Abstract
Smith-Lemli-Opitz syndrome (SLOS) is an inborn error of cholesterol synthesis due to mutations of DHCR7. DHCR7 catalyzes the reduction of 7-dehydrocholesterol to yield cholesterol in the final step of cholesterol biosynthesis. Phenotypically patients with SLOS have multiple malformations, cognitive deficits, and behavioral difficulties. Impaired DHCR7 activity results in the accumulation of 7DHC and frequently decreased cholesterol in blood and tissues. Dietary cholesterol supplementation has become standard therapy for SLOS, and anecdotal reports suggest rapid, marked clinical improvement of behavior problems. Although reported in the literature, beneficial behavioral effects of dietary cholesterol supplementation have not been formally documented through a randomized clinical trial. To address this we initiated a double-masked, placebo-controlled, cross-over trial to test the hypothesis that dietary cholesterol supplementation has rapid beneficial effects on behavior. Our primary outcome measure was the hyperactivity subscale of the Aberrant Behavior Checklist (ABC). Hyperactivity is a symptom that has been reported to respond rapidly to dietary cholesterol supplementation. Secondary outcome measures included the total ABC score and other ABC subscale scores. Ten subjects completed this study. Although the trial was done under conditions similar to those reported to induce marked behavioral changes in SLOS patients, we observed no differences between treatment and placebo phases. The results of this study call into question anecdotal reports supporting rapid behavioral benefits previously reported for dietary cholesterol supplementation in SLOS and underscore the need for a larger placebo-controlled trial.
Keywords: Smith-Lemli-Opitz syndrome, SLOS, RSH syndrome, cholesterol, DHCR7, Aberrant Behavior Checklist, randomized controlled clinical trial
INTRODUCTION
Smith-Lemli-Opitz syndrome (SLOS) is an autosomal recessive inborn error of cholesterol due to mutation of the 7-dehydrocholesterol reductase gene (DHCR7) [Fitzky et al., 1998; Wassif et al., 1998; Waterham et al., 1998]. DHCR7 catalyzes the conversion of 7-dehydrocholesterol (7DHC) to cholesterol (Fig. 1A). Impaired DHCR7 enzymatic activity typically results in decreased cholesterol and increased 7DHC levels in serum and tissue. The SLOS phenotype is extremely broad (reviewed in [Porter, 2008]). However, typical physical findings include craniofacial anomalies (microcephaly, ptosis, small upturned nose, cleft palate/bifid uvula, and micrognathia), growth failure, limb anomalies (postaxial polydactyly, short proximally placed thumbs, single palmar creases, and cutaneous syndactyly of the second and third toes), brain malformations (holoprosencephaly and agenesis of the corpus callosum), congenital heart defects, gastrointestinal defects (pyloric stenosis and colonic agangliosis), and genital anomalies (hypospadias and ambiguous genitalia). SLOS also has a distinct behavioral phenotype which includes autistic manifestations [Tierney et al., 2001; Sikora et al., 2006].
Figure 1. Aberrant Behavior Checklist scores in cholesterol treated and untreated Smith-Lemli-Opitz syndrome patients.
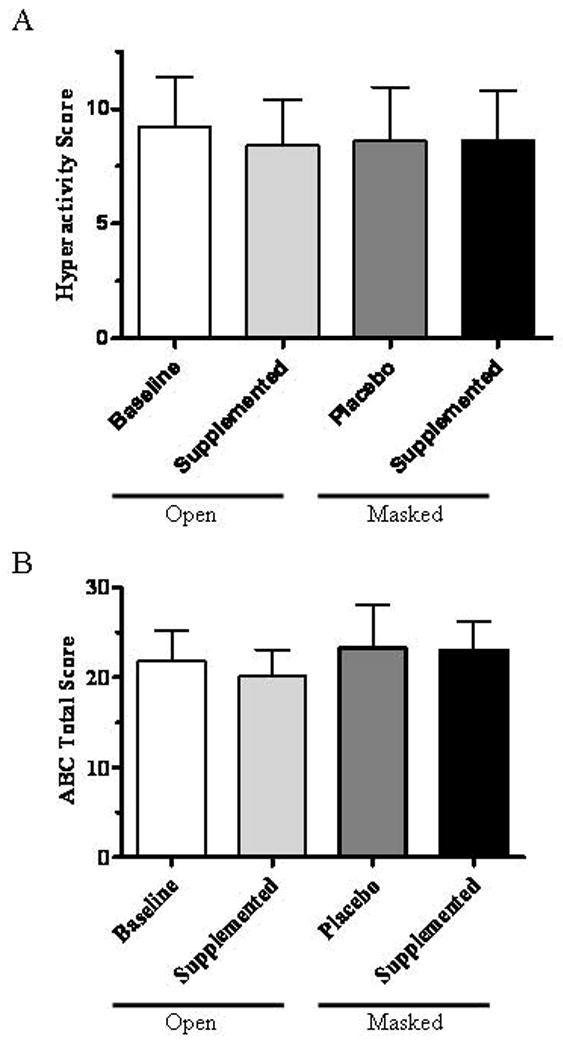
A) The mean and SEM of the hyperactivity subscale scores at the start of the study (9.2 ± 2.2), after open label supplementation with 150 mg/kg cholesterol (8.4 ± 2.0), or during either of the masked phases (8.6 ± 2.3 for the placebo phase and 8.6 ± 2.2 for the supplemented phase) did not differ significantly. B) The mean and SEM of the total ABC scores at the start of the study (21.8 ± 3.5), after open label supplementation with 150 mg/kg cholesterol (20.2 ± 2.9), or during either of the masked phases (23.2 ± 4.8 for the placebo phase and 23.0 ± 3.2 for the supplemented phase) did not differ significantly.
Once the biochemical defect in cholesterol synthesis was identified, dietary cholesterol supplementation was attempted. A series of anecdotal reports suggest marked clinical improvement and parents frequently report improved behavior within days to weeks of starting cholesterol supplementation. In six patients, after initiation of dietary cholesterol therapy, Elias et al. [1997] observed rapid improvement of hyperactivity, self-injurious behavior and irritability. Martin et al. [2001] described significant improvement in hyperactivity, aggression and self-injurious behavior within 48 hours of initiating dietary cholesterol therapy in a case report. Kelley and Hennekam [2000] reported that irritability and sleep disorders improved rapidly after initiation of therapy in contrast to other behavioral issues such as tactile sensitivity which improved over a longer period of time. Furthermore, consistent with what is described by many caretakers, they also reported that behavioral abnormalities return when dietary cholesterol therapy is interrupted. Although the idea that cholesterol supplementation improves behavior in SLOS patients has become firmly entrenched, this conclusion of efficacy is only supported by uncontrolled anecdotal evidence. Thus, we initiated a placebo-controlled study to test the hypothesis that dietary cholesterol supplementation rapidly improves various aspects of the SLOS behavioral phenotype.
MATERIALS and METHODS
Our trial used a double-blind, placebo-controlled, cross-over design, and the structure is depicted in Fig. 1B. The trial was composed of five two-week phases. Two phases, phases 2 and 4, were double-blinded. During these phases, subjects were either on placebo (Papetti Foods “Better ‘n Eggs” egg substitute) or pasteurized egg yolk (two large yolk equivalents, Papetti Foods “Easy Eggs Liquid Egg Yolks”). During the interval phases (1, 3 and 5) subjects were maintained on their baseline therapy of 150 mg/kg/d of cholesterol (Spectrum Pharmaceutical, Irvine CA) suspended in Ora-Plus (Paddock Laboratories, Minneapolis MN). Although interval periods of no cholesterol therapy would have been desirable, this design would not have been accepted by caretakers. Our primary outcome measure was the hyperactivity subscale of the Aberrant Behavior Checklist (ABC). Hyperactivity is a symptom that has been reported to respond rapidly to dietary cholesterol supplementation. The ABC is a validated behavioral assessment tool that has five behavioral domains (irritability, lethargy, stereotypy, hyperactivity, and inappropriate behavior) [Aman et al., 1985a, b]. Secondary outcome measures included the total ABC score as well as the domain scores for irritability, lethargy, stereotypy and inappropriate behavior. The ABC checklist was completed by a single caretaker at baseline and then after each phase (weeks 2, 4, 6, 8 and 10). Thirteen subjects were enrolled between October 2005 and July 2008, and 10 subjects completed the study. One patient completed the study but the assessment forms were lost prior to being returned to the study coordinator. Two subjects were withdrawn during the study due to failure to complete and return the assessment forms.
RESULTS
Demographic information for participants that completed the study is shown in Table I. Mean and median ages were 11.4 and 12.1 years, respectively, and age ranged from 4.6 to 19.9 years. The patients in this study all had mild to classical SLOS with severity scores [Kelley and Hennekam, 2000] that ranged from 6 to 28 with a median score of 17. Our primary outcome measure was the ABC hyperactivity subscale. Comparing hyperactivity scores at baseline (week 0), after the interval phases (weeks 2, 6, 10), after placebo (week 4 or 8) and after cholesterol supplementation (week 4 or 8), we found no significant differences (Fig. 1A). Similarly, a comparison of total ABC scores at baseline (week 0), after the interval phases (weeks 2, 6, 10), after placebo (week 4 or 8) and after cholesterol supplementation (week 4 or 8) showed no significant differences (Fig. 1B). Specifically, we observed no significant differences between the scores for the masked phases for either the hyperactivity subscore (p=1.0, paired t-test) or the total score (p=0.97, paired t-test). Fig. 2 presents a comparison of the treated and untreated masked phases for the five ABC subscales. None showed significant differences.
Table 1.
Patient Demographics
| Patient | Age (years) | Gender | Severity Score1 | Cholesterol mg/dl2 | 7-DHC mcg/ml2 | Genotype |
|---|---|---|---|---|---|---|
| 1 | 5.4 | Male | 6 | 36 | 128 | c.964-1G>C/p.A247V |
| 2 | 4.6 | Female | 22 | 55 | 410 | p.T93M/p.R443C |
| 3 | 9.8 | Female | 6 | 100 | 26 | p.P51S/p.W151X |
| 4 | 6.3 | Female | 28 | 56 | 273 | c.964-1G>C/p.T93M |
| 5 | 11.9 | Female | 17 | 70 | 135 | c.964-1G>C/p.Y318N |
| 6 | 16.1 | Male | 11 | 117 | 59 | pC380Y/p.E448K |
| 7 | 13.0 | Male | 6 | 89 | 52 | c.964-1G>C/pT289I |
| 8 | 12.3 | Male | 28 | 18 | 80 | p.V326L/unk3 |
| 9 | 14.3 | Female | 17 | 94 | 81 | p.R242C/p.W177R |
| 10 | 19.9 | Female | 22 | 79 | 142 | c.964-1G>C/p.R352W |
See reference [Kelley and Hennekam, 2000]
Initial Values
Unknown. Sequencing of genomic DNA showed that the patient is heterozygous for the pV326L mutation. A second mutation was not detected in either coding regions or splice site junctions.
Figure 2. Aberrant Behavior Checklist Subscale scores.
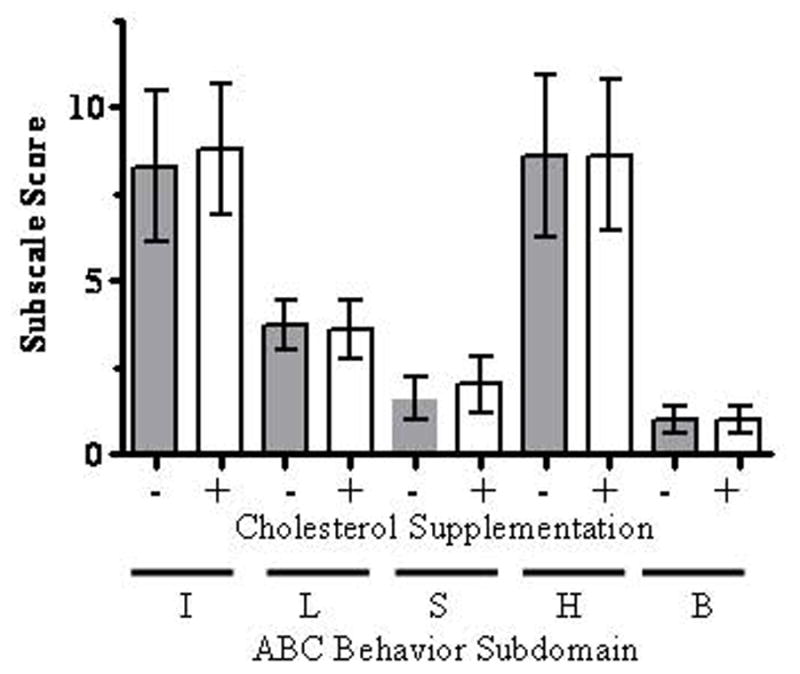
Subscale scores for irritability (I), lethargy (L), stereotypy (S), hyperactivity (H), and inappropriate behavior (B) did not differ significantly between the masked phases. Data are presented as the mean and SEM.
Due to the large range of scores, we were concerned that average values may have obscured changes in individual subjects. Thus, we compared the change in scores between the cholesterol-treated phase and the placebo-treated phase. A negative value would indicate improved behavior during the cholesterol supplementation phase. Comparing values obtained after the masked placebo and cholesterol treated phases, the hyperactivity subscale scores improved in two subjects, worsened in one, and were essentially unchanged in seven. Similarly total ABC scores improved in four subjects, worsened in four subjects and were unchanged in two subjects. Fig. 3A shows the change in total score between the untreated and treated phases, and Fig. 3B plots the data for the five ABC behavioral domains.
Figure 3. Individual changes in Aberrant Behavior Checklist scores.
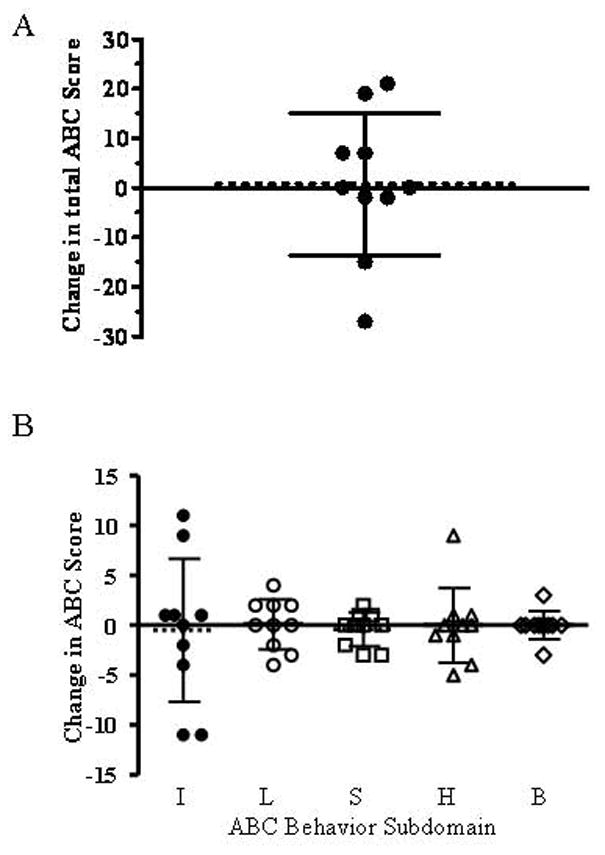
The change in the individual subjects total ABC score (A) and subdomain scores (B) are presented. Decreased scores indicate improved behavior. The mean score is indicated by a dashed line and the error bars indicate the standard deviation. Subdomain scales include irritability (I), lethargy (L), stereotypy (S), hyperactivity (H), and inappropriate behavior (B). Mean values were −0.5, 0.1, −0.4, 0, and 0 points respectively.
DISCUSSION
Dietary cholesterol therapy has become standard-of-care for SLOS. Dietary cholesterol supplementation will supplement the peripheral cholesterol deficiency and thus, likely improves the health of patients with SLOS [Kelley and Hennekam, 2000]. However, dietary cholesterol does not cross the blood-brain barrier (reviewed by [Dietschy, 2009]), thus it is not clear how dietary cholesterol supplementation could functionally have positive effects on mental development or behavior. In addition, the very slow turnover rates of brain cholesterol, especially in myelin, further complicate attempts to explain how dietary cholesterol could directly have a rapid functional effect on the central nervous system. Given these known confounding issues, various potential mechanisms, such as neuroactive steroid production, have been invoked to try to explain anecdotal reports of rapid improvement in behavior with dietary cholesterol supplementation [Marcos et al., 2004]. Unfortunately, the anecdotal reports of improved learning and behavior have not been documented in formal studies, thus placebo effects are a potential confounding factor. Sikora et al. [2004] did not observe any improvement in development quotients, and our current data do not support the hypothesis that rapid behavioral improvement occurs in response to dietary cholesterol supplementation in SLOS patients. The current study has several limitations. First, due to low enrollment, it is underpowered. Power to detect a 50% change in the hyperactivity domain and ABC total score were 45% and 67%, respectively. We initially planned to evaluate forty subjects; however, even though this was an “at home” study, recruitment proved to be extremely difficult. Major contributing factors to the poor recruitment were assumptions by caretakers that dietary cholesterol therapy has already been proven to be effective in improving behavior and concern that withdrawing cholesterol therapy would result in major behavioral disturbances. These caretaker biases underscore the critical need for well-controlled studies before therapeutic interventions become established as “standard-of-care” based on anecdotal reports of efficacy. A second limitation is that the two-week time period may not have been sufficient to demonstrate differences. Although this time period was consistent with anecdotal reports of rapid change [Elias et al., 1997; Kelley and Hennekam, 2000; Martin et al., 2001] and replicates the conditions under which parents frequently report behavioral changes related to cholesterol supplementation, it is possible that longer phases would be necessary to observe behavioral changes. Again, trial design was limited by preexisting bias and what would be an acceptable period of time off of cholesterol supplementation for caretakers. Finally, the ABC may not be a sensitive instrument to detect the behavioral changes that were previously reported. However, the irritability and hyperactivity domains of the ABC ascertain symptoms that have been reported to show rapid changes upon cholesterol supplementation. Although a negative conclusion can always be critiqued, one needs to consider the results of our study in the context of the evidence supporting the idea that dietary cholesterol supplementation has rapid positive effects on behavior in SLOS. This study raises the concern that the previous anecdotal reports simply reflect biased reporting of placebo effects. It also clearly demonstrates, due to the finding that no parents opted out of a blinded phase, that cholesterol therapy can be withdrawn for at least two weeks without major behavioral disturbances. Thus, this study can be used to encourage parents to participate in future trials requiring placebo controls. Cholesterol replacement therapy is clearly indicated for health reasons in many SLOS children. However, it is not clear that perceived behavioral benefits provide a valid rational for cholesterol supplementation in mild SLOS patients with normal cholesterol levels. These issues will only be resolved by a properly powered placebo-controlled trial. The continued acceptance of therapeutic efficacy based on non-controlled, anecdotal reports only serves to delay the development of therapeutic interventions that hold the potential of directly affecting brain cholesterol metabolism.
Acknowledgments
This work was supported by both intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Kennedy Krieger Institute. The authors would especially like to thank the children and parents who participated in this study.
References
- Aman MG, Singh NN, Stewart AW, Field CJ. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985a;89:485–491. [PubMed] [Google Scholar]
- Aman MG, Singh NN, Stewart AW, Field CJ. Psychometric characteristics of the aberrant behavior checklist. Am J Ment Defic. 1985b;89:492–502. [PubMed] [Google Scholar]
- Dietschy JM. Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol Chem. 2009;390:287–293. doi: 10.1515/BC.2009.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias ER, Irons MB, Hurley AD, Tint GS, Salen G. Clinical effects of cholesterol supplementation in six patients with the Smith-Lemli-Opitz syndrome (SLOS) Am J Med Genet. 1997;68:305–310. doi: 10.1002/(sici)1096-8628(19970131)68:3<305::aid-ajmg11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Fitzky BU, Witsch-Baumgartner M, Erdel M, Lee JN, Paik YK, Glossmann H, Utermann G, Moebius FF. Mutations in thedelta7-sterol reductase gene in patients with the Smith-Lemli-Opitz syndrome. Proc Natl Acad Sci USA. 1998;95:8181–8186. doi: 10.1073/pnas.95.14.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley RI, Hennekam RC. The Smith-Lemli-Opitz syndrome. J Med Genet. 2000;37:321–335. doi: 10.1136/jmg.37.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos J, Guo LW, Wilson WK, Porter FD, Shackleton C. The implications of 7-dehydrosterol-7-reductase deficiency (Smith-Lemli-Opitz syndrome) to neurosteroid production. Steroids. 2004;69:51–60. doi: 10.1016/j.steroids.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Martin A, Koenig K, Scahill L, Tierney E, Porter FD, Nwokoro NA. Smith-Lemli-Opitz syndrome. J Am Acad Child Adolesc Psychiatry. 2001;40:506–507. doi: 10.1097/00004583-200105000-00008. [DOI] [PubMed] [Google Scholar]
- Porter FD. Smith-Lemli-Opitz syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet. 2008;16:535–541. doi: 10.1038/ejhg.2008.10. [DOI] [PubMed] [Google Scholar]
- Sikora DM, Pettit-Kekel K, Penfield J, Merkens LS, Steiner RD. The near universal presence of autism spectrum disorders in children with Smith-Lemli-Opitz syndrome. Am J Med Genet A. 2006;140:1511–1518. doi: 10.1002/ajmg.a.31294. [DOI] [PubMed] [Google Scholar]
- Sikora DM, Ruggiero M, Petit-Kekel K, Merkens LS, Connor WE, Steiner RD. Cholesterol supplementation does not improve developmental progress in Smith-Lemli-Opitz syndrome. J Pediatr. 2004;144:783–791. doi: 10.1016/j.jpeds.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Tierney E, Nwokoro NA, Porter FD, Freund LS, Ghuman JK, Kelley RI. Behavior phenotype in the RSH/Smith-Lemli-Opitz syndrome. Am J Med Genet. 2001;98:191–200. doi: 10.1002/1096-8628(20010115)98:2<191::aid-ajmg1030>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Wassif CA, Maslen C, Kachilele-Linjewile S, Lin D, Linck LM, Connor WE, Steiner RD, Porter FD. Mutations in the human sterol delta7-reductase gene at 11q12–13 cause Smith-Lemli-Opitz syndrome. Am J Hum Genet. 1998;63:55–62. doi: 10.1086/301936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Wijburg FA, Hennekam RC, Vreken P, Poll-The BT, Dorland L, Duran M, Jira PE, Smeitink JA, Wevers RA, Wanders RJ. Smith-Lemli-Opitz syndrome is caused by mutations in the 7-dehydrocholesterol reductase gene. Am J Hum Genet. 1998;63:329–338. doi: 10.1086/301982. [DOI] [PMC free article] [PubMed] [Google Scholar]


