Abstract
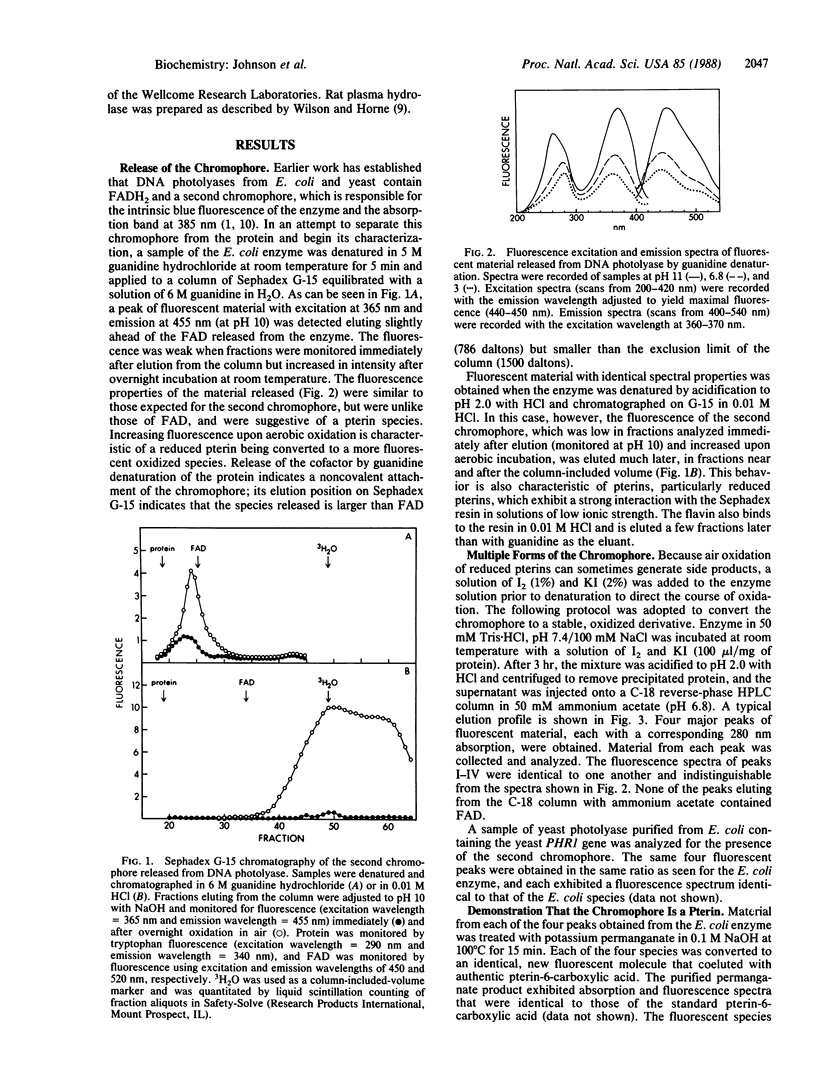
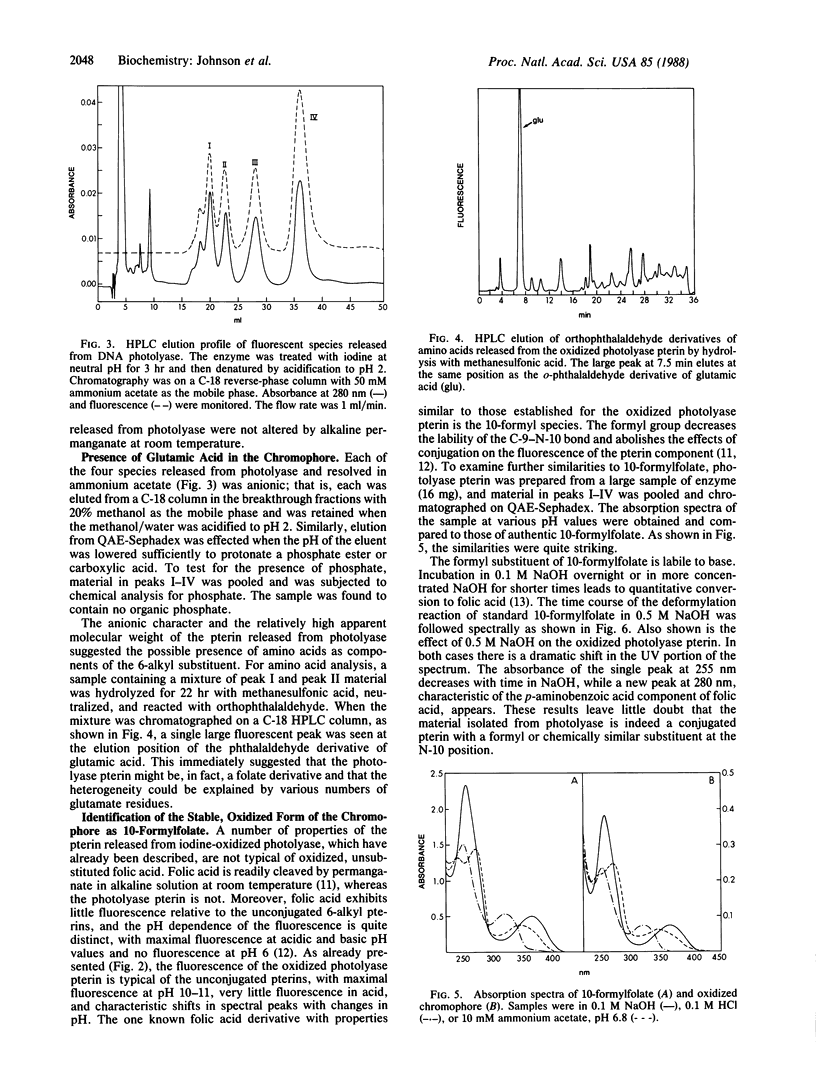
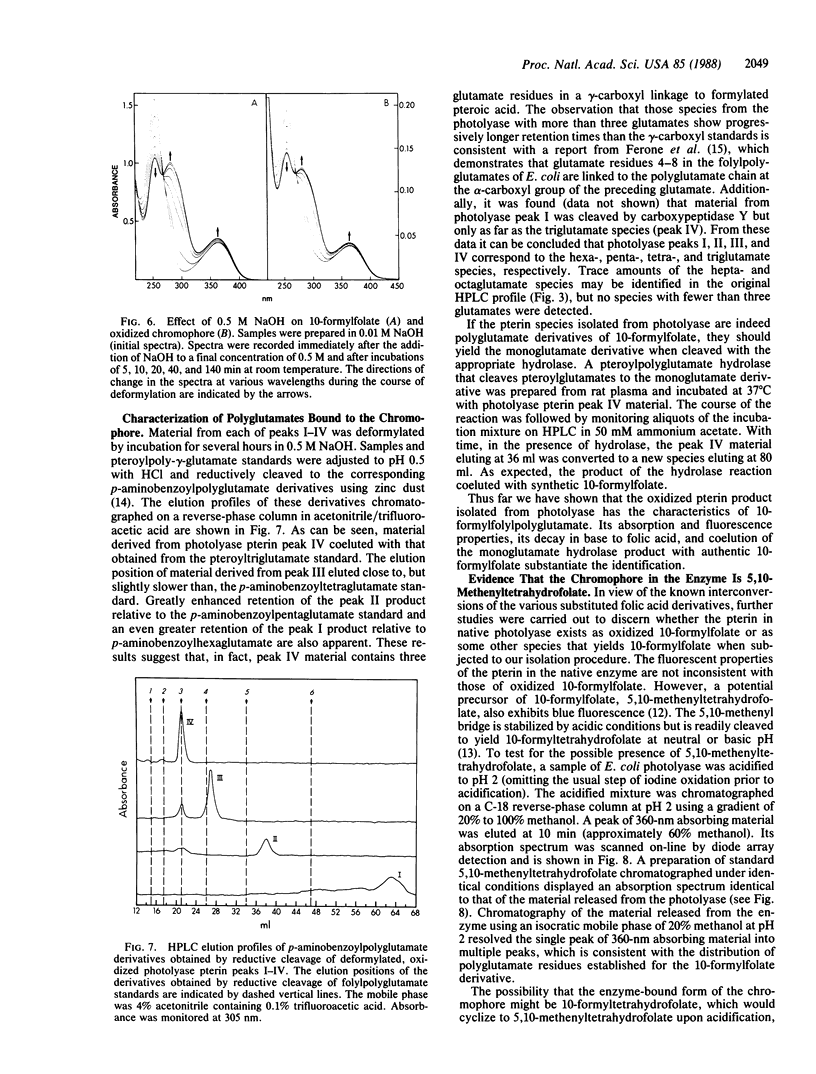
Denaturation of DNA photolyase (deoxyribodipyrimidine photolyase, EC 4.1.99.3) from Escherichia coli with guanidine hydrochloride or acidification to pH 2 released, in addition to FAD, a chromophore with the spectral and chromatographic properties of a reduced pterin. Treatment of the enzyme with iodine prior to acidification converted the chromophore to a stable, oxidized derivative, which was resolved by HPLC into four species with identical spectral properties. The same species, in the same distribution, were obtained from the yeast enzyme. The material isolated from the iodine-oxidized enzyme was shown to be a pterin by conversion to pterin-6-carboxylic acid with alkaline permanganate and was found to release glutamate upon acid hydrolysis. The presence of 10-formylfolate in the isolated, oxidized chromophore was demonstrated by absorption and fluorescence spectroscopy and by deformylation and conversion to folic acid. Analysis of the distribution of polyglutamates revealed that the four species identified by HPLC corresponded to the tri-, tetra-, penta-, and hexaglutamate derivatives of 10-formylfolate. The results were consistent with gamma linkages in the triglutamate derivative with additional glutamates linked via the alpha-carboxyl group of the preceding residue. Treatment with rat plasma hydrolase produced the monoglutamate derivative of 10-formylfolate. The native, enzyme-bound form of the folate cofactor was identified as 5,10-methenyltetrahydrofolylpolyglutamate by effecting release and isolation at low pH to protect the 5,10-methenyl bridge and preserve the reduced pyrazine ring structure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ferone R., Hanlon M. H., Singer S. C., Hunt D. F. alpha-Carboxyl-linked glutamates in the folylpolyglutamates of Escherichia coli. J Biol Chem. 1986 Dec 15;261(35):16356–16362. [PubMed] [Google Scholar]
- Foo S. K., Cichowicz D. J., Shane B. Cleavage of naturally occurring folates to unsubstituted p-aminobenzoylpoly-gamma-glutamates. Anal Biochem. 1980 Sep 1;107(1):109–115. doi: 10.1016/0003-2697(80)90499-6. [DOI] [PubMed] [Google Scholar]
- Jorns M. S., Baldwin E. T., Sancar G. B., Sancar A. Action mechanism of Escherichia coli DNA photolyase. II. Role of the chromophores in catalysis. J Biol Chem. 1987 Jan 5;262(1):486–491. [PubMed] [Google Scholar]
- Jorns M. S., Sancar G. B., Sancar A. Identification of a neutral flavin radical and characterization of a second chromophore in Escherichia coli DNA photolyase. Biochemistry. 1984 Jun 5;23(12):2673–2679. doi: 10.1021/bi00307a021. [DOI] [PubMed] [Google Scholar]
- Lewis G. P., Rowe P. B. Oxidative and reductive cleavage of folates--a critical appraisal. Anal Biochem. 1979 Feb;93(1):91–97. [PubMed] [Google Scholar]
- Payne G., Heelis P. F., Rohrs B. R., Sancar A. The active form of Escherichia coli DNA photolyase contains a fully reduced flavin and not a flavin radical, both in vivo and in vitro. Biochemistry. 1987 Nov 3;26(22):7121–7127. doi: 10.1021/bi00396a038. [DOI] [PubMed] [Google Scholar]
- Sancar A., Smith F. W., Sancar G. B. Purification of Escherichia coli DNA photolyase. J Biol Chem. 1984 May 10;259(9):6028–6032. [PubMed] [Google Scholar]
- Sancar G. B., Jorns M. S., Payne G., Fluke D. J., Rupert C. S., Sancar A. Action mechanism of Escherichia coli DNA photolyase. III. Photolysis of the enzyme-substrate complex and the absolute action spectrum. J Biol Chem. 1987 Jan 5;262(1):492–498. [PubMed] [Google Scholar]
- Sancar G. B., Smith F. W., Heelis P. F. Purification of the yeast PHR1 photolyase from an Escherichia coli overproducing strain and characterization of the intrinsic chromophores of the enzyme. J Biol Chem. 1987 Nov 15;262(32):15457–15465. [PubMed] [Google Scholar]
- UYEDA K., RABINOWITZ J. C. Fluorescence properties of tetrahydrofolate and related compounds. Anal Biochem. 1963 Jul;6:100–108. doi: 10.1016/0003-2697(63)90012-5. [DOI] [PubMed] [Google Scholar]
- Wilson S. D., Horne D. W. Use of glycerol-cryoprotected Lactobacillus casei for microbiological assay of folic acid. Clin Chem. 1982 May;28(5):1198–1200. [PubMed] [Google Scholar]


