207–215. doi: 10.1007/s004220000205. [DOI] [PubMed] [Google Scholar]
- Changizi MA, Shimojo S. Parcellation and area-area connectivity as a function of neocortex size. Brain, Behav Evolut. 2005;66:88–98. doi: 10.1159/000085942. [DOI] [PubMed] [Google Scholar]
- Cherniak C. The bounded brain: toward quantitative neuroanatomy. J Cogni Neurosci. 1990;2:58–68. doi: 10.1162/jocn.1990.2.1.58. [DOI] [PubMed] [Google Scholar]
- Cherniak C. Component placement optimization in the brain. J Neurosci. 1994;14:2418–2427. doi: 10.1523/JNEUROSCI.14-04-02418.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniak C. Neural component placement. Trends Neurosci. 1995;18:522–527. doi: 10.1016/0166-2236(95)98373-7. [DOI] [PubMed] [Google Scholar]
- Cherniak C, Changizi M, Kang DW. Large-scale optimization of neuron arbors. Phys Rev. 1999;59:6001–6009. doi: 10.1103/physreve.59.6001. [DOI] [PubMed] [Google Scholar]
- Clark DA, Mitra PP, Wang SS-H. Scalable architecture in mammalian brains. Nature. 2001;411:189–193. doi: 10.1038/35075564. [DOI] [PubMed] [Google Scholar]
- Deco G, Rolls ET. Object-based visual neglect: a computational hypothesis. Euro J Neurosci. 2002;16:1994–2000. doi: 10.1046/j.1460-9568.2002.02279.x. [DOI] [PubMed] [Google Scholar]
- Friede RL. Der quantitative Anteil der Glia an der Cortexentwicklung. Acta Anatomica. 1954;20:290–296. [PubMed] [Google Scholar]
- Friede RL. The relationship of body size, nerve cell size, axon length, and glial density in the cerebellum. Proc Natl Acad Sci USA. 1963;49:187–193. doi: 10.1073/pnas.49.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede RL, Van Houten WH. Neuronal extension and glial supply: Functional significance of glia. Proc Natl Acad Sci USA. 1962;48:817–821. doi: 10.1073/pnas.48.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel LM, Murre JMJ. The design and evolution of modular neural network architectures. Neural Netw. 1994;7:985–1004. doi: 10.1016/S0893-6080(05)80155-8. [DOI] [Google Scholar]
- Haug H. Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: a stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant) Am J Anat. 1987;180:126–142. doi: 10.1002/aja.1001800203. [DOI] [PubMed] [Google Scholar]
- Hawkins A, Olszewski J. Glia/ nerve cell index for cortex of the whale. Science. 1957;126:76–77. doi: 10.1126/science.126.3263.76. [DOI] [PubMed] [Google Scholar]
- Hendry SHC, Schwark HD, Jones EG, Yan J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 1987;7:1503–1519. doi: 10.1523/JNEUROSCI.07-05-01503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman MA. Size and shape of the cerebral cortex in mammals I. The cortical surface. Brain Behav Evolut. 1985;27:28–40. doi: 10.1159/000118718. [DOI] [PubMed] [Google Scholar]
- Hofman MA. On the evolution and geometry of the brain in mammals. Progress Neurobiol. 1989;32:137–158. doi: 10.1016/0301-0082(89)90013-0. [DOI] [PubMed] [Google Scholar]
- Kaas JH. The segregation of function in the nervous system: Why do sensory systems have so many subdivisions? In: Neff WD, editor. Contributions to sensory physiology, vol 7. New York: Academic Press; 1982. pp. 201–240. [Google Scholar]
- Kaas JH. Understanding brain evolution. Nature Neurosci. 2005;8:539. doi: 10.1038/nn0505-539. [DOI] [Google Scholar]
- Karbowski J. How does connectivity between cortical areas depend on brain size? Implications for efficient computation. J Comput Neurosci. 2003;15:347–356. doi: 10.1023/A:1027467911225. [DOI] [PubMed] [Google Scholar]
- Li Z. Contextual influences in V1 as a basis for pop out and asymmetry in visual search. Proc Natl Acad Sci USA. 1999;96:10539–10535. doi: 10.1073/pnas.96.18.10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew TM, Mwamengele GLM, Dantzer V. Comparative morphmetry of the mammalian brain: estimates of cerebral volumes and cortical surface areas obtained from macroscopic slices. J Anat. 1990;172:191–200. [PMC free article] [PubMed] [Google Scholar]
- Mitchison G. Neuronal branching patterns and the economy of cortical wiring. Proc Roy Sci Lond. 1991;245:151–158. doi: 10.1098/rspb.1991.0102. [DOI] [PubMed] [Google Scholar]
- Mitchison G. Axonal trees and cortical architecture. Trends Neurosci. 1992;14:122–126. doi: 10.1016/0166-2236(92)90352-9. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. An organizing principle for cerebral function: The unit module and the distributed system. In: Edelman GM, Mountcastle VB, editors. The mindful brain. Cambridge: MIT Press; 1978. [Google Scholar]
- Murre JMJ. Categorization and learning in modular neural networks. Hillsdale: Lawrence Erlbaum; 1992. [Google Scholar]
- Murre JMJ, Sturdy DPF. The connectivity of the brain: multi-level quantitative analysis. Biol Cybern. 1995;73:529–545. doi: 10.1007/BF00199545. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Ten Donkelaar HJ, Nicholson C. The central nervous system of vertebrates. Heidelberg: Springer; 1998. [Google Scholar]
- Pakkenberg B, Gundersen HJG. Neocortical neuron number in humans: effect of sex and age. J Comparat Neurol. 1997;384:312–320. doi: 10.1002/(SICI)1096-9861(19970728)384:2<312::AID-CNE10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Palm G. Neural assemblies: an alternative approach to artificial intelligence. Berlin: Springer; 1982. [Google Scholar]
- Prothero J. Cortical scaling in mammals: a repeating units model. J Brain Res. 1997;38:195–207. [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: A hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;9:383–388. doi: 10.1016/0166-2236(95)93934-P. [DOI] [PubMed] [Google Scholar]
- Rockel AJ, Hiorns RW, Powell TPS. The basic uniformity in structure of the neocortex. Brain. 1980;103:221–244. doi: 10.1093/brain/103.2.221. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Lamme VAF, Spekreijse H, Bosch H. Figure-ground segregation in a recurrent network architecture. J Cogn Neurosci. 2002;14:525–537. doi: 10.1162/08989290260045756. [DOI] [PubMed] [Google Scholar]
- Ruppin E, Scwartz EL, Yeshurun Y. Examining the volume-efficiency of the cortical architecture in a multi-processor network. Biol Cybern. 1993;70:89–94. doi: 10.1007/BF00202570. [DOI] [PubMed] [Google Scholar]
- Shankle WR, Landing BH, Rafii MS, Schiano A, Chen JM, Hara J. Evidence for a postnatal doubling of neuron number in the developing human cerebral cortex between 15 months and 6 years. J Theor Biol. 1998;191:115–140. doi: 10.1006/jtbi.1997.0570. [DOI] [PubMed] [Google Scholar]
- Skoglund TS, Pascher R, Berthold C-H. Heterogeneity in the columnar number of neurons in different neocortical areas in the rat. Neurosci Lett. 1996;208:97–100. doi: 10.1016/0304-3940(96)12569-6. [DOI] [PubMed] [Google Scholar]
- Stolzenburg J-U, Reichenbach A, Neumann M. Size and density of glial and neuronal cells within the cerebral neocortex of various insectivorian species. Glia. 1989;2:78–84. doi: 10.1002/glia.440020203. [DOI] [PubMed] [Google Scholar]
- Striedter GF. Principles of brain evolution. Sunderland: Sinauer Associates; 2005. [Google Scholar]
- Szentágothai J. The ‘module-concept’ in cerebral cortex architecture. Brain Res. 1975;95:475–496. doi: 10.1016/0006-8993(75)90122-5. [DOI] [PubMed] [Google Scholar]
- Tower DB. Distribution of cerebral fluids and electrolytes in vivo and in vitro. In: Klatzo I, Seitelberger F, editors. Brain edema. New York: Springer; 1967. pp. 303–332. [Google Scholar]
- Tower DB, Young OM. The activities of butyrylcholinesterase and carbonic anhydrase, the rate of anaerobic glycolysis, and the question of a constant density of glial cells in cerebral cortices of various mammalian species from mouse to whale. J Neurochem. 1973;20:269–278. doi: 10.1111/j.1471-4159.1973.tb12126.x. [DOI] [PubMed] [Google Scholar]
- Wen Q, Chklovskii DB. Segregation of the brain into gray and white matter: a design minimizing conduction delays. PLoS Comput Biol. 2005;1:617–630. doi: 10.1371/journal.pcbi.0010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MP. Objective analysis of the topological organization of the primate cortical visual system. Nature. 1992;358:152–155. doi: 10.1038/358152a0. [DOI] [PubMed] [Google Scholar]


