Abstract
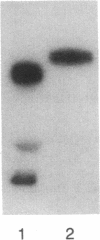
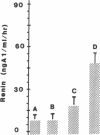
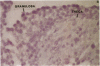
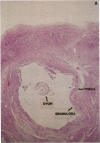
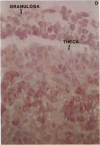
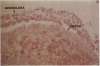
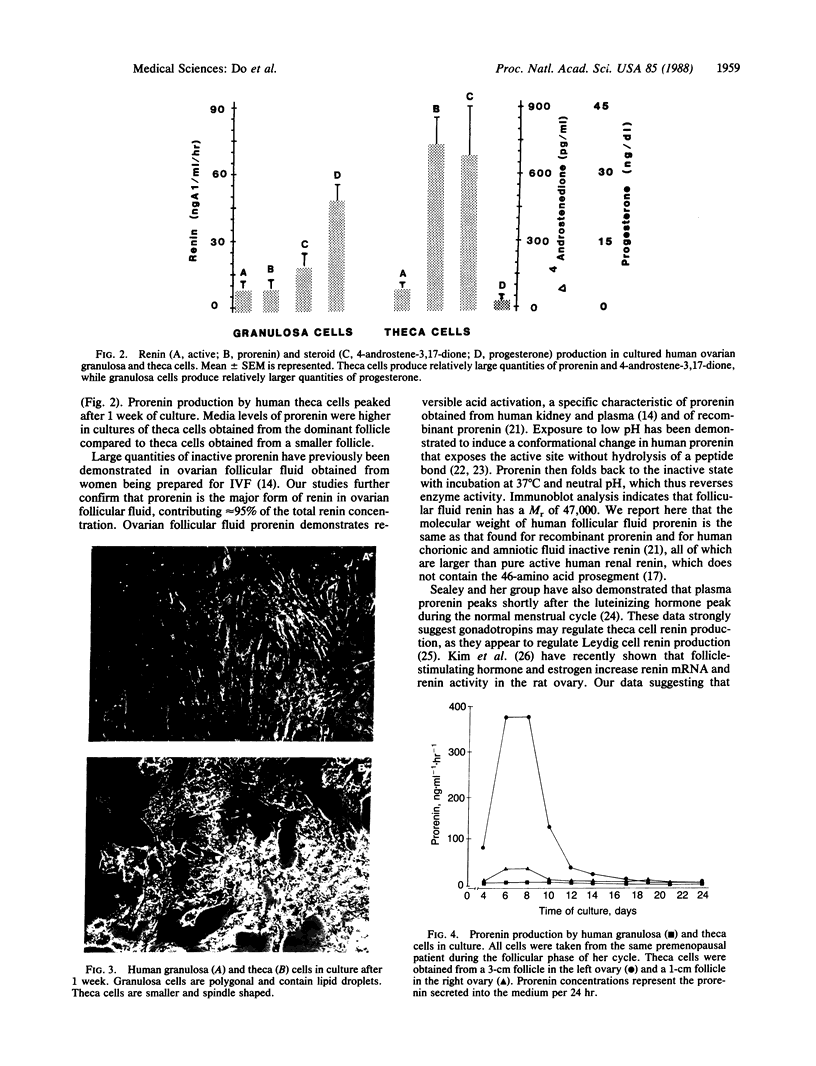
Human ovarian follicular fluid contains renin-like activity. In normal women, circulating levels of prorenin, the biosynthetic precursor of renin (EC 3.4.23.15), change in parallel with changes in progesterone during the menstrual cycle. Therefore, the ovary has been implicated as a source of plasma prorenin. In the present studies, we report the finding of high concentrations of prorenin in human ovarian follicular fluid (3000 ng.ml-1.hr-1 vs. 10-40 ng.ml-1.hr-1 in normal human plasma) obtained from follicles of women prepared for in vitro fertilization. The inactive renin-like enzyme was identified as prorenin by its activation characteristics, its molecular weight of 47,000, which is the same as that for recombinant prorenin, and its cross-reactivity with human renal renin antibodies. Culture of isolated human theca cells and isolated granulosa cells indicated that prorenin is secreted by theca cells but not by granulosa cells. Prorenin production by theca cells peaked during the first 10 days of culture and gradually decreased by 17 days. Active renin levels were 10% or less of the prorenin levels. Prorenin was barely detectable in medium from granulosa cells cultured for 24 days. Immunohistochemical staining of human ovaries (n = 5) with anti-human renin antibody demonstrated the presence of renin primarily in theca cells. These studies suggest that the theca cell is the source of the large quantities of prorenin in human ovarian follicular fluid.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker G. M., Galen F. X., Devaux C., Foote S., Papernik E., Pesty A., Menard J., Corvol P. Human chorionic cells in primary culture: a model for renin biosynthesis. J Clin Endocrinol Metab. 1982 Nov;55(5):902–909. doi: 10.1210/jcem-55-5-902. [DOI] [PubMed] [Google Scholar]
- Aguilera G., Hyde C. L., Catt K. J. Angiotensin II receptors and prolactin release in pituitary lactotrophs. Endocrinology. 1982 Oct;111(4):1045–1050. doi: 10.1210/endo-111-4-1045. [DOI] [PubMed] [Google Scholar]
- Culler M. D., Tarlatzis B. C., Lightman A., Fernandez L. A., Decherney A. H., Negro-Vilar A., Naftolin F. Angiotensin II-like immunoreactivity in human ovarian follicular fluid. J Clin Endocrinol Metab. 1986 Mar;62(3):613–615. doi: 10.1210/jcem-62-3-613. [DOI] [PubMed] [Google Scholar]
- Derkx F. H., Schalekamp M. P., Schalekamp M. A. Two-step prorenin-renin conversion. Isolation of an intermediary form of activated prorenin. J Biol Chem. 1987 Feb 25;262(6):2472–2477. [PubMed] [Google Scholar]
- Deschepper C. F., Mellon S. H., Cumin F., Baxter J. D., Ganong W. F. Analysis by immunocytochemistry and in situ hybridization of renin and its mRNA in kidney, testis, adrenal, and pituitary of the rat. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7552–7556. doi: 10.1073/pnas.83.19.7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Y. S., Shinagawa T., Tam H., Inagami T., Hsueh W. A. Characterization of pure human renal renin. Evidence for a subunit structure. J Biol Chem. 1987 Jan 25;262(3):1037–1043. [PubMed] [Google Scholar]
- Fernandez L. A., Tarlatzis B. C., Rzasa P. J., Caride V. J., Laufer N., Negro-Vilar A. F., DeCherney A. H., Naftolin F. Renin-like activity in ovarian follicular fluid. Fertil Steril. 1985 Aug;44(2):219–223. doi: 10.1016/s0015-0282(16)48740-6. [DOI] [PubMed] [Google Scholar]
- Fernandez L. A., Twickler J., Mead A. Neovascularization produced by angiotensin II. J Lab Clin Med. 1985 Feb;105(2):141–145. [PubMed] [Google Scholar]
- Furman A., Rotmensch S., Dor J., Venter A., Mashiach S., Vlodavsky I., Amsterdam A. Culture of human granulosa cells from an in vitro fertilization program: effects of extracellular matrix on morphology and cyclic adenosine 3',5' monophosphate production. Fertil Steril. 1986 Sep;46(3):514–517. doi: 10.1016/s0015-0282(16)49596-8. [DOI] [PubMed] [Google Scholar]
- GROSS F., SCHAECHTELIN G., ZIEGLER M., BERGER M. A RENIN-LIKE SUBSTANCE IN THE PLACENTA AND UTERUS OF THE RABBIT. Lancet. 1964 Apr 25;1(7339):914–916. doi: 10.1016/s0140-6736(64)91637-x. [DOI] [PubMed] [Google Scholar]
- Glorioso N., Atlas S. A., Laragh J. H., Jewelewicz R., Sealey J. E. Prorenin in high concentrations in human ovarian follicular fluid. Science. 1986 Sep 26;233(4771):1422–1424. doi: 10.1126/science.3529392. [DOI] [PubMed] [Google Scholar]
- Hsueh W. A., Carlson E. J., Dzau V. J. Characterization of inactive renin from human kidney and plasma. Evidence of a renal source of circulating inactive renin. J Clin Invest. 1983 Mar;71(3):506–517. doi: 10.1172/JCI110795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain A., Bumpus F. M., De Silva P., Speth R. C. Localization of angiotensin II receptors in ovarian follicles and the identification of angiotensin II in rat ovaries. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2489–2493. doi: 10.1073/pnas.84.8.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb D. K., Dorrington J. H. Human granulosa and thecal cells secrete distinct protein profiles. Fertil Steril. 1987 Aug;48(2):243–248. doi: 10.1016/s0015-0282(16)59350-9. [DOI] [PubMed] [Google Scholar]
- Lobo R. A., diZerega G. S., Marrs R. P. Follicular fluid steroid levels in dysmature and mature follicles from spontaneous and hyperstimulated cycles in normal and anovulatory women. J Clin Endocrinol Metab. 1985 Jan;60(1):81–87. doi: 10.1210/jcem-60-1-81. [DOI] [PubMed] [Google Scholar]
- Naruse K., Murakoshi M., Osamura R. Y., Naruse M., Toma H., Watanabe K., Demura H., Inagami T., Shizume K. Immunohistological evidence for renin in human endocrine tissues. J Clin Endocrinol Metab. 1985 Jul;61(1):172–177. doi: 10.1210/jcem-61-1-172. [DOI] [PubMed] [Google Scholar]
- Ohkubo H., Nakayama K., Tanaka T., Nakanishi S. Tissue distribution of rat angiotensinogen mRNA and structural analysis of its heterogeneity. J Biol Chem. 1986 Jan 5;261(1):319–323. [PubMed] [Google Scholar]
- Pandey K. N., Inagami T. Regulation of renin angiotensins by gonadotropic hormones in cultured murine Leydig tumor cells. Release of angiotensin but not renin. J Biol Chem. 1986 Mar 25;261(9):3934–3938. [PubMed] [Google Scholar]
- Pandey K. N., Maki M., Inagami T. Detection of renin mRNA in mouse testis by hybridization with renin cDNA probe. Biochem Biophys Res Commun. 1984 Dec 14;125(2):662–667. doi: 10.1016/0006-291x(84)90590-4. [DOI] [PubMed] [Google Scholar]
- Pandey K. N., Misono K. S., Inagami T. Evidence for intracellular formation of angiotensins: coexistence of renin and angiotensin-converting enzyme in Leydig cells of rat testis. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1337–1343. doi: 10.1016/0006-291x(84)91238-5. [DOI] [PubMed] [Google Scholar]
- Pucell A. G., Bumpus F. M., Husain A. Rat ovarian angiotensin II receptors. Characterization and coupling to estrogen secretion. J Biol Chem. 1987 May 25;262(15):7076–7080. [PubMed] [Google Scholar]
- Re R. N. Cellular biology of the renin-angiotensin systems. Arch Intern Med. 1984 Oct;144(10):2037–2041. [PubMed] [Google Scholar]
- Sealey J. E., Atlas S. A., Glorioso N., Manapat H., Laragh J. H. Cyclical secretion of prorenin during the menstrual cycle: synchronization with luteinizing hormone and progesterone. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8705–8709. doi: 10.1073/pnas.82.24.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealey J. E., Atlas S. A., Laragh J. H. Prorenin and other large molecular weight forms of renin. Endocr Rev. 1980 Fall;1(4):365–391. doi: 10.1210/edrv-1-4-365. [DOI] [PubMed] [Google Scholar]
- Yu R., Anderton J., Skinner S. L., Best J. B. Renin in anephric man. Case report with physiologic studies. Am J Med. 1972 May;52(5):707–711. doi: 10.1016/0002-9343(72)90061-7. [DOI] [PubMed] [Google Scholar]