Abstract
In humans and mice, mutations in the Ret gene result in Hirschsprung's disease and renal defects. In the embryonic kidney, binding of Ret to its ligand, Gdnf, induces a program of epithelial cell remodeling that controls primary branch formation and branching morphogenesis within the kidney. Our previous studies showed that transcription factors belonging to the retinoic acid (RA) receptor family are crucial for controlling Ret expression in the ureteric bud; however, the mechanism by which retinoid-signaling acts has remained unclear. In the current study, we show that expression of a dominant-negative RA receptor in mouse ureteric bud cells abolishes Ret expression and Ret-dependent functions including ureteric bud formation and branching morphogenesis, indicating that RA-receptor signaling in ureteric bud cells is crucial for renal development. Conversely, we find that RA-receptor signaling in ureteric bud cells depends mainly on RA generated in nearby stromal cells by retinaldehyde dehydrogenase 2, an enzyme required for most fetal RA synthesis. Together, these studies suggest that renal development depends on paracrine RA signaling between stromal mesenchyme and ureteric bud cells that regulates Ret expression both during ureteric bud formation and within the developing collecting duct system.
Keywords: Ret expression, Kidney development, Retinoic acid, Mouse
INTRODUCTION
Kidney development depends on reciprocal signaling between metanephric mesenchyme that differentiates into the nephron, the ureteric bud epithelium that forms the collecting duct system, and stromal mesenchyme that differentiates into the renal interstitium. As the fetal kidney grows, nephron induction and branching morphogenesis occur continuously in the nephrogenic zone in the outer cortex, while further differentiation occurs in deeper regions of the kidney. In the nephrogenic zone, ureteric bud tips generate signals important for survival and maintenance of nephron progenitors while, at the same time, signals from metanephric mesenchyme, which trans-differentiates into the epithelial components of the nephron, induce ureteric bud tips to grow and branch (Grobstein, 1953). Surrounding ureteric bud branches and nephron progenitors, cortical stromal cells generate signals required for nephron differentiation and branching morphogenesis (Hatini et al., 1996; Levinson et al., 2005).
Retinoic acid (RA), the active form of vitamin A, is crucial for formation of most organs and tissues, including the kidney. In rodents, maternal vitamin A deficiency results in renal hypoplasia, the severity of which depends on the extent of vitamin A deprivation (Lelievre-Pegorier et al., 1998; Quadro et al., 2005; Wilson, 1953). RA is synthesized from retinol, an inactive precursor, by enzymes belonging to the retinol dehydrogenase and retinaldehyde dehydrogenase (Aldh1a, hereafter referred to as Raldh) families. Retinol dehydrogenases mediate the first step in RA synthesis that converts retinol to retinaldehyde and, with the exception of Rdh10, are widely expressed (Duester et al., 2003). However, enzymes belonging to the Raldh family that convert retinaldehyde to retinoic acid are selectively expressed in the embryo at many sites of active RA signaling, where they are thought to be crucial for regulating the availability of RA (Duester, 2008; Duester et al., 2003; Lin et al., 2003; Marlier and Gilbert, 2004; Niederreither and Dolle, 2008). Gene targeting and studies with RA reporter mice suggest that Raldh2 accounts for most RA synthesis in the embryo (Mic et al., 2002; Niederreither et al., 1999; Zhao et al., 2009). In the embryonic kidney, Raldh2 is localized in stromal mesenchyme of the outer cortex and Raldh3 is expressed in the ureteric bud (Batourina et al., 2001; Mic et al., 2002; Niederreither et al., 2002). It is unknown, however, whether one or both of these enzymes are important for kidney organogenesis.
Once RA becomes available in cells, it binds to and activates retinoic acid receptors (Rars), a family of transcription factors that control gene expression via retinoid response elements in regulatory sequences of target genes. The RA receptor family includes 8 members encoded by 3 genes, Rara, Rarb and Rarg, that are widely expressed during development (Dolle et al., 1990; Leid et al., 1992; Ruberte et al., 1990). Inactivation of individual Rars results in few embryonic abnormalities (Lohnes et al., 1993; Lufkin et al., 1993; Luo et al., 1995; Mendelsohn et al., 1994c). However, compound mutants lacking multiple Rar family members display a set of malformations that mirror those induced by maternal vitamin A deficiency (Ghyselinck et al., 1998; Kastner et al., 1995; Lohnes et al., 1994; Luo et al., 1996; Mendelsohn et al., 1994b). Hence, RA signaling in the embryo is either functionally redundant, requiring a sufficient level of RA receptor expression in a given cell type, or depends on the concerted activity of more than one receptor species.
Our previous studies of Rara–/–;Rarb2–/– mutants revealed abnormalities in many tissues, including the kidney. At birth, the nephrogenic zone, which in wild-type animals contains nephron progenitors, stromal cells and ureteric bud tips, was absent. (Mendelsohn et al., 1999; Mendelsohn et al., 1994b). Ret, a gene required for formation of the ureteric bud and its branching in the kidney (Schuchardt et al., 1996), was downregulated in mutant kidneys and branching morphogenesis was impaired (Mendelsohn et al., 1999). The observation that all of these phenotypes could be rescued by forced expression of Ret in ureteric bud cells of Rara–/–;Rarb2–/– mutants suggests that a major function of RA during renal development is in regulating Ret, while Ret signaling acts downstream of RA, regulating branching and stromal cell patterning (Batourina et al., 2001). The observation that renal hypoplasia and abnormal stromal cell patterning were also observed in mutants lacking Ret9, one of two Ret isoforms (de Graaff et al., 2001), supports the conclusion that branching and stromal cell abnormalities are linked to impaired Ret signaling in the ureteric bud.
Despite what we have learned, a number of questions remain. For example, it is unclear whether branching morphogenesis and Ret expression depend on RA generated by Raldh3 in ureteric bud cells, by Raldh2 in stromal cells or both, and it is unclear whether RA receptor signaling regulates Ret via an autocrine pathway in ureteric bud cells or by a paracrine mechanism via mesenchyme. The observation previously, that inactivation of Rara and Rarb2 together, but not separately, resulted in renal abnormalities, led us to suggest that their concerted function was required in a given renal cell type for regulating branching morphogenesis and Ret expression. In situ hybridization studies revealed that Rara and Rarb2 were colocalized in stromal cells, but not in other cell types, suggesting that stromal cells were the prime mediators of RA signaling (Mendelsohn et al., 1999; Dolle et al., 1990; Visel et al., 2004). Based on these findings, we proposed that RA receptor signaling in stromal cells was important for generating secreted signals that control Ret expression and branching in ureteric bud cells (Mendelsohn et al., 1999). More recently however, microarray studies reveal that Rara and Rarb2 are also colocalized in the ureteric bud (Schmidt-Ott et al., 2005) (GUDMAP, http://www.gudmap.org/). Hence, Rara and Rarb2 regulation of Ret could occur either via ureteric bud cells or stromal cells.
To begin to address these questions in the current study, we analyzed mutants lacking Raldh2 and Raldh3 to test the requirement for RA-synthesis in the stroma and in ureteric bud cells, respectively. We find that inactivation of Raldh3 has little, if any, effect on kidney development, whereas inactivation of Raldh2 results in phenotypes that are similar to, but slightly less severe than, those in Rara–/–;Rarb2–/– mutants. Deletion of both Raldh2 and Raldh3 together increased the severity of malformations, suggesting that Raldh2 is the major source of RA required for kidney development, but that, in its absence, Raldh3 can generate sufficient levels of RA to partially rescue renal morphogenesis.
The observation here that RA can induce branching and Ret expression in ureteric buds cultured without mesenchyme, suggests that RA, but not other stromal cell signals, is normally important for regulating branching morphogenesis. We find that in the absence of either Gdnf or RA, Ret expression was undetectable and branching was inhibited. Hence, RA and Gdnf are likely to be required independently: RA for inducing expression of Ret in ureteric bud cells and Gdnf for activating Ret RTK (receptor tyrosine kinase) signaling that controls ureteric bud remodeling. Finally, using a dominant-negative mouse model, we show that blocking RA receptor signaling in ureteric bud cells results in renal hypoplasia and renal agenesis, supporting the idea that RA receptors are required in ureteric bud cells rather than in stroma. Together, these studies reveal a novel stromal-ureteric bud signaling pathway that is required for formation of the renal collecting duct system and in which RA secreted from cortical stroma induces the expression of Ret in ureteric bud cells by activating RA receptor signaling. This pathway is likely to be conserved and utilized at other sites in the developing embryo.
MATERIALS AND METHODS
Mouse strains and genotyping
All matings were with Swiss-Webster mice (Taconic). Embryonic day 0.5 (E0.5) was considered to be noon on the day when a vaginal plug was observed. Littermates were used for all experiments in which normal and mutant embryos were compared. The Hoxb7-Cre line was generated in the McMahon laboratory (Yu et al., 2002). RARE-hsp68-lacZ mice were a generous gift from the Rossant laboratory (Rossant et al., 1991). Genotyping was done by PCR of the tail or yolk sac. PCR genotyping of the HoxB7-Cre mice was performed using primers 5′-TGATGAGGTTCGCAAGAACC-3′ and 5′-CCATGAGTGAACGAACCTGG-3′, generating a 400 bp product. RARE-hsp68-lacZ mice were genotyped using primers 5′-CGTCGTCCCCTCAAACTGGCAGATGC-3′ and 5′-TTCGGCGCTCCACAGTTTCGGGTTTTC-3′ generating a 570 bp product. RARaDN mice were genotyped using primers 5′-ATGGTGTACACGTGTCACC-3′ and 5′-CACCTTCTCAATGAGCTCC-3′. For the wild-type allele we used the primers 5′-TGGCTCGTGTCAAAGAACTG-3′ and 5′-TGGTCGGTAGAAAGGCAGAG-3′. The mutant and wild-type bands were 210 bp and 426 bp, respectively. All PCR protocols were performed using a DNA Thermal Cycler PTC-100 (BIO-RAD, Hercules, CA, USA) with 40 cycles of 94°C for 30 seconds, 53.5°C for 30 seconds and 72°C for 40 seconds, except for the RARaDN mutant, where we performed 45 cycles of 94°C for 30 seconds, 54.5°C for 30 seconds and 72°C for 40 seconds.
RA rescue
For the generation of Raldh2–/– and Raldh2–/–;Raldh3–/– embryos, retinoic acid (RA) supplementation was performed as previously described (Batourina et al., 2005). The experimental design used in these studies was approved by the institutional animal care and use committee at Columbia University.
Immunochemistry, histology and non-radioactive in situ hybridization
For histology and in situ analysis, embryos were dissected into ice cold PBS and then transferred to 4% paraformaldehyde (PFA) and fixed overnight at 4°C. Following fixation, embryos were transferred to 30% sucrose overnight at 4°C and then embedded in OCT. Sagittal cryosections (14 mm) were cut, dried and processed directly or stored at –80°C. Histology was performed using Hematoxylin and Eosin staining. Non-radioactive in situ hybridizations and lacZ histochemistry were performed as previously described (Mendelsohn et al., 1999).
Quantitation of branching morphogenesis
Hoxb7-GFP;Raldh2–/– and control kidneys were cultured for 3 days to allow explants to flatten, then were fixed in 4% PFA. Images were generated from each sample at low magnification and ureteric bud tips were counted by dividing each sample into four parts. The Student's t-test was used for statistical analysis.
Organ culture
Whole kidneys were cultured under conditions previously described (Mendelsohn et al., 1999). To isolate ureteric buds, kidneys from E11 embryos were placed in DMEM supplemented with collagenase and incubated at 37°C for 15 minutes to dissociate the metanephric mesenchyme from the ureteric bud then transferred to medium supplemented with DNAse and mechanically separated from the surrounding mesenchyme with fine needles. Isolated ureteric buds were then transferred to growth-reduced Matrigel (BD)-coated Transwell (Costar) filters and embedded in growth-reduced Matrigel. Cultures were in DMEM with or without 125 ng/ml Gdnf and 200 nM all-trans-RA and 9-cis RA.
RT-PCR
Total RNA was isolated from isolated E11 ureteric buds cultured for 32 hours under the conditions described above. RNA was extracted with Trizol reagent (Invitrogen) according to manufacturer's protocol. The quality and concentration of isolated RNA was assessed using an RNA600 Pico Assay Kit (Agilent Technologies). Each RNA dilution (10 ng to 1 mg) was used as a template for first-strand cDNA synthesis using oligo (dT) as a primer, in the presence of Superscript III reverse transcriptase (Invitrogen). One microliter of cDNA was used as a template for the PCR reaction using Platinum Blue PCR Super Mix (Invitrogen). Each experiment was performed in triplicate using the following primers to detect expression: Foxd1, 5′-CTCCTCCGTGTCCTCGTCCG-3′ and 5′-AGTTTAGCTCAGAGGGTCCA-3′ generating a product of 286 bp; Raldh2, 5′-TGGTGGAACGGGACAGGGCA-3′ and 5′-AGCGATTGCTGCCCCTGCTG-3′ generating a 418 bp product; Gdnf, 5′-TACGGAGACCGGATCCGAGG-3′ and 5′-CAGGCATATTGGAGTCACTGG-3′ generating a 207 bp product; Ret, 5′-GCTATGCCCAGATCGGGAAAG-3′ and 5′-TCAGTAATGGATGTCCCCTCC-3′ generating a 280 bp product; and actin, 5′-CTAAGGCCAACCGTGAAAAG-3′ and 5′-TCTCAGCTGTGGTGGTGAAG-3′ generating a 282 bp product. β-actin-specific primers were included in all PCR reactions as an internal control. PCR conditions were 35 cycles of: denaturation at 94°C for 2 minutes, annealing at 55°C for 30 seconds and extension at 72°C for 1 minute. Amplified products were separated on a 1.2% agarose gel using a 1 kb DNA ladder (GIBCO-BRL) for size comparison.
Generation of a Rosa26-RARa403 dominant-negative mouse
The human Rara truncation mutant (RARaT403) (Damm et al., 1993) was isolated from the pCAGGS expression vector (Novitch et al., 2003) and subcloned into the multiple cloning site of the pBIGT vector (Srinivas et al., 2001) using SalI and ApaI enzymes to generate pBIGT-RARaT403. Next, the pBIGT-RARaT403 vector was digested with PacI and AscI to release the floxed neo-tpa and RARaT403 cDNA insert, and the resulting fragment was cloned into the pROSA26PA plasmid, which contained the ROSA26 genomic targeting arms, to generate the final targeting vector, pROSA26PA-BIGT-RARaT403. This plasmid was then linearized with KpnI and electroporated into 129/SvEv embryonic stem (ES) cells. Following selection with G418 for 10 days, 96 colonies were picked, expanded and their ES cell genomic DNA screened by genomic Southern blot hybridization after digestion with EcoRV. A 5′ probe (Srinivas et al., 2001) was used for Southern blot hybridizations and correctly targeted clones were identified by the presence of an 11 kb wild-type band and a 3.8 kb targeted band, which reflects the targeted allele and includes an additional EcoRV site. One clone, E9, was expanded and used to generate the RaraDN mouse line.
RESULTS
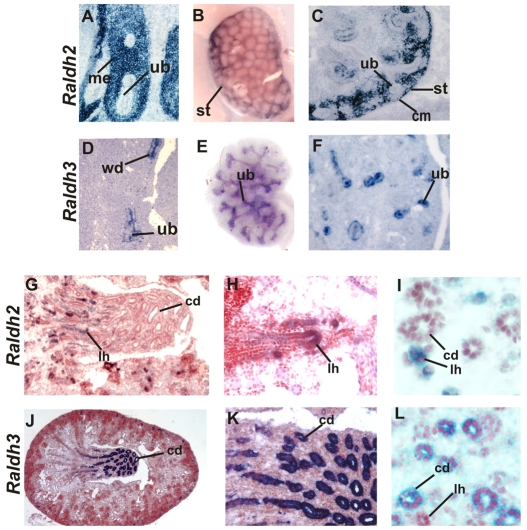
To begin to study the mechanism by which RA signaling controls renal development, we mapped the distribution of Raldh2 and Raldh3, RA-synthesizing enzymes which account for most of the RA in the embryo. Raldh2 expression was strong at E11 in mesenchyme and at later stages became restricted to cortical stroma in the nephrogenic zone (Fig. 1A,B). Raldh2 was also present in comma shaped bodies, then in the differentiating glomerulus in the visceral layer and in podocytes, a pattern similar to that reported in the rat (Marlier and Gilbert, 2004) (data not shown). Our analysis revealed an additional site of Raldh2 expression in the Loop of Henle: in the hairpin turn that is thought to be a site of tubule elongation (Fig. 1C-E).
Fig. 1.
Raldh2 and Raldh3 are selectively expressed in cortical stroma and in the ureteric bud during kidney development. (A-C,G-I) Raldh2 expression in a section from an E11 wild-type embryo (A), a wholemount E14 wild-type kidney (B), a section of an E14 wild-type embryonic kidney (C), a section of an E18 wild-type kidney (G), a higher magnification of G (H), and a section through an E18 wild-type embryonic kidney (I). (D-F,J-L) Raldh3 expression in a section of an E11 wild-type embryonic kidney (D), a wholemount E14 wild-type embryonic kidney (E), a section of an E14 wild-type embryonic kidney (F), a section of an E18 wild-type embryonic kidney (J), a higher magnification J (K), and a section of an E18 wild-type embryo (L). cd, collecting duct; cm, cap mesenchyme; lh, Loop of Henle; me, mesenchyme; st, stroma; ub, ureteric bud; wd, Wolffian duct. Magnifications: 40× in I,L; 20× in A,C,D,F,H,K; 10× in G; 5× in B,E,J.
Raldh3 expression was observed exclusively in epithelial cells beginning at E11 in the ureteric bud tips and trunk (Mic et al., 2002; Niederreither et al., 2002) (Fig. 1F,G). As the papilla began to form, Raldh3 expression became particularly intense in the large collecting ducts adjacent to the Loop of Henle where Raldh2 was localized (Fig. 1H-J). The localized expression of RA-synthesizing enzymes in the Loops of Henle and large collecting ducts suggests that in addition to a role in branching morphogenesis, they might also control RA-dependent functions required for medullary patterning.
Branching morphogenesis depends mainly on RA synthesized in cortical stroma
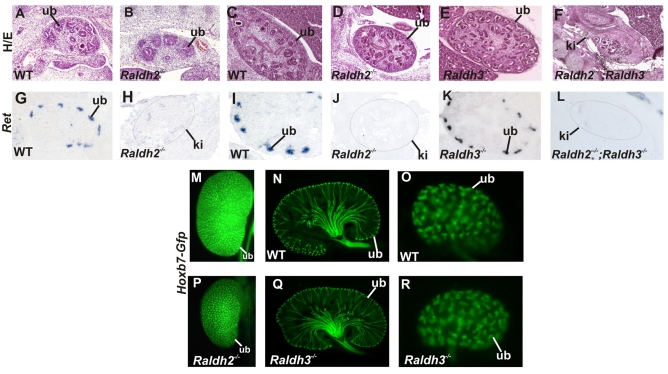
The distribution of RA-synthesizing enzymes in the embryonic kidney suggests that Ret expression and branching depend on Raldh2 in cortical stromal cells, Raldh3 in ureteric bud cells, or both. To address this question, we analyzed renal development in mutant mice lacking either Raldh2 or Raldh3. To bypass lethality due to heart defects, Raldh2 mutants were rescued by adding a small amount of RA to the maternal diet between E7 and E9 as described (Niederreither et al., 1999). Histological analysis at E12.5 revealed that branching had progressed to a similar extent in Raldh2 mutants compared with wild-type controls (Fig. 2A,B). By E14.5 however, mutant kidneys were greatly reduced in size compared with controls but displayed relatively normal architecture (Fig. 2C,D). By contrast, kidney development was apparently normal in mutants lacking Raldh3 (Fig. 2C,E).
Fig. 2.
Renal development depends mainly on Raldh2. (A-F) A Hematoxylin and Eosin (H/E)-stained section through an E12 kidney from a wild-type embryo (A), an E12 Raldh2 mutant embryonic kidney (B), an E14 wild-type kidney (C), an E14 Raldh2 mutant kidney (D), an E14 Raldh3 mutant kidney (E), and an E14 kidney from a Raldh2–/–;Raldh3–/– mutant (F). (G-L) In situ hybridization showing Ret expression in an E12 wild-type kidney (G), an E12 Raldh2 mutant kidney (H), an E14 wild-type kidney (I), an E14 Raldh2 mutant kidney (J), an E14 Raldh3 mutant kidney (K), and an E14 Raldh2–/–;Raldh3–/– compound mutant kidney (L). (M) A wholemount E18 kidney from a wild-type Hoxb7-Gfp transgenic embryo. (N) A vibratome section through an E18 Hoxb7-Gfp wild-type embryo. (O) A wholemount E14 control Hoxb7-Gfp embryonic kidney. (P) A wholemount E18 Hoxb7-Gfp;Raldh2–/– kidney. (Q) A vibratome section through an E18 Hoxb7-Gfp;Raldh3–/– embryonic kidney. (R) A wholemount Hoxb7-Gfp;Raldh3–/– E14 kidney. ki, kidney; ub, ureteric bud. Magnifications: 10× in A-L,O,R; 5× in M,N,P,Q.
The observation from our previous studies that forced expression of Ret in the ureteric bud could rescue renal development in Rara–/–;Rarb2–/– mutants suggests that RA signaling is likely to be crucial for Ret expression. To determine whether RA-synthesized by Raldh2 is also important for expression of Ret, we performed in situ hybridization analysis to determine whether Ret expression was altered in Raldh2 mutant kidneys. Analysis of embryonic kidneys from E12.5 wild-type and Raldh2 mutants revealed robust expression of Ret in the ureteric buds of wild-type embryos, which was nearly undetectable in Raldh2 mutants (Fig. 2G,H). At E14, Ret expression was greatly reduced in the ureteric buds from Raldh2 mutant kidneys compared with controls (Fig. 2I,J). Analysis of Raldh3 mutants revealed wild-type levels of Ret expression (Fig. 2K), consistent with the apparently normal renal development observed in this line.
Loss of Ret expression is likely to result in reduced levels of branching morphogenesis. To determine whether this was the case, Raldh2 mutants were crossed with Hoxb7-Gfp mice, a transgenic line expressing Gfp throughout the renal collecting duct system (Srinivas et al., 1999). Analysis of Hoxb7-Gfp;Raldh2–/– mutant kidneys at E18 and earlier stages revealed that branch numbers were reduced by 50% compared with wild type littermates (Fig. 2M,P; n=5; data not shown); an 80% reduction in branch numbers was observed in Rara–/–;Rarb2–/– mutants (Mendelsohn et al., 1999). Conversely, analysis of Raldh3–/–;Hoxb7-Gfp+/– mutant kidneys revealed branch numbers and ureteric bud patterning that were comparable to controls and consistent with normal histology and Ret expression in this line (Fig. 2N,O,Q,R).
One explanation for the relatively mild phenotype in Raldh2 mutants compared with RA-receptor knockouts could be that Raldh3, which is expressed in the ureteric bud, might provide sufficient RA to partially rescue renal development. To assess whether this was the case, we analyzed compound mutants lacking both Raldh2 and Raldh3 to determine whether removing Raldh3 increases the severity of renal phenotypes in Raldh2 mutants. Histological analysis of Raldh2–/–;Raldh3–/– mutant kidneys indeed revealed a more severe pattern of abnormalities reminiscent of those in Rara–/–;Rarb2–/– knockouts. Raldh2–/–;Raldh3–/– mutant kidneys were greatly reduced in size compared with mutants lacking Raldh2 alone, contained few ureteric bud branches or developing nephrons and Ret expression in mutant ureteric buds was essentially undetectable (Fig. 2F,L). Hence, Raldh3 is unlikely to be required normally for renal morphogenesis. However, the observation that deletion of Raldh3 together with Raldh2 increases the severity of renal malformations compared with those in single Raldh2 knockouts, suggests that RA generated by Raldh3 can marginally support development.
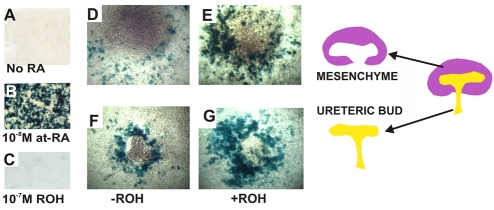
One possible explanation for the apparent minimal requirement for Raldh3 during kidney formation is that there might be less RA synthesis in ureteric bud cells or that RA synthesized in ureteric bud cells is less stable than RA synthesized in stroma. To address this question, we examined whether isolated ureteric bud explants and mesenchymes were capable of synthesizing RA. To detect RA, we performed co-culture experiments, plating isolated ureteric buds and mesenchymes on a lawn of F9-RARE-lacZ cells, a cell line containing a stably integrated RA-response element fused to the lacZ gene that is activated by nanomolar quantities of RA (Wagner et al., 1992). After 16 hours of culture, lacZ activity was undetectable in F9-RARE-lacz cells maintained in serum-free medium without added RA (Fig. 3A), indicating that reporter activity was RA-dependent. Addition of 10 nM RA to the culture medium resulted in robust lacZ expression (Fig. 3B); however, there was no reporter activity detected in cultures with 10–7 M retinol, an inactive RA precursor (Fig. 3C). Hence, reporter activity in F9-RARE-lacZ cells is RA-dependent and is not activated by retinol at a concentration of 10–7 M.
Fig. 3.
RA is synthesized by both the ureteric bud and mesenchyme in the embryonic kidney. (A-C) LacZ expression in F9-RARE;lacZ reporter cells cultured for 16 hours in medium without added retinoic acid (RA) (A), with 10–8 M at 9-cis RA (B) and with 10–7 M retinol (C), an inactive RA precursor. (D-G) LacZ expression in explants cultured for 16 hours on lawns of F9-RARE;lacZ reporter cells: E11 mesenchyme without a source of vitamin A (retinol) (D), E11 mesenchyme with 10–7 M retinol (E), E11 ureteric buds plated without retinol (F) and E11 ureteric buds with 10–7 M retinol (G). The schematic on the right shows separated metanephric mesenchyme (purple, assayed for lacZ activity in 3D,E) and ureteric bud explants (yellow, assayed for lacZ activity in 3F,G). Magnification: 20× in A-G.
To determine whether isolated ureteric buds and mesenchymes secrete RA, we cultured explants on lawns of F9-RARE-lacZ cells in serum-free medium then assayed co-cultures for lacZ expression. After 16 hours, lacZ staining was present in a ring of cells surrounding both mesenchyme and ureteric bud explants (Fig. 3D,F), indicating that both tissues secrete at least nanomolar amounts of RA that are sufficient to activate reporter expression. To determine whether ureteric buds and mesenchyme contain active RA-synthesizing enzymes, we investigated whether explants could convert retinol, an inactive RA precursor, to RA at sufficient levels to induce reporter activity. Explants were plated on lawns of F9-RARE-lacZ cells with 10–7 M retinol then stained for lacZ activity. We found that retinol stimulated lacZ expression in both ureteric bud and mesenchymal explants (Fig. 3F,G). Although both ureteric bud and mesenchymal compartments synthesize and secrete RA, it is nonetheless possible that stromal cells secrete higher levels of RA compared with ureteric bud cells. As F9-RARE-lacZ cells are extremely sensitive to RA, ureteric bud cells might generate RA at concentrations that activate RA-reporter-driven lacZ expression that is too low to efficiently activate RA-dependent transcription of Ret in ureteric bud cells.
RA, but not other stromal cell-derived signals, is likely to be crucial for inducing Ret expression and ureteric bud branching
The observations from microarray analyses that Rara and Rarb2 are co-localized both in the ureteric bud and in stromal cells, suggests that RA-receptor activity could be important in either cell type. To begin to address this question, we investigated whether stromal cell-derived signals were necessary for Ret expression and branching by culturing ureteric buds in the absence of mesenchyme. Isolated ureteric buds from E11 embryos were plated in Matrigel in serum-free medium with added Gdnf (Meyer et al., 2004) for 32 hours either with or without added RA. In control experiments, RT-PCR analysis of explants did not reveal detectable expression of Raldh2 or Foxd1, which are localized in the cortical stroma, or Gdnf, which is localized in metanephric mesenchyme (see Fig. S1 in the supplementary material), suggesting that ureteric bud cultures were not significantly contaminated with mesenchyme.
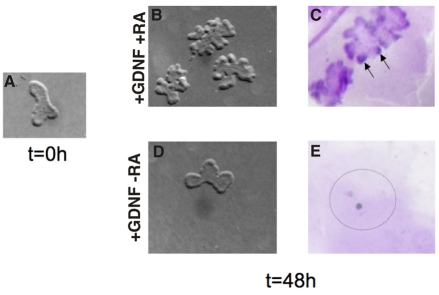
In cultures containing both RA and Gdnf, ureteric buds underwent extensive branching and Ret expression was upregulated (Fig. 4; see Fig. S1 in the supplementary material). However, in cultures lacking either RA or Gdnf, branching was blocked and Ret was undetectable (Fig. 4; see Fig. S1 in the supplementary material). These observations suggest that branching and Ret expression depend on RA and Gdnf and do not require additional signals from the stromal mesenchyme. The observations that RA and Gdnf can activate branching and Ret expression together, but not separately, suggests that these two signaling pathways act independently in ureteric bud cells, with RA inducing Ret expression in ureteric bud cells and Gdnf activating tyrosine-kinase signaling via the Ret receptor.
Fig. 4.
RA is sufficient for maintaining Ret expression and branching of isolated ureteric buds in the absence of mesenchyme. (A) A wholemount E11 ureteric bud prior to culture. (B) E11 ureteric buds cultured in serum-free medium including Gdnf, with added retinoic acid (RA). (C) In situ hybridization showing Ret expression in ureteric buds cultured for 48 hours in medium containing Gdnf and RA. (D) A wholemount ureteric bud cultured for 48 hours in medium with Gdnf and without added RA. (E) Undetectable Ret expression in an E11 ureteric bud cultured for 48 hours in medium with Gdnf and without RA. Magnification: 20× in A-E.
RA-receptor-mediated signaling is required in the ureteric bud for maintaining Ret expression and branching
The observation that Ret can be induced in isolated ureteric buds in the absence of stromal mesenchyme suggests that, in contrast to our original model, RA receptors are likely to be required for Ret expression and branching in ureteric bud cells rather than in stroma. To directly address this question, we tested whether inhibition of RA-receptor signaling in the renal collecting duct system would lead to abnormalities similar to those seen in Rara–/–;Rarb2–/– mutants.
RaraT403 is a truncated form of human RARa that lacks the carboxyl terminal sequence that is normally required for RA-dependent transcriptional activation. Numerous studies show that this mutant receptor inhibits endogenous RA-mediated transcription of target genes in a dose-dependent manner (Blumberg et al., 1997; Damm et al., 1993; Rajaii et al., 2008). Inhibition of RA-receptor signaling would be likely to generate lethal phenotypes, hence we generated a line of mice carrying a dormant RaraT403 transgene (hereafter called RaraDN) inserted into the ROSA26R locus (Soriano, 1999). The transgene is preceded by a floxed transcriptional and translational STOP sequence that is excised in cells expressing the Cre recombinase, activating the expression of the mutant RA receptor (see Fig. S2 in the supplementary material).
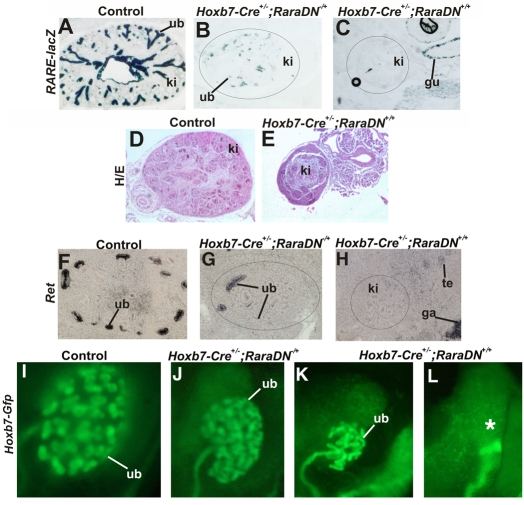
To ensure that the RaraDN protein was synthesized and actively suppressed RA signaling in the ureteric bud, we first tested whether its expression could efficiently block RARE-lacZ expression in RA-reporter mice (Giguere et al., 1990), a line containing a stably integrated RARE-driven lacZ transgene, whose expression in the ureteric bud is RA-dependent (see Fig. S3 in the supplementary material). Analysis of E14 control embryos revealed abundant lacZ expression in the ureteric bud tree (Fig. 5A). In E14 Hoxb7-Cre–/–;RaraDNflox/+;RARE-lacZ embryos expressing a single allele of the RaraDN transgene, lacZ expression in the ureteric bud was greatly reduced but still detectable (Fig. 5B), indicating that RA-signaling was not completely blocked. However, in kidneys from mice expressing both alleles of the RaraDN transgene (Hoxb7-Cre–/–;RaraDNflox/flox;RARE-lacZ animals), lacZ activity was undetectable, indicating that, as seen by other groups, this RA dominant-negative mutant can block endogenous RA-receptor-dependent signaling in a dose-dependent manner.
Fig. 5.
Expression of RaraDN in ureteric bud cells inhibits Ret expression and branching in a dose-dependent manner. (A) A lacZ-stained section from a control (Hoxb7-Cre–/–;RaraDNflox/flox;RARE-lacZ) embryo. (B) A lacZ-stained section of an embryo expressing one allele (Hoxb7-Cre–/+;RaraDNflox/+;RARE-lacZ) of the RaraDN transgene. (C) Undetectable lacZ expression in kidneys in an embryo expressing two alleles (Hoxb7-Cre–/+;RaraDNflox/flox;RARE-lacZ) of the RaraDN transgene. (D) A Hematoxylin and Eosin (H/E)-stained section from an E14 Hoxb7-Cre–/–;RaraDNflox/flox control embryonic kidney. (E) An H/E-stained section through a kidney from an Hoxb7-Cre–/+;RaraDNflox/flox embryo. (F) In situ hybridization analysis of a control (Hoxb7-Cre–/–;RaraDNflox/+) embryonic kidney. (G) In situ hybridization analysis of an embryo expressing one allele (Hoxb7-Cre–/+;RaraDNflox/+) of the RaraDN transgene. (H) Undetectable Ret expression in a sectioned kidney from an E14 (Hoxb7-Cre–/+;RaraDNflox/flox embryo) expressing two alleles of the RaraDN transgene. (I) Hoxb7-Gfp expression in a control (Hoxb7-Cre–/–;RaraDNflox/flox) E14 embryonic kidney (J) Reduced branching morphogenesis in a Hoxb7-Cre–/+;RaraDNflox/+;Hoxb7-Gfp E14 kidney from an embryo expressing one allele of the dominant-negative transgene. (K,L) Renal hypoplasia and renal agenesis, respectively, in Hoxb7-Cre–/+;RaraDNflox/flox;Hoxb7-Gfp E14 embryonic kidneys from animals expressing two alleles of dominant-negative RaraDN transgene. The asterisk in (L) denotes renal agenesis. gu, gut; ki, kidney; te, testes; ub, ureteric bud. Magnification: 10× in A-L.
Histological analysis revealed that expression of both alleles of the RaraDN mutant also resulted in a phenotype very similar to that in Rara–/–;Rarb2–/– mutants; Hoxb7-Cre;RaraDNflox/flox embryonic kidneys were greatly reduced in size compared with controls, had few ureteric bud branches or nephrons and displayed severe patterning abnormalities (Fig. 5D,E). To determine whether, as in Rara–/–;Rarb2–/– and Raldh2 mutants, these phenotypes were due to loss of Ret expression in ureteric bud cells, we analyzed kidneys from dominant-negative mutants to determine whether Ret was downregulated. This analysis revealed abundant expression of Ret in the ureteric bud tips of controls, which was downregulated in most ureteric bud tips, but still detectable in others (Fig. 5F,G). Consistent with the observation that the RaraDN mutant functions in a dose-dependent manner, expression of two alleles of the RaraDN transgene in ureteric bud cells resulted in undetectable Ret expression (Fig. 5H).
To determine whether branching defects were more severe in kidneys from embryos expressing two alleles of the RaraDN transgene compared with those expressing a single allele, Hoxb7-Cre;RaraDN mice were intercrossed with Hoxb7-Gfp mice to enable us to clearly visualize branching morphogenesis. Analysis of kidneys from at E14 Hoxb7-Cre;RaraDNflox/+;Hoxb7-Gfp embryos expressing one allele of the RaraDN receptor revealed renal hypoplasia with a 50-80% reduction in branch numbers (n=11; Fig. 5I,J), whereas in embryos expressing two alleles of the RA receptor mutant (Hoxb7-Cre;RaraDNflox/flox;Hoxb7-Gfp mutants), we observed fewer branch numbers and renal agenesis (n=5/6; Fig. 5L).
Renal agenesis was not observed previously in Rara–/–;Rarb2–/– mutants but is the most common phenotype (second to renal hypoplasia) in Ret mutants (Schuchardt et al., 1996), indicating that both ureteric bud formation and branching are Ret-dependent events. Hence, as in the kidney, RA signaling might normally be important for maintaining Ret expression during ureteric bud outgrowth, and loss of Ret expression in the emerging ureteric bud of Hoxb7-Cre;RaraDNflox/flox mutants might be the underlying cause of renal agenesis. Ureteric buds sprout from the caudal-most portion of the Wolffian duct between E10.5 and E11, then invade kidney mesenchyme and begin to branch. To determine whether renal agenesis in Hoxb7-Cre;RaraDNflox/flox was linked to defective ureteric bud outgrowth, we analyzed Hoxb7-Cre;RaraDNflox/flox;Hoxb7-Gfp mutants at E10 and E11, to determine whether ureteric bud formation was impaired. At E10 in control embryos, the ureteric bud had begun to extend from the Wolffian duct, whereas 1 day later, it had invaded kidney mesenchyme and undergone 1 to 2 generations of branching (Fig. 6A,C). In Hoxb7-Cre+/–;RaraDNflox/flox;Hoxb7-Gfp embryos, the ureteric bud was smaller at E10.5 than in control embryos, and at E11 it had barely begun to branch, suggesting that primary ureteric bud formation was indeed abnormal. To determine whether this phenotype was linked to downregulation of Ret, we analyzed E10.5 Hoxb7-Cre;RaraDNflox/flox embryos and controls for Ret expression by wholemount in situ hybridization. In controls not expressing RaraDN (Hoxb7-Cre–/–;RaraDNflox/flox embryos), Ret was abundant in the Wolffian ducts and in the emerging ureteric bud (Fig. 6E). However, ureteric bud formation was delayed and Ret expression was greatly reduced in the Wolffian ducts of Hoxb7-Cre+/–;RaraDNflox/flox mutants. These results suggest that RA signaling is likely to be important at the onset of kidney formation for primary ureteric bud outgrowth and also within the embryonic kidney for branching morphogenesis, in both cases acting as a crucial regulator of Ret expression.
Fig. 6.

RA signaling is important for primary ureteric bud formation as well as for branching morphogenesis within the kidney. (A,B) Ureteric bud formation in a wholemount E10.5 control (Hoxb7-Cre–/–;RaraDNflox/flox;Hoxb7-Gfp) embryo (A) and an E10.5 Hoxb7-Cre–/+;RaraDNflox/flox;Hoxb7-Gfp embryo expressing two alleles of the RaraDN transgene (B). (C,D) Gfp expression in an E11.5 control embryo (C) and an E11.5 Hoxb7-Cre–/–;RaraDNflox/flox;Hoxb7-Gfp embryo (D). (E,F) Wholemount in situ hybridization showing Ret expression in the emerging ureteric bud of a control (Hoxb7-Cre–/–;RaraDNflox/flox) E10.5 embryo (E) and an E10.5 Hoxb7-Cre–/+;RaraDNflox/flox;Hoxb7-Gfp embryo (F). The black arrows point to the caudal Wolffian ducts. Magnification: 20× in A-F.
DISCUSSION
Studies performed in rodents demonstrate that maternal vitamin A is required for formation of most organs and tissues including the kidney (Lelievre-Pegorier et al., 1998; Quadro et al., 2005; Wilson, 1948). Our previous analysis of animals lacking two RA receptor family members (Rara–/–;Rarb2–/– mice) revealed a similar pattern of defects as that reported in vitamin A deficiency studies (Batourina et al., 2001; Mendelsohn et al., 1994a). The mutant kidney displayed reduced branch numbers, abnormal patterning and downregulation of Ret, a gene that is important both for ureteric bud formation and for branching morphogenesis within the developing kidney (Schuchardt et al., 1996). That forced expression of Ret in the ureteric bud cells of Rara–/–;Rarb2–/– mutants rescued these kidney defects suggests that a major role of RA-receptor signaling during renal development is to regulate Ret expression in the ureteric bud (Batourina et al., 2001).
RA-dependent transcription is regulated via a switch-like mechanism; in the absence of RA, Rars can bind to enhancers in regulatory sequences of target genes where they repress basal transcription, but when bound by RA, receptors undergo a conformational change and become potent transactivators. Recent studies suggest that in many cases, RA signaling occurs via a paracrine mechanism where RA receptors expressed in one cell type are activated by RA that is generated in a neighboring cell type (Duester, 2008). Our studies suggest that renal development also depends on paracrine signaling, in this case between stromal cells that synthesize RA and ureteric bud cells that respond to RA. This pathway in turn regulates ureteric bud formation and branching by controlling the expression of Ret.
Raldh2 in cortical stroma generates a crucial source of RA that maintains renal development.
Among the Raldh family members, only Raldh2 and Raldh3 are expressed at robust levels in the mouse embryonic kidney, where they are localized in cortical stroma and ureteric bud cells respectively (Fig. 1) (Niederreither et al., 2002). Paradoxically, we find that deletion of Raldh3 has little, if any, effect on renal development, whereas deletion of Raldh2 results in defects that are similar to, but less severe than, those in RA receptor knockout mice, including reduced kidney size, impaired branching and downregulation of Ret in the ureteric bud. The decreased severity of renal defects in Raldh2 mutants compared with those in RA receptor knockouts suggests that Raldh3 provides a source of RA that can partially rescue renal development. This suggestion is supported by the observation here that removal of Raldh3 on a Raldh2 mutant background increased the severity of kidney defects to the level of those found in Rara–/–;Rarb2–/– knockout mice.
Our findings are in general agreement with those of other groups; Raldh2 mutants display a spectrum of defects in organogenesis that mirror those observed in vitamin A deficiency (Duester, 2008; Niederreither and Dolle, 2008; Wilson, 1953). However, despite its robust and widespread expression in the embryo, malformations in Raldh3 mutants are limited to the nasal passage, eyes and brain (Dupe et al., 2003; Halilagic et al., 2007; Molotkova et al., 2007). The observation from our studies and from those of others that deletion of Raldh2 and Raldh3 together can increase the severity of embryonic phenotypes compared with those in mutants lacking either enzyme alone, together with studies in RA reporter mice (Molotkov et al., 2006), demonstrates that Raldh3 is a bona fide RA-synthesizing enzyme. One explanation for the disparity between its expression and function could be that although Raldh3 is able to synthesize sufficient quantities of RA to activate the expression of sensitive RA reporters, RA synthesis might be at levels that are insufficient to fully activate transcriptional programs in the developing embryo. Whether Raldh3 or other RA-synthesizing enzymes play a role in post-natal renal development is an interesting question that we will further pursue.
What renal cell types are important for mediating RA-dependent signaling?
We found previously that inactivation of Rara and Rarb2 together, but not separately, generated renal malformations including defective branching and downregulation of Ret in ureteric bud cells (Mendelsohn et al., 1999). In situ hybridization analysis revealed that Rarb2 and Rara were colocalized in stromal cells but not in other renal cell types, suggesting that their concerted function was required in the stromal cell compartment for inducing expression of secreted signals that control Ret expression in ureteric bud cells (Mendelsohn et al., 1999). However, more recent microarray studies reveal Rarb expression both in stroma and in ureteric bud cells (Schmidt-Ott et al., 2005) (GUDMAP, http://www.gudmap.org/); therefore, RA-receptor signaling could be via ureteric bud cells or via stromal cells. The finding that without stromal mesenchyme, ureteric buds can be induced by RA to express Ret and branch, suggests that RA, but not other stromal-cell signals, regulates Ret expression by activating RA-receptor-dependent transcription in ureteric bud cells (Fig. 7). Direct evidence for this model comes from experiments in which we demonstrate that blocking RA-dependent transcriptional activity in ureteric bud cells by expressing a dominant-negative RA receptor results in phenotypes resembling those in Rara–/–;Rarb2–/– mutants and in Ret mutants (Fig. 5).
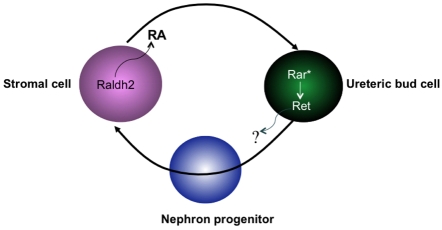
Fig. 7.
Model showing Ret regulation in ureteric bud cells by paracrine RA signaling. Secreted retinoic acid (RA) synthesized by Raldh2 in cortical stroma binds to RA receptors in ureteric bud cells and activates Rar-dependent signaling (Rar*). RA-dependent transcription induces Ret expression in ureteric bud cells, either directly or indirectly.
How does RA regulate Ret in ureteric bud cells?
RA regulates Ret in the embryonic kidneys of several species, including rodents (Gilbert, 2002) and amphibians (Osafune et al., 2002). Withdrawal of RA or impaired RA signaling results in downregulation of Ret in the ureteric bud (Mendelsohn et al., 1999); however, even at high concentrations, RA is unable to expand the domain of Ret expression or induce ectopic expression of Ret in embryonic kidneys (Batourina et al., 2001). These findings suggest that RA is likely to be required for maintaining Ret, but does not normally activate de novo Ret expression in ureteric bud cells. The question of whether RA receptors act by binding to enhancers in Ret regulatory sequences or by inducing expression of an intermediary transcription factor in ureteric bud cells is unclear. Several candidate RA response elements have been identified in the Ret promoter; however, none of these has been shown to drive RA-dependent expression (Grice et al., 2005; Patrone et al., 1997), suggesting that RA might control Ret indirectly. Potential direct activators of Ret include Pax2 (Brophy et al., 2001), Hox genes (Lui et al., 2008), Ttf1 (Garcia-Barcelo et al., 2007), Nkx2.1 (Garcia-Barcelo et al., 2008) and Nurr1 (Nr4a2 – Mouse Genome Informatics) (Wallen et al., 2001), all of which are localized in the developing kidney.
RA signaling regulates formation of the primary ureteric bud from the Wolffian duct and its branching morphogenesis within the developing kidney via Ret
Ret is a gene that, when mutated in humans, can cause renal defects, Hirschsprung's disease and cancer (Dressler, 2008; Moore and Zaahl, 2008; Runeberg-Roos and Saarma, 2007). The majority of Ret mutant mice display renal agenesis due to defects at the first stage of branch formation, whereas a minority display renal hypoplasia due to impaired ureteric bud branching within the kidney (Schuchardt et al., 1996). The expression of the RaraDN mutant receptor in ureteric bud cells produces a set of renal phenotypes that overlap with those observed in Ret knockouts, whose severity depends on the gene dosage. Mutants expressing one RaraDN allele display renal hypoplasia, reduced branching and reduced Ret expression in the embryonic kidney compared with mutants expressing two RaraDN alleles that display renal agenesis or severe hypoplasia and downregulation of Ret both at early stages in the emerging ureteric bud and at later stages within the kidney (Fig. 5; Fig. 6). The observation that RA and Ret act in a similar manner in the lower urinary tract, regulating ureter insertion via remodeling of the common nephric duct (the portion of the Wolffian duct below the ureteric bud) (Batourina et al., 2005), suggests that this conserved signaling pathway controls epithelial cell remodeling at multiple stages of urinary tract formation: during primary ureteric bud outgrowth from the Wolffian duct, during ureteric bud branching within the kidney and during ureter insertion when common nephric duct remodeling is required for proper insertion of ureters in the bladder.
Studies in rodents suggest that even mild vitamin A deficiency can lead to impaired branching and a reduction in the number of nephrons (Lelievre-Pegorier et al., 1998; Moreau et al., 1998). In this case, renal size and architecture appeared normal but fewer nephrons formed, a phenotype which is probably owing to impaired branching morphogenesis and partial loss of Ret expression in the ureteric bud (Moreau et al., 1998). In humans, nephron deficits are associated with increased risk of hypertension (Brenner and Mackenzie, 1997). That mild (sub-clinical) vitamin A deficiency, which is widely found in the human population, might also lead to nephron deficits is a real possibility (Bhat and Manolescu, 2008).
Acknowledgements
We thank Qais Al-awqati, Jon Barasch and Doris Herzlinger for helpful discussions; Andy McMahon and Akio Kobayashi for the Hoxb7-Cre and comments on the manuscript; Ben Novitch and David Lohnes for plasmids; Janet Rossant for the generous gift of the RARE-lacZ reporter line; Tom Jessell and Michael Wagner for the F9-RARE-lacZ cells; and Fang Hua Li for technical assistance. This work was supported by grants from the Irma T. Hirchl foundation [DK55388 and (ARRA) DK082963]. C.R. was supported by a T32 training grant from the NIH (HL007343). L.S. was supported by a grant from the American Heart Association. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.040287/-/DC1
References
- Batourina E., Gim S., Bello N., Shy M., Clagett-Dame M., Srinivas S., Costantini F., Mendelsohn C. (2001). Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat. Genet. 27, 74-78 [DOI] [PubMed] [Google Scholar]
- Batourina E., Tsai S., Lambert S., Sprenkle P., Viana R., Dutta S., Hensle T., Wang F., Niederreither K., McMahon A. P., et al. (2005). Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat. Genet. 37, 1082-1089 [DOI] [PubMed] [Google Scholar]
- Bhat P. V., Manolescu D. C. (2008). Role of vitamin A in determining nephron mass and possible relationship to hypertension. J. Nutr. 138, 1407-1410 [DOI] [PubMed] [Google Scholar]
- Blumberg B., Bolado J., Jr, Moreno T. A., Kintner C., Evans R. M., Papalopulu N. (1997). An essential role for retinoid signaling in anteroposterior neural patterning. Development 124, 373-379 [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Mackenzie H. S. (1997). Nephron mass as a risk factor for progression of renal disease. Kidney Int. Suppl. 63, S124-S127 [PubMed] [Google Scholar]
- Brophy P. D., Ostrom L., Lang K. M., Dressler G. R. (2001). Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development 128, 4747-4756 [DOI] [PubMed] [Google Scholar]
- Damm K., Heyman R. A., Umesono K., Evans R. M. (1993). Functional inhibition of retinoic acid response by dominant negative retinoic acid receptor mutants. Proc. Natl. Acad. Sci. USA 90, 2989-2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaff E., Srinivas S., Kilkenny C., D'Agati V., Mankoo B. S., Costantini F., Pachnis V. (2001). Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev. 15, 2433-2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle P., Ruberte E., Leroy P., Morriss-Kay G., Chambon P. (1990). Retinoic acid receptors and cellular retinoid binding proteins. I. A systematic study of their differential pattern of transcription during mouse organogenesis. Development 110, 1133-1151 [DOI] [PubMed] [Google Scholar]
- Dressler G. R. (2008). Epigenetics, development, and the kidney. J. Am. Soc. Nephrol. 19, 2060-2067 [DOI] [PubMed] [Google Scholar]
- Duester G. (2008). Retinoic acid synthesis and signaling during early organogenesis. Cell 134, 921-931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G., Mic F. A., Molotkov A. (2003). Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem. Biol. Interact. 143-144, 201-210 [DOI] [PubMed] [Google Scholar]
- Dupe V., Matt N., Garnier J. M., Chambon P., Mark M., Ghyselinck N. B. (2003). A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc. Natl. Acad. Sci. USA 100, 14036-14041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Barcelo M. M., Lau D. K., Ngan E. S., Leon T. Y., Liu T. T., So M. T., Miao X. P., Lui V. C., Wong K. K., Ganster R. W., et al. (2007). Evaluation of the thyroid transcription factor-1 gene (TITF1) as a Hirschsprung's disease locus. Ann. Hum. Genet. 71, 746-754 [DOI] [PubMed] [Google Scholar]
- Garcia-Barcelo M. M., Lau D. K., Ngan E. S., Leon T. Y., Liu T., So M., Miao X., Lui V. C., Wong K. K., Ganster R. W., et al. (2008). Evaluation of the NK2 homeobox 1 gene (NKX2-1) as a Hirschsprung's disease locus. Ann. Hum. Genet. 72, 170-177 [DOI] [PubMed] [Google Scholar]
- Ghyselinck N. B., Wendling O., Messaddeq N., Dierich A., Lampron C., Decimo D., Viville S., Chambon P., Mark M. (1998). Contribution of retinoic acid receptor beta isoforms to the formation of the conotruncal septum of the embryonic heart. Dev. Biol. 198, 303-318 [PubMed] [Google Scholar]
- Giguere V., Shago M., Zirngibl R., Tate P., Rossant J., Varmuza S. (1990). Identification of a novel isoform of the retinoic acid receptor gamma expressed in the mouse embryo. Mol. Cell. Biol. 10, 2335-2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert T. (2002). Vitamin A and kidney development. Nephrol. Dial. Transplant 17Suppl. 9, 78-80 [DOI] [PubMed] [Google Scholar]
- Grice E. A., Rochelle E. S., Green E. D., Chakravarti A., McCallion A. S. (2005). Evaluation of the RET regulatory landscape reveals the biological relevance of a HSCR-implicated enhancer. Hum. Mol. Genet. 14, 3837-3845 [DOI] [PubMed] [Google Scholar]
- Grobstein C. (1953). Inductive epithelio-mesenchymal interaction in cultured organ rudiments. Science 113, 52-55 [DOI] [PubMed] [Google Scholar]
- Halilagic A., Ribes V., Ghyselinck N. B., Zile M. H., Dolle P., Studer M. (2007). Retinoids control anterior and dorsal properties in the developing forebrain. Dev. Biol. 303, 362-375 [DOI] [PubMed] [Google Scholar]
- Hatini V., Huh S. O., Herzlinger D., Soares V. C., Lai E. (1996). Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 10, 1467-1478 [DOI] [PubMed] [Google Scholar]
- Kastner P., Mark M., Chambon P. (1995). Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell 83, 859-869 [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Chambon P. (1992). Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem. Sci. 17, 427-433 [DOI] [PubMed] [Google Scholar]
- Lelievre-Pegorier M., Vilar J., Ferrier M. L., Moreau E., Freund N., Gilbert T., Merlet-Benichou C. (1998). Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int. 54, 1455-1462 [DOI] [PubMed] [Google Scholar]
- Levinson R. S., Batourina E., Choi C., Vorontchikhina M., Kitajewski J., Mendelsohn C. L. (2005). Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development 132, 529-539 [DOI] [PubMed] [Google Scholar]
- Lin M., Zhang M., Abraham M., Smith S. M., Napoli J. L. (2003). Mouse retinal dehydrogenase 4 (RALDH4), molecular cloning, cellular expression, and activity in 9-cis-retinoic acid biosynthesis in intact cells. J. Biol. Chem. 278, 9856-9861 [DOI] [PubMed] [Google Scholar]
- Lohnes D., Kastner P., Dierich A., Mark M., LeMeur M., Chambon P. (1993). Function of retinoic acid receptor gamma in the mouse. Cell 73, 643-658 [DOI] [PubMed] [Google Scholar]
- Lohnes D., Mark M., Mendelsohn C., Dolle P., Dierich A., Gorry P., Gansmuller A., Chambon P. (1994). Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development 120, 2723-2748 [DOI] [PubMed] [Google Scholar]
- Lufkin T., Lohnes D., Mark M., Dierich A., Gorry P., Gaub M. P., LeMeur M., Chambon P. (1993). High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc. Natl. Acad. Sci. USA 90, 7225-7229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui V. C., Cheng W. W., Leon T. Y., Lau D. K., Garcia-Barcelo M. M., Miao X. P., Kam M. K., So M. T., Chen Y., Wall N. A., et al. (2008). Perturbation of hoxb5 signaling in vagal neural crests down-regulates ret leading to intestinal hypoganglionosis in mice. Gastroenterology 134, 1104-1115 [DOI] [PubMed] [Google Scholar]
- Luo J., Pasceri P., Conlon R. A., Rossant J., Giguere V. (1995). Mice lacking all isoforms of retinoic acid receptor beta develop normally and are susceptible to the teratogenic effects of retinoic acid. Mech. Dev. 53, 61-71 [DOI] [PubMed] [Google Scholar]
- Luo J., Sucov H. M., Bader J. A., Evans R. M., Giguere V. (1996). Compound mutants for retinoic acid receptor (RAR) beta and RAR alpha 1 reveal developmental functions for multiple RAR beta isoforms. Mech. Dev. 55, 33-44 [DOI] [PubMed] [Google Scholar]
- Marlier A., Gilbert T. (2004). Expression of retinoic acid-synthesizing and -metabolizing enzymes during nephrogenesis in the rat. Gene Expr. Patterns 5, 179-185 [DOI] [PubMed] [Google Scholar]
- Mendelsohn C., Larkin S., Mark M., LeMeur M., Clifford J., Zelent A., Chambon P. (1994a). RAR beta isoforms: distinct transcriptional control by retinoic acid and specific spatial patterns of promoter activity during mouse embryonic development. Mech. Dev. 45, 227-241 [DOI] [PubMed] [Google Scholar]
- Mendelsohn C., Lohnes D., Decimo D., Lufkin T., LeMeur M., Chambon P., Mark M. (1994b). Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development 120, 2749-2771 [DOI] [PubMed] [Google Scholar]
- Mendelsohn C., Mark M., Dolle P., Dierich A., Gaub M. P., Krust A., Lampron C., Chambon P. (1994c). Retinoic acid receptor beta 2 (RAR beta 2) null mutant mice appear normal. Dev. Biol. 166, 246-258 [DOI] [PubMed] [Google Scholar]
- Mendelsohn C., Batourina E., Fung S., Gilbert T., Dodd J. (1999). Stromal cells mediate retinoid-dependent functions essential for renal development. Development 126, 1139-1148 [DOI] [PubMed] [Google Scholar]
- Meyer T. N., Schwesinger C., Bush K. T., Stuart R. O., Rose D. W., Shah M. M., Vaughn D. A., Steer D. L., Nigam S. K. (2004). Spatiotemporal regulation of morphogenetic molecules during in vitro branching of the isolated ureteric bud: toward a model of branching through budding in the developing kidney. Dev. Biol. 275, 44-67 [DOI] [PubMed] [Google Scholar]
- Mic F. A., Molotkov A., Fan X., Cuenca A. E., Duester G. (2000). RALDH3, a retinaldehyde dehydrogenase that generates retinoic acid, is expressed in the ventral retina, otic vesicle and olfactory pit during mouse development. Mech. Dev. 97, 227-230 [DOI] [PubMed] [Google Scholar]
- Molotkov A., Molotkova N., Duester G. (2006). Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development 133, 1901-1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkova N., Molotkov A., Duester G. (2007). Role of retinoic acid during forebrain development begins late when Raldh3 generates retinoic acid in the ventral subventricular zone. Dev. Biol. 303, 601-610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. W., Zaahl M. G. (2008). Multiple endocrine neoplasia syndromes, children, Hirschsprung's disease and RET. Pediatr. Surg. Int. 24, 521-530 [DOI] [PubMed] [Google Scholar]
- Moreau E., Vilar J., Lelievre-Pegorier M., Merlet-Benichou C., Gilbert T. (1998). Regulation of c-ret expression by retinoic acid in rat metanephros: implication in nephron mass control. Am. J. Physiol. 275, F938-F945 [DOI] [PubMed] [Google Scholar]
- Niederreither K., Dolle P. (2008). Retinoic acid in development: towards an integrated view. Nat. Rev. Genet. 9, 541-553 [DOI] [PubMed] [Google Scholar]
- Niederreither K., Subbarayan V., Dolle P., Chambon P. (1999). Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 21, 444-448 [DOI] [PubMed] [Google Scholar]
- Niederreither K., Fraulob V., Garnier J. M., Chambon P., Dolle P. (2002). Differential expression of retinoic acid-synthesizing (RALDH) enzymes during fetal development and organ differentiation in the mouse. Mech. Dev. 110, 165-171 [DOI] [PubMed] [Google Scholar]
- Novitch B. G., Wichterle H., Jessell T. M., Sockanathan S. (2003). A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron 40, 81-95 [DOI] [PubMed] [Google Scholar]
- Osafune K., Nishinakamura R., Komazaki S., Asashima M. (2002). In vitro induction of the pronephric duct in Xenopus explants. Dev. Growth Differ. 44, 161-167 [DOI] [PubMed] [Google Scholar]
- Patrone G., Puliti A., Bocciardi R., Ravazzolo R., Romeo G. (1997). Sequence and characterisation of the RET proto-oncogene 5′ flanking region: analysis of retinoic acid responsiveness at the transcriptional level. FEBS Lett. 419, 76-82 [DOI] [PubMed] [Google Scholar]
- Quadro L., Hamberger L., Gottesman M. E., Wang F., Colantuoni V., Blaner W. S., Mendelsohn C. L. (2005). Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology 146, 4479-4490 [DOI] [PubMed] [Google Scholar]
- Rajaii F., Bitzer Z. T., Xu Q., Sockanathan S. (2008). Expression of the dominant negative retinoid receptor, RAR403, alters telencephalic progenitor proliferation, survival, and cell fate specification. Dev. Biol. 316, 371-382 [DOI] [PubMed] [Google Scholar]
- Rossant J., Zirngibl R., Cado D., Shago M., Giguere V. (1991). Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 5, 1333-1344 [DOI] [PubMed] [Google Scholar]
- Ruberte E., Dolle P., Krust A., Zelent A., Morriss-Kay G., Chambon P. (1990). Specific spatial and temporal distribution of retinoic acid receptor gamma transcripts during mouse embryogenesis. Development 108, 213-222 [DOI] [PubMed] [Google Scholar]
- Runeberg-Roos P., Saarma M. (2007). Neurotrophic factor receptor RET: structure, cell biology, and inherited diseases. Ann. Med. 39, 572-580 [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott K. M., Chen X., Paragas N., Levinson R. S., Mendelsohn C. L., Barasch J. (2006). c-kit delineates a distinct domain of progenitors in the developing kidney. Dev. Biol. 299, 238-249 [DOI] [PubMed] [Google Scholar]
- Schuchardt A., D'Agati V., Pachnis V., Costantini F. (1996). Renal agenesis and hypodysplasia in ret-k-mutant mice result from defects in ureteric bud development. Development 122, 1919-1929 [DOI] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70-71 [DOI] [PubMed] [Google Scholar]
- Srinivas S., Goldberg M. R., Watanabe T., D'Agati V., al-Awqati Q., Costantini F. (1999). Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Dev. Genet. 24, 241-251 [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C. S., William C. M., Tanabe Y., Jessell T. M., Costantini F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visel A., Thaller C., Eichele G. (2004). GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 32, D552-D556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M., Han B., Jessell T. M. (1992). Regional differences in retinoid release from embryonic neural tissue detected by an in vitro reporter assay. Development 116, 55-66 [DOI] [PubMed] [Google Scholar]
- Wallen A. A., Castro D. S., Zetterstrom R. H., Karlen M., Olson L., Ericson J., Perlmann T. (2001). Orphan nuclear receptor Nurr1 is essential for Ret expression in midbrain dopamine neurons and in the brain stem. Mol. Cell Neurosci. 18, 649-663 [DOI] [PubMed] [Google Scholar]
- Wilson J. G., Wackany J. (1948). Malformations in the genito-urinary tract induced by maternal vitamin A deficiency in the rat. Am. J. Anat. 83, 357-407 [DOI] [PubMed] [Google Scholar]
- Wilson J. G., Roth C. V., Warkany J. (1953). An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am. J. Anat. 92, 189-217 [DOI] [PubMed] [Google Scholar]
- Yu J., Carroll T. J., McMahon A. P. (2002). Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129, 5301-5312 [DOI] [PubMed] [Google Scholar]
- Zhao D., McCaffery P., Ivins K. J., Neve R. L., Hogan P., Chin W. W., Drager U. C. (1996). Molecular identification of a major retinoic-acid-synthesizing enzyme, a retinaldehyde-specific dehydrogenase. Eur. J. Biochem. 240, 15-22 [DOI] [PubMed] [Google Scholar]
- Zhao X., Sirbu I. O., Mic F. A., Molotkova N., Molotkov A., Kumar S., Duester G. (2009). Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning. Curr. Biol. 19, 1050-1057 [DOI] [PMC free article] [PubMed] [Google Scholar]