Abstract
DNA methylation may be a component of a multilevel control mechanism that regulates eukaryotic gene expression. We used synthetic oligonucleotides to investigate the effect of cytosine methylation on the binding of the transcription factor Sp1 to its target sequence (a G + C-rich sequence known as a "GC box"). Concatemers of double-stranded 14-mers containing a GC box successfully competed with the human metallothionein IIA promoter for binding to Sp1 in DNase I protection experiments. The presence of 5-methylcytosine in the CpG sequence of the GC box did not influence Sp1 binding. The result was confirmed using double-stranded 20-mers containing 16 base pairs of complementary sequence. Electrophoretic gel retardation analysis of annealed 28-mers containing a GC box incubated with an Sp1-containing HeLa cell nuclear extract demonstrated the formation of DNA-protein complexes; formation of these complexes was not inhibited when an oligomer without a GC box was used as a competitor. Once again, the presence of a 5-methylcytosine residue in the GC box did not influence the binding of the protein to DNA. The results therefore preclude a direct effect of cytosine methylation on Sp1-DNA interactions.
Full text
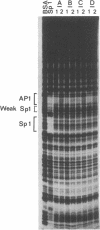
PDF


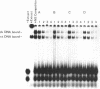
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Buchanan R. L., Gralla J. D. Factor interactions at simian virus 40 GC-box promoter elements in intact nuclei. Mol Cell Biol. 1987 Apr;7(4):1554–1558. doi: 10.1128/mcb.7.4.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschhausen G., Wittig B., Graessmann M., Graessmann A. Chromatin structure is required to block transcription of the methylated herpes simplex virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1177–1181. doi: 10.1073/pnas.84.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger M., Hurst J., Flavell R. A. DNA methylation and the regulation of globin gene expression. Cell. 1983 Aug;34(1):197–206. doi: 10.1016/0092-8674(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Christman J. K., Mendelsohn N., Herzog D., Schneiderman N. Effect of 5-azacytidine on differentiation and DNA methylation in human promyelocytic leukemia cells (HL-60). Cancer Res. 1983 Feb;43(2):763–769. [PubMed] [Google Scholar]
- Constantinides P. G., Taylor S. M., Jones P. A. Phenotypic conversion of cultured mouse embryo cells by aza pyrimidine nucleosides. Dev Biol. 1978 Sep;66(1):57–71. doi: 10.1016/0012-1606(78)90273-7. [DOI] [PubMed] [Google Scholar]
- Darmon M., Nicolas J. F., Lamblin D. 5-Azacytidine is able to induce the conversion of teratocarcinoma-derived mesenchymal cells into epithelia cells. EMBO J. 1984 May;3(5):961–967. doi: 10.1002/j.1460-2075.1984.tb01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Martin P. L., Shastry B. S., Roeder R. G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Isolation of transcription factors that discriminate between different promoters recognized by RNA polymerase II. Cell. 1983 Mar;32(3):669–680. doi: 10.1016/0092-8674(83)90053-3. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Flatau E., Bogenmann E., Jones P. A. Variable 5-methylcytosine levels in human tumor cell lines and fresh pediatric tumor explants. Cancer Res. 1983 Oct;43(10):4901–4905. [PubMed] [Google Scholar]
- Gidoni D., Dynan W. S., Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. 1984 Nov 29-Dec 5Nature. 312(5993):409–413. doi: 10.1038/312409a0. [DOI] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann M., Graessmann A., Wagner H., Werner E., Simon D. Complete DNA methylation does not prevent polyoma and simian virus 40 virus early gene expression. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6470–6474. doi: 10.1073/pnas.80.21.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heguy A., West A., Richards R. I., Karin M. Structure and tissue-specific expression of the human metallothionein IB gene. Mol Cell Biol. 1986 Jun;6(6):2149–2157. doi: 10.1128/mcb.6.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. H., Wang R., Gama-Sosa M. A., Shenoy S., Ehrlich M. A protein from human placental nuclei binds preferentially to 5-methylcytosine-rich DNA. Nature. 1984 Mar 15;308(5956):293–295. doi: 10.1038/308293a0. [DOI] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Ishii S., Kadonaga J. T., Tjian R., Brady J. N., Merlino G. T., Pastan I. Binding of the Sp1 transcription factor by the human Harvey ras1 proto-oncogene promoter. Science. 1986 Jun 13;232(4756):1410–1413. doi: 10.1126/science.3012774. [DOI] [PubMed] [Google Scholar]
- Ishii S., Merlino G. T., Pastan I. Promoter region of the human Harvey ras proto-oncogene: similarity to the EGF receptor proto-oncogene promoter. Science. 1985 Dec 20;230(4732):1378–1381. doi: 10.1126/science.2999983. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Morris J. A. Induction of prolactin-deficient variants of GH3 rat pituitary tumor cells by ethyl methanesulfonate: reversion by 5-azacytidine, a DNA methylation inhibitor. Proc Natl Acad Sci U S A. 1982 May;79(9):2967–2970. doi: 10.1073/pnas.79.9.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Luciw P. A., Tjian R. Activation of the AIDS retrovirus promoter by the cellular transcription factor, Sp1. Science. 1986 May 9;232(4751):755–759. doi: 10.1126/science.3008338. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M., Mohandas T., Shapiro L. J. Cell cycle-specific reactivation of an inactive X-chromosome locus by 5-azadeoxycytidine. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1215–1219. doi: 10.1073/pnas.79.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Heguy A., Dietlin T., Cooke T. Metal-responsive elements act as positive modulators of human metallothionein-IIA enhancer activity. Mol Cell Biol. 1987 Feb;7(2):606–613. doi: 10.1128/mcb.7.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Keshet I., Lieman-Hurwitz J., Cedar H. DNA methylation affects the formation of active chromatin. Cell. 1986 Feb 28;44(4):535–543. doi: 10.1016/0092-8674(86)90263-1. [DOI] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Role of an adenovirus E2 promoter binding factor in E1A-mediated coordinate gene control. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2180–2184. doi: 10.1073/pnas.84.8.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N. C. The effects of 5-azacytidine on the expression of the rat growth hormone gene. Methylation modulates but does not control growth hormone gene activity. J Biol Chem. 1984 Sep 25;259(18):11601–11606. [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Lieberman M. W., Beach L. R., Palmiter R. D. Ultraviolet radiation-induced metallothionein-I gene activation is associated with extensive DNA demethylation. Cell. 1983 Nov;35(1):207–214. doi: 10.1016/0092-8674(83)90223-4. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Heguy A., Karin M. Structural and functional analysis of the human metallothionein-IA gene: differential induction by metal ions and glucocorticoids. Cell. 1984 May;37(1):263–272. doi: 10.1016/0092-8674(84)90322-2. [DOI] [PubMed] [Google Scholar]
- Schneider R., Gander I., Müller U., Mertz R., Winnacker E. L. A sensitive and rapid gel retention assay for nuclear factor I and other DNA-binding proteins in crude nuclear extracts. Nucleic Acids Res. 1986 Feb 11;14(3):1303–1317. doi: 10.1093/nar/14.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R., Gruenbaum Y., Pollack Y., Razin A., Cedar H. Clonal inheritance of the pattern of DNA methylation in mouse cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):61–65. doi: 10.1073/pnas.79.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]