Abstract
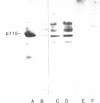
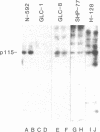
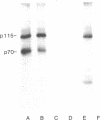
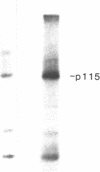
It has been hypothesized that bombesin-like peptides produced by small cell lung carcinomas may sustain deregulated proliferation through an autocrine mechanism. We have shown that the neuropeptide bombesin leads to the activation of a protein-tyrosine kinase that phosphorylates a 115-kDa protein (p115) associated with the bombesin receptor complex in mouse Swiss 3T3 fibroblasts. We now report that phosphotyrosine antibodies recognize a 115-kDa protein, phosphorylated on tyrosine, in four human small cell lung carcinoma cell lines producing bombesin but not in a nonproducer "variant" line. p115 from detergent-treated small cell lung carcinoma cells binds to bombesin-Sepharose and can be phosphorylated on tyrosine in the presence of radiolabeled ATP and Mn2+. As for the p115 immunoprecipitated from mouse fibroblast, the small cell lung carcinoma p115 can be phosphorylated in an immunocomplex kinase assay. However, the latter does not require the presence of exogenous bombesin for activity. Binding data, obtained by using radiolabeled ligand, suggest receptor occupancy in the cell lines producing bombesin. These observations are consistent with the hypothesis that proliferation in some human small cell lung carcinoma lines is under autocrine control, regulated through activation of bombesin receptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANASTASI A., ERSPAMER V. The isolation and amino acid sequence of eledoisin, the active endecapeptide of the posterior salivary glands of Eledone. Arch Biochem Biophys. 1963 Apr;101:56–65. doi: 10.1016/0003-9861(63)90533-2. [DOI] [PubMed] [Google Scholar]
- Anastasi A., Erspamer V., Bucci M. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia. 1971 Feb 15;27(2):166–167. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Westermark B., Ek B., Heldin C. H. Coexpression of a PDGF-like growth factor and PDGF receptors in a human osteosarcoma cell line: implications for autocrine receptor activation. Cell. 1984 Dec;39(3 Pt 2):447–457. doi: 10.1016/0092-8674(84)90452-5. [DOI] [PubMed] [Google Scholar]
- Bonikos D. S., Benson K. G. Endocrine cells of bronchial and bronchiolar epithelium. Am J Med. 1977 Nov;63(5):765–771. doi: 10.1016/0002-9343(77)90163-2. [DOI] [PubMed] [Google Scholar]
- Broccardo M., Falconieri Erspamer G., Melchiorri P., Negri L., de Castiglione R. Relative potency of bombesin-like peptides. Br J Pharmacol. 1975 Oct;55(2):221–227. doi: 10.1111/j.1476-5381.1975.tb07631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Blay J., Irvine R. F., Heslop J. P., Berridge M. J. Reduction of epidermal growth factor receptor affinity by heterologous ligands: evidence for a mechanism involving the breakdown of phosphoinositides and the activation of protein kinase C. Biochem Biophys Res Commun. 1984 Aug 30;123(1):377–384. doi: 10.1016/0006-291x(84)90424-8. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carney D. N., Cuttitta F., Moody T. W., Minna J. D. Selective stimulation of small cell lung cancer clonal growth by bombesin and gastrin-releasing peptide. Cancer Res. 1987 Feb 1;47(3):821–825. [PubMed] [Google Scholar]
- Cirillo D. M., Gaudino G., Naldini L., Comoglio P. M. Receptor for bombesin with associated tyrosine kinase activity. Mol Cell Biol. 1986 Dec;6(12):4641–4649. doi: 10.1128/mcb.6.12.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons D. R., Van Wyk J. J. Evidence for a functional role of endogenously produced somatomedinlike peptides in the regulation of DNA synthesis in cultured human fibroblasts and porcine smooth muscle cells. J Clin Invest. 1985 Jun;75(6):1914–1918. doi: 10.1172/JCI111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Carpenter G. Human epidermal growth factor: isolation and chemical and biological properties. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1317–1321. doi: 10.1073/pnas.72.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoglio P. M., Di Renzo M. F., Tarone G., Giancotti F. G., Naldini L., Marchisio P. C. Detection of phosphotyrosine-containing proteins in the detergent-insoluble fraction of RSV-transformed fibroblasts by azobenzene phosphonate antibodies. EMBO J. 1984 Mar;3(3):483–489. doi: 10.1002/j.1460-2075.1984.tb01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Autocrine growth factors in human small cell lung cancer. Cancer Surv. 1985;4(4):707–727. [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Di Renzo M. F., Ferracini R., Naldini L., Giordano S., Comoglio P. M. Immunological detection of proteins phosphorylated at tyrosine in cells stimulated by growth factors or transformed by retroviral-oncogene-coded tyrosine kinases. Eur J Biochem. 1986 Jul 15;158(2):383–391. doi: 10.1111/j.1432-1033.1986.tb09765.x. [DOI] [PubMed] [Google Scholar]
- Erisman M. D., Linnoila R. I., Hernandez O., DiAugustine R. P., Lazarus L. H. Human lung small-cell carcinoma contains bombesin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2379–2383. doi: 10.1073/pnas.79.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher E. R., Paulson J. D. A new in vitro cell line established from human large cell variant of oat cell lung cancer. Cancer Res. 1978 Nov;38(11 Pt 1):3830–3835. [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Ross A. H., Eisen H. N. Characterization and use of monoclonal antibodies for isolation of phosphotyrosyl proteins from retrovirus-transformed cells and growth factor-stimulated cells. Mol Cell Biol. 1983 Aug;3(8):1343–1352. doi: 10.1128/mcb.3.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris R. M., Hazan R., Villines J., Moody T. W., Schlessinger J. Identification of the bombesin receptor on murine and human cells by cross-linking experiments. J Biol Chem. 1987 Aug 15;262(23):11215–11220. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letterio J. J., Coughlin S. R., Williams L. T. Pertussis toxin-sensitive pathway in the stimulation of c-myc expression and DNA synthesis by bombesin. Science. 1986 Nov 28;234(4780):1117–1119. doi: 10.1126/science.3465038. [DOI] [PubMed] [Google Scholar]
- Little C. D., Nau M. M., Carney D. N., Gazdar A. F., Minna J. D. Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature. 1983 Nov 10;306(5939):194–196. doi: 10.1038/306194a0. [DOI] [PubMed] [Google Scholar]
- McDonald T. J., Jörnvall H., Nilsson G., Vagne M., Ghatei M., Bloom S. R., Mutt V. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun. 1979 Sep 12;90(1):227–233. doi: 10.1016/0006-291x(79)91614-0. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Bertness V., Carney D. N. Bombesin-like peptides and receptors in human tumor cell lines. Peptides. 1983 Sep-Oct;4(5):683–686. doi: 10.1016/0196-9781(83)90018-9. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Pert C. B., Gazdar A. F., Carney D. N., Minna J. D. High levels of intracellular bombesin characterize human small-cell lung carcinoma. Science. 1981 Dec 11;214(4526):1246–1248. doi: 10.1126/science.6272398. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Pert C. B., Rivier J., Brown M. R. Bomebesin: specific binding to rat brain membranes. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5372–5376. doi: 10.1073/pnas.75.11.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Palumbo A. P., Rossino P., Comoglio P. M. Bombesin stimulation of c-fos and c-myc gene expression in cultures of Swiss 3T3 cells. Exp Cell Res. 1986 Nov;167(1):276–280. doi: 10.1016/0014-4827(86)90226-0. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Ross A. H., Baltimore D., Eisen H. N. Phosphotyrosine-containing proteins isolated by affinity chromatography with antibodies to a synthetic hapten. Nature. 1981 Dec 17;294(5842):654–656. doi: 10.1038/294654a0. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Willey J. C., Lechner J. F., Harris C. C. Bombesin and the C-terminal tetradecapeptide of gastrin-releasing peptide are growth factors for normal human bronchial epithelial cells. Exp Cell Res. 1984 Jul;153(1):245–248. doi: 10.1016/0014-4827(84)90466-x. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Tremble P. M., Lavin M. F., Sunday M. E. Platelet-derived growth factor receptors form a high affinity state in membrane preparations. Kinetics and affinity cross-linking studies. J Biol Chem. 1984 Apr 25;259(8):5287–5294. [PubMed] [Google Scholar]
- Wood S. M., Wood J. R., Ghatei M. A., Lee Y. C., O'Shaughnessy D., Bloom S. R. Bombesin, somatostatin and neurotensin-like immunoreactivity in bronchial carcinoma. J Clin Endocrinol Metab. 1981 Dec;53(6):1310–1312. doi: 10.1210/jcem-53-6-1310. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Abe K., Kameya T., Adachi I., Taguchi S., Otsubo K., Yanaihara N. Production and molecular size heterogeneity of immunoreactive gastrin-releasing peptide in fetal and adult lungs and primary lung tumors. Cancer Res. 1983 Aug;43(8):3932–3939. [PubMed] [Google Scholar]
- Yang K., Ulich T., Taylor I., Cheng L., Lewin K. J. Pulmonary carcinoids. Immunohistochemical demonstration of brain-gut peptides. Cancer. 1983 Sep 1;52(5):819–823. doi: 10.1002/1097-0142(19830901)52:5<819::aid-cncr2820520512>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. High-affinity receptors for peptides of the bombesin family in Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7616–7620. doi: 10.1073/pnas.82.22.7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Identification of a receptor for peptides of the bombesin family in Swiss 3T3 cells by affinity cross-linking. J Biol Chem. 1987 Mar 25;262(9):3947–3950. [PubMed] [Google Scholar]
- Zippel R., Sturani E., Toschi L., Naldini L., Alberghina L., Comoglio P. M. In vivo phosphorylation and dephosphorylation of the platelet-derived growth factor receptor studied by immunoblot analysis with phosphotyrosine antibodies. Biochim Biophys Acta. 1986 Mar 19;881(1):54–61. doi: 10.1016/0304-4165(86)90096-6. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leij L., Postmus P. E., Buys C. H., Elema J. D., Ramaekers F., Poppema S., Brouwer M., van der Veen A. Y., Mesander G., The T. H. Characterization of three new variant type cell lines derived from small cell carcinoma of the lung. Cancer Res. 1985 Dec;45(12 Pt 1):6024–6033. [PubMed] [Google Scholar]