Abstract
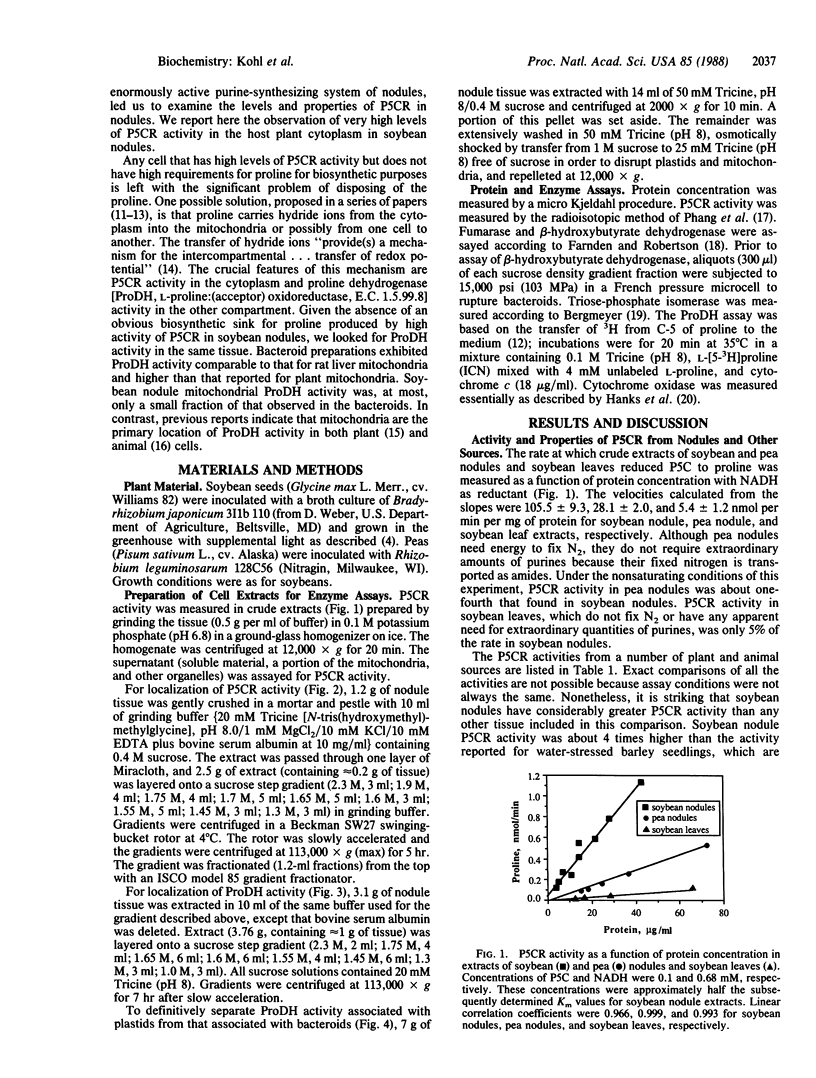
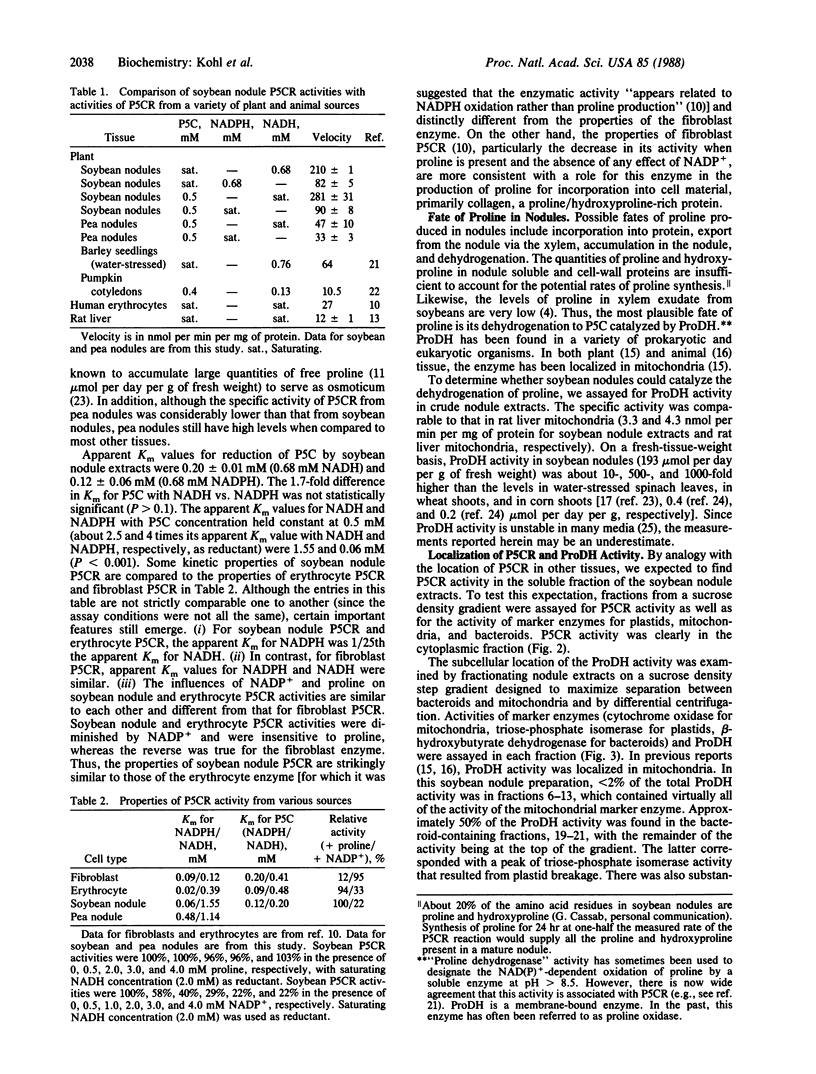
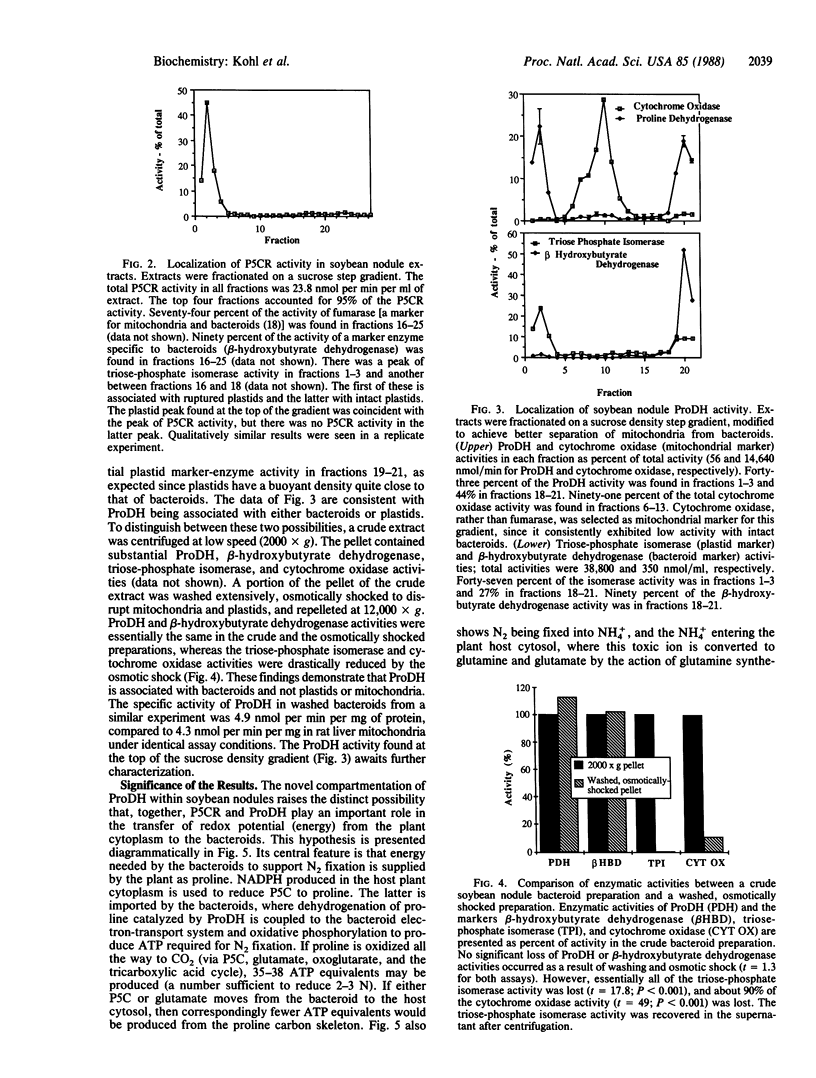
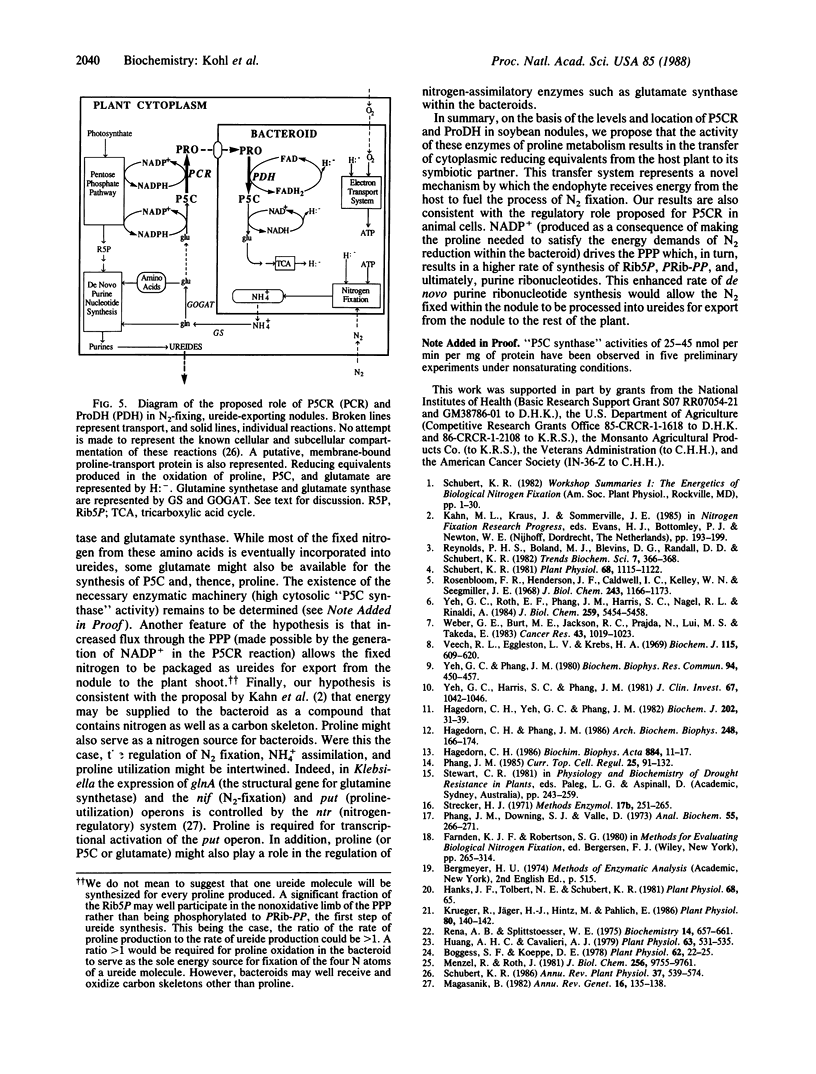
N2-fixing root nodules of soybean (Glycine max L. Merr.) convert atmospheric N2 to ammonia(um) in an energy-intensive enzymatic reaction. These nodules synthesize large quantities of purines because nitrogen fixed by bacteria contained within this tissue is transferred to the shoots in the form of ureides, which are degradation products of purines. In animal systems, it has been proposed that proline biosynthesis by pyrroline-5-carboxylate reductase (P5CR) is used to generate the NADP+ required for the synthesis of the purine precursor ribose 5-phosphate. We have examined the levels, properties, and location of P5CR and proline dehydrogenase (ProDH) in soybean nodules. Nodule P5CR was found in the plant cytosol. Its activity was substantially higher than that reported for other animal and plant tissues and is 4-fold higher than in pea (Pisum sativum) nodules (which export amides). The Km for NADPH was lower by a factor of 25 than the Km for NADH, while the Vmax with NADPH was one-third of that with NADH. P5CR activity was diminished by NADP+ but not by proline. These characteristics are consistent with a role for P5CR in supporting nodule purine biosynthesis rather than in producing proline for incorporation into protein. ProDH activity was divided between the bacteroids and plant cytosol, but less than 2% was in the mitochondria-rich fractions. The specific activity of ProDH in soybean nodule bacteroids was comparable to that in rat liver mitochondria. In addition, we propose that some of the proline synthesized in the plant cytosol by P5CR is catabolized within the bacteroids by ProDH and that this represents a novel mechanism for transferring energy from the plant to its endosymbiont.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boggess S. F., Koeppe D. E. Oxidation of proline by plant mitochondria. Plant Physiol. 1978 Jul;62(1):22–25. doi: 10.1104/pp.62.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn C. H. Demonstration of a NADPH-linked delta 1-pyrroline-5-carboxylate-proline shuttle in a cell-free rat liver system. Biochim Biophys Acta. 1986 Oct 29;884(1):11–17. doi: 10.1016/0304-4165(86)90220-5. [DOI] [PubMed] [Google Scholar]
- Hagedorn C. H., Phang J. M. Catalytic transfer of hydride ions from NADPH to oxygen by the interconversions of proline and delta 1-pyrroline-5-carboxylate. Arch Biochem Biophys. 1986 Jul;248(1):166–174. doi: 10.1016/0003-9861(86)90413-3. [DOI] [PubMed] [Google Scholar]
- Hagedorn C. H., Yeh G. C., Phang J. M. Transfer of 1-pyrroline-5-carboxylate as oxidizing potential from hepatocytes to erythrocytes. Biochem J. 1982 Jan 15;202(1):31–39. doi: 10.1042/bj2020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks J. F., Tolbert N. E., Schubert K. R. Localization of enzymes of ureide biosynthesis in peroxisomes and microsomes of nodules. Plant Physiol. 1981 Jul;68(1):65–69. doi: 10.1104/pp.68.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Cavalieri A. J. Proline Oxidase and Water Stress-induced Proline Accumulation in Spinach Leaves. Plant Physiol. 1979 Mar;63(3):531–535. doi: 10.1104/pp.63.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger R., Jäger H. J., Hintz M., Pahlich E. Purification to homogeneity of pyrroline-5-carboxylate reductase of barley. Plant Physiol. 1986 Jan;80(1):142–144. doi: 10.1104/pp.80.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B. Genetic control of nitrogen assimilation in bacteria. Annu Rev Genet. 1982;16:135–168. doi: 10.1146/annurev.ge.16.120182.001031. [DOI] [PubMed] [Google Scholar]
- Menzel R., Roth J. Purification of the putA gene product. A bifunctional membrane-bound protein from Salmonella typhimurium responsible for the two-step oxidation of proline to glutamate. J Biol Chem. 1981 Sep 25;256(18):9755–9761. [PubMed] [Google Scholar]
- Phang J. M., Downing S. J., Valle D. A radioisotopic assay for delta1-pyrroline-5-carboxylate reductase. Anal Biochem. 1973 Sep;55(1):266–271. doi: 10.1016/0003-2697(73)90312-6. [DOI] [PubMed] [Google Scholar]
- Phang J. M. The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr Top Cell Regul. 1985;25:91–132. doi: 10.1016/b978-0-12-152825-6.50008-4. [DOI] [PubMed] [Google Scholar]
- Rosenbloom F. M., Henderson J. F., Caldwell I. C., Kelley W. N., Seegmiller J. E. Biochemical bases of accelerated purine biosynthesis de novo in human fibroblasts lacking hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1968 Mar 25;243(6):1166–1173. [PubMed] [Google Scholar]
- Schubert K. R. Enzymes of Purine Biosynthesis and Catabolism in Glycine max: I. COMPARISON OF ACTIVITIES WITH N(2) FIXATION AND COMPOSITION OF XYLEM EXUDATE DURING NODULE DEVELOPMENT. Plant Physiol. 1981 Nov;68(5):1115–1122. doi: 10.1104/pp.68.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Burt M. E., Jackson R. C., Prajda N., Lui M. S., Takeda E. Purine and pyrimidine enzymic programs and nucleotide pattern in sarcoma. Cancer Res. 1983 Mar;43(3):1019–1023. [PubMed] [Google Scholar]
- Yeh G. C., Harris S. C., Phang J. M. Pyrroline-5-carboxylate reductase in human erythrocytes. J Clin Invest. 1981 Apr;67(4):1042–1046. doi: 10.1172/JCI110115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh G. C., Phang J. M. The function of pyrroline-5-carboxylate reductase in human erythrocytes. Biochem Biophys Res Commun. 1980 May 30;94(2):450–457. doi: 10.1016/0006-291x(80)91252-8. [DOI] [PubMed] [Google Scholar]
- Yeh G. C., Roth E. F., Jr, Phang J. M., Harris S. C., Nagel R. L., Rinaldi A. The effect of pyrroline-5-carboxylic acid on nucleotide metabolism in erythrocytes from normal and glucose-6-phosphate dehydrogenase-deficient subjects. J Biol Chem. 1984 May 10;259(9):5454–5458. [PubMed] [Google Scholar]


