Abstract
Nonalcoholic Steatohepatitis (NASH) is a chronic progressive liver disease which is strongly associated with obesity. Currently, there is no approved therapy for NASH. Weight reduction is typically recommended but efficacy data is lacking. We performed a randomized controlled trial to examine the effects of lifestyle intervention using a combination of diet, exercise and behavior modification with a goal of 7-10% weight reduction on clinical parameters of NASH. The primary outcome measure was the change in NASH histologic activity score (NAS) after 48 weeks of intervention. Thirty one overweight or obese individuals (Body mass index 25-40 kg/m2) with biopsy proven NASH were randomized in a 2:1 ratio to receive intensive lifestyle intervention (LS) or structured education (control). After 48 weeks of intervention, participants assigned to LS lost an average of 9.3% of their weight vs 0.2% in the control group (p=0.003). A higher proportion of participants in the LS group had a reduction of NAS ≥ 3 points or had post treatment NAS ≤ 2 as compared to the control group (72% vs 30%, p=0.03). NAS improved significantly in the LS group (from 4.4 to 2.0) in comparison to the control group (from 4.9 to 3.5) (p=0.05). Percent weight reduction correlated significantly with improvement in NAS (r=0.497, p=0.007). Participants who achieved study weight loss goal (≥7%), compared to those lost <7%, had significant improvements in steatosis (−1.36 vs −0.41, p<0.001), lobular inflammation (−0.82 vs −0.24, p=0.03), ballooning injury (−1.27 vs −0.53, p=0.03) and NAS (−3.45 vs −1.18, p<0.001). Conclusion: Weight reduction achieved through lifestyle intervention leads to improvements in liver histology in NASH.
Keywords: nonalcoholic steatohepatitis, fatty liver, steatosis, lifestyle intervention, weight loss, cirrhosis
Nonalcoholic steatohepatitis (NASH) is a chronic liver disease characterized by accumulation of fat in the liver accompanied by necroinflammation and hepatocellular injury(1). Despite being one of the most common chronic liver diseases in the U.S., there is currently no approved pharmacologic therapy for this condition(2). An effective medical treatment of NASH is clearly needed since without treatment this disease can progress to cirrhosis and liver failure in a significant proportion of cases(3). Several pharmaceutical interventions have been evaluated but none has been approved for general use(4, 5). Clinical trials of insulin-sensitizing agents such as thiazolidinediones have shown promising results(6-8) but side effects and the need for long term therapy may limit widespread acceptance(9).
Obesity is considered one of the most important risk factors for this condition(10). Weight reduction is generally recommended as an initial step in the management of NASH(11). However, the efficacy of weight reduction for the treatment of NASH has not been carefully evaluated(12, 13). Prior studies of the effects of weight reduction on NASH have been uncontrolled, used poorly defined patient populations and non-standardized weight loss interventions, and lacked a well-accepted primary outcome for NASH(12, 13). The objective of this study was to conduct a randomized controlled trial of a year-long weight reduction in the management of NASH using a standardized state-of-the-art lifestyle intervention program. Overweight or obese individuals with biopsy-proven NASH were randomized to receive either standard medical care and educational sessions related to NASH, healthy eating, weight loss, and exercise (control group); or to an intensive weight management with a goal of at least 7-10 % weight reduction (lifestyle intervention group). The weight loss intervention was modeled on interventions that have been successful in other overweight populations(14) and was similar to the programs implemented in DPP (Diabetes Prevention Program)(15, 16) and Look AHEAD(17, 18), an ongoing study with overweight individuals with type 2 diabetes. We hypothesized that a 7-10% weight reduction through intensive lifestyle intervention would lead to improvements of biochemical and histological features of NASH. The primary outcome measure was improvement in NASH activity score (NAS) of at least 3 points or post treatment-NAS of 2 points or less.
Methods
Participants
We recruited 65 participants between January 2005 to February 2007 through newspaper advertisement, and contacts with local physicians in the Rhode Island area. To be eligible, participants were required to have elevated alanine or aspartate aminotransferase values (ALT >41 or AST>34 U/L), body mass index between 25-40 Kg/m2 and no evidence of another form of liver disease. All participants were required to complete a 2-week run-in period consisting of completion of self-monitoring records of diet and exercise. Major exclusion criteria were significant alcohol consumption (> 1 standard drink per day), contraindications to obtaining a liver biopsy, inability to walk 2 blocks or a quarter of a mile without stopping, pregnancy, engagement in an active weight loss program or taking weight-loss medication, substance abuse, and significant psychiatric problems.
After a successful completion of a 2-week run-in period, a liver biopsy was performed. Only participants who fulfilled the histologic criteria for steatohepatitis were enrolled in the weight management programs. Evidence of steatohepatitis on liver biopsy was defined as presence of (a) macrovesicular steatosis, (b) lobular inflammation and (c) acinar zone 3 hepatocellular injury or ballooning degeneration(19). Presence of all 3 components is required for study inclusion. Additionally helpful, but not required, features included the presence of Mallory's hyalin and perisinusoidal fibrosis that predominantly involved zone 3. The protocol was approved by the institutional review board at the Rhode Island Hospital, Providence; written informed consent was obtained from all participants.
Study Design
Participants who fulfilled all inclusion criteria and had no exclusion criteria were randomly assigned to a lifestyle intervention group or a control group in a 2:1 ratio. Randomization was performed using a random number generator developed by the project statistician with a target enrollment of 30 participants. Sample size was calculated to detect a difference in weight change of 7.5% between the intervention and control group using a two-sided test with α = .05 and power = .8. Previous studies using the same lifestyle intervention achieved a 9.1±5.3% weight loss at 1 year and less than 1% weight loss in control group. There were no available data at the time of study design to estimate histologic response with lifestyle intervention or control. Randomization process was conducted by a project staff who was blinded to the randomization sequence. Data collection was obtained by trained staff who were not aware of the group assignment or sequence of measurement.
All participants, regardless of group assignment were seen by a hepatologist (study principal investigator) every 12 weeks and had a standard care of their liver disease. Fasting (12-hour) blood sample was obtained at each visit. At the end of the 48-week intervention, participants underwent a repeat liver biopsy to compare with their pre-intervention biopsy. Participants were given an honorarium of $100 at completion of the trial.
Interventions
1) Control group
Participants in this group attended small group sessions providing basic education about NASH, and about principles of healthy eating, physical activity and weight control. These sessions occurred every 12 weeks and were conducted by a Master's level nutritionist or health educator. Participants were not taught specific behavioral self-regulation skills to help them change behaviors. Providing basic education about diet and exercise has produced minimal weight loss in other clinical trials(16, 20). The educational sessions were included in this study in order to provide standard care to these patients and to maximize subject retention.
2) Lifestyle Intervention
Participants randomized to the Lifestyle Intervention received an intensive, state-of-the-art weight loss intervention based on strategies used successfully in the Diabetes Prevention Program (DPP), Look AHEAD, and in several behavioral trials(21, 22). The intervention focused on changing both eating and exercise habits with a goal of producing a 7-10% weight loss within the first 6 months and then maintaining this weight loss. Participants who were able to lose more than 10% of their body weight were encouraged to do so.
Participants were seen in small groups (3 to 5 members) conducted by a Master's level nutritionist or health educator. Groups met weekly for the first 6 months and then bi-weekly for months 7-12. All participants were given the same curriculum, using a standardized treatment manual based on the DPP and updated to include treatment strategies shown recently to improve weight loss (e.g. portion-controlled diets(21) and higher exercise goals(22)).
The lifestyle intervention focused on diet, exercise, and behavior modification:
Diet
All participants were assigned a calorie goal based on their starting weight (1000-1200 kcal/day if baseline weight < 200 lb or 1200-1500/day if baseline weight > 200 lb) and a daily fat gram goal designed to produce a 25% fat diet (28 –33 g for 1000-1200 kcal diet or 33-42 g for 1200-1500 kcal diet). These calorie and fat goals have produced weight losses of 0.5 – 1.0 kg/week in previous studies(21, 23). The dietary recommendations in this study were consistent with the recommendations of the American Heart Association, the American Diabetic Association, and the American College of Sports Medicine. During the first 8 weeks of the program, participants were given meal plans that provided different options for meals and snacks that would fit within their calorie goals and included use of commercially available portion-controlled foods such as Slimfast and Lean Cuisine. The use of a portion-controlled foods is based on several recent studies indicating that this approach promotes dietary adherence and consequently weight loss through at least 12-18 months(20-22, 24). Over time, participants transitioned to more self-selected diets. Participants were taught the basic principles of healthy eating from the Food Guide Pyramid and encouraged to increase fiber and decrease saturated fats and cholesterol intake.
Physical activity
The physical activity intervention relied heavily on unsupervised exercise, since that has been more effective for long-term adherence than supervised exercise(25). The program focused on moderate intensity activities, with particular emphasis on walking. All participants were given pedometers and encouraged to gradually increase their walking until reaching 10,000 steps per day. Other activities such as bicycling, aerobic dance, and strength training were also encouraged. Participants were instructed to gradually progress to a goal of 200 minutes per week of moderate intensity physical activity (achieving this goal by the end of the first 6 months). The 200-minute goal is selected in preference to a 150-or 175-minute goal since greater amounts of activity have been associated with better long-term weight loss results(26). To improve adherence to exercise, participants were encouraged to accumulate exercise through multiple short bouts of exercise (at least 10 min in duration).
Behavioral strategies
Behavioral strategies were used to produce and maintain changes in diet and activity. All participants were encouraged to self-monitor their eating and exercise (recording all foods eaten, and calories and fat grams in their foods and minutes of activity) daily throughout the entire weight loss program. Self-monitoring records were reviewed weekly by the therapist in collaboration with the participant to identify areas of progress and areas where further change would be advantageous. Other key behavioral strategies such as stimulus control techniques, problem solving(27) and relapse prevention(28) were taught in the individual weekly group sessions. Participants set individual behavioral goals with the case manager and brainstormed solutions to any barriers to achieving the weight loss, activity or dietary goals.
Management of Diabetes medications during study period
Clinical trials suggest that insulin sensitizing agents (thiazolidinediones and metformin) may have biochemical and histological effects on NASH. To avoid the potential confounding effects from these medications, participants were not allowed to start on any of these medications during the entire study period. Participants were allowed to start a new medication for management of hyperglycemia if medically necessary. Sulfonylureas, meglitinides and insulin were available options. Participants who were already taking TZD or metformin must be on a stable regimen for at least 6 months prior to study enrollment and initial liver biopsy. The dose of these medications must remain stable during the study. The rationale was that patients who have been on these medications and continue to have active NASH should be allowed to participate in the study to maximize generalizability. These medications should have minimal or no effect on hepatic histology during the study period.
Exercise and reduced caloric consumption can produce hypoglycemia in patients with type 2 diabetes who are on insulin or sulfonylureas. Dose adjustment of these medications was conducted according to study protocol.
Study End Points
All liver biopsies were read by an expert hepatopathologist who was not aware of the treatment assignment or clinical information. Weighted kappa scores showed a high degree of intra-rater agreement for these findings (Steatosis grade: 0.85, Fibrosis stage: 0.79, Lobular inflammation: 0.91, and Ballooning degeneration: 0.7). The primary end point was an improvement in NASH Activity Score (NAS) after 48 weeks of intervention as determined by liver biopsies performed before and at the end of treatment. The definition of histologic improvement was a reduction in NAS by at least 3 points or post-treatment NAS of 2 points or less. The NAS ranges from 0 to 8 (highest activity) and is calculated as the sum of scores of the three components of the histologic scoring system [NAS = steatosis (0–3) + lobular inflammation (0–3) + hepatocyte ballooning (0–2)]. The score was derived as a simple sum of the three component scores that were independently associated with the distinction between NASH and non-NASH. The histologic scoring system was developed and validated by the NASH Clinical Research Network (NASH-CRN) pathology committee and currently recommended for NASH-related clinical trials(19).
Statistical Analyses
Statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS 14.0 for Windows). Comparisons between treatment groups on relevant baseline variables and demographic characteristics were conducted using analysis of variance for continuous variables and chi-square tests for categorical variables. Analysis of covariance, using baseline values as covariates, was used to compare the LS and Control groups on changes in weight, waist circumference, liver chemistry, insulin sensitivity, lipid profile variables, HbA1C levels and histologic variables. Chi-square tests were used for all cross-sectional tests of proportions, and correlations (Pearson's r) were used to examine the relationships between percent weight change and changes in ALT values, degree of hepatic steatosis, and NASH activity score (NAS).
Results
Characteristics of the Participants
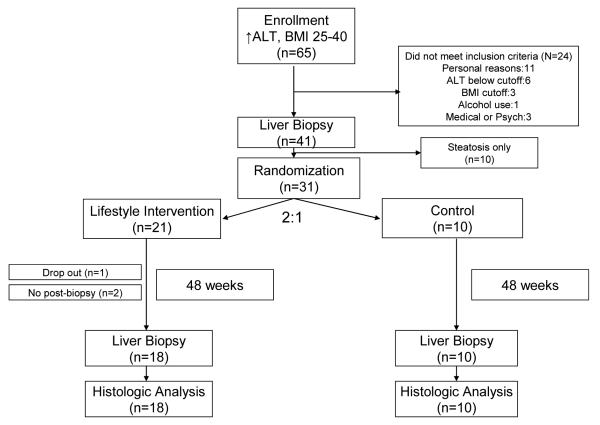
Sixty five subjects were enrolled into the screening phase of the study, 31 subjects completed the screening evaluation and underwent randomization (see figure 1). The baseline characteristics of the participants who underwent randomization are shown in Table 1. The mean age was 48 years and the mean BMI was 34 kg/m2. Most participants (71%) were men. Twenty-six participants (86%) were whites, 4 participants (9%) were Hispanics and 1 participant (5%) was American Indian/Alaska Native. About half of the participants (48%) had Type 2 diabetes and 74% fulfilled the diagnostic criteria for the metabolic syndrome (29). Twenty one participants were assigned to the lifestyle intervention group and 10 participants were assigned to the control group. None of the baseline characteristics differed significantly between the two groups.
Figure 1.
Enrollment of study participants.
Table 1.
Baseline Characteristics of Participants
| Variable (SD) | Control (n=10) | LS (n=21) | P Value |
|---|---|---|---|
| Age (yr) | 47.6 (12.0) | 48.9 (10.9) | 0.78 |
| Sex (M/F) | 8/2 | 14/7 | 0.68 |
| Weight (kg) | 102.9 (20.2) | 98.9 (23.9) | 0.66 |
| BMI (Kg/m2) | 33.7 (4.7) | 33.9 (5.3) | 0.91 |
| Waist Circumference (cm) |
111.3 (11.2) | 109.7 (14.0) | 0.74 |
| ALT (U/L) | 85.5 (36.5) | 85.6 (38.8) | 0.99 |
| AST (U/L) | 66.0 (46.3) | 57.5 (24.9) | 0.51 |
| Cholesterol (mg/dL) | 235.2 (56.8) | 204.8 (50.0) | 0.14 |
| LDL (mg/dL) | 156.1 (47.7) | 126.2 (41.0) | 0.10 |
| HDL (mg/dL) | 39.9 (9.7) | 41.4 (10.2) | 0.70 |
| Triglycerides (mg/dL) | 208.4 (104.8) | 193.1 (120.0) | 0.73 |
| Glucose (mg/dL) | 152.0 (87.2) | 121.1 (28.1) | 0.16 |
| HbA1C (%) | 6.8 (2.5) | 6.3 (0.9) | 0.43 |
| Insulin (μU/ml) | 20.2 (13.3) | 23.7 (12.2) | 0.48 |
| HOMA | 7.3 (6.2) | 7.3 (4.5) | 0.99 |
| Metabolic syndrome (%) |
6 (60%) | 17 (81%) | 0.38 |
| Diabetes (%) | 4 (40%) | 11 (52%) | 0.52 |
| Metformin (%) | 3 (30%) | 6 (29%) | 0.94 |
| TZD (%) | 0 | 2 (10%) | 0.82 |
| Insulin (%) | 0 | 1 (5%) | 1.00 |
Data expressed as number (%) or mean (SD)
Thirty participants (97%) completed the study. One participant (3%) in the lifestyle intervention group withdrew from the study after 3 months. All other participants adhered to the study protocol follow-up schedule. Post intervention liver biopsy was completed in 28/31 (90%) of participants, 18/21 (86%) in the lifestyle intervention group and 10/10 (100%) in the control group. The reasons for the lack of follow up biopsy were anticoagulation therapy (1), technical difficulty (1) and withdrawal from the study (1).
Weight Change
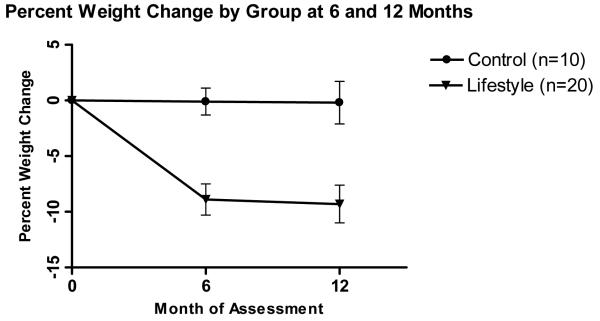
The mean weight change over the 48-week period was −8.7 kg (95% CI, −11.7 to −5.6) in the lifestyle intervention group as compared with −0.5 kg (95% CI, −4.8 to 3.8) in the control group (p=0.005) (Table 2). Percent weight reduction (SD) of participants in the lifestyle group was significantly greater than participants in the control group at 24 weeks (8.9 [6.3]% vs 0.1 [3.7]%, p<0.001) and at 48 weeks (9.3 [7.5]% vs 0.2 [6.1]%, p=0.003) (Figure 2a) Eight participants (40%) in the lifestyle intervention group achieved a ≥10% weight reduction while no participant (0%) in the control group achieved this degree of weight reduction (p=0.02).
Table 2.
Change in physical and biochemical parameters after 48 weeks of intervention*
| Variable | Group | Baseline | End of Study | P-value# |
|---|---|---|---|---|
| Weight (Kg) | Control | 102.9 (20.2) | 102.4 (19.2) | 0.005 |
| LS | 98.9 (24.5) | 90.2 (25.9) | ||
| BMI (kg/m2) | Control | 33.7 (4.7) | 33.6 (5.0) | 0.004 |
| LS | 33.6 (5.3) | 30.6 (6.2) | ||
| Waist Circ (cm) | Control | 111.3 (11.2) | 111.6 (11.2) | 0.004 |
| LS | 109.3 (14.3) | 101.8 (18.4) | ||
| ALT (U/L) | Control | 85.5 (36.5) | 69.0 (38.5) | 0.01 |
| LS | 84.1 (39.1) | 41.7 (20.8) | ||
| AST (U/L) | Control | 66.0 (46.3) | 48.0 (17.4) | 0.11 |
| LS | 56.9 (25.4) | 36.7 (15.0) | ||
| Glucose (mg/dL) |
Control | 152.0 (87.2) | 121.4 (37.1) | 0.88 |
| LS | 121.6 (28.7) | 111.7 (22.6) | ||
| HbA1C (%0 | Control | 6.8 (2.5) | 6.1 (1.2) | 0.52 |
| LS | 6.3 (1.0) | 5.8 (0.7) | ||
| HOMA | Control | 7.3 (6.2) | 7.4 (5.6) | 0.37 |
| LS | 7.4 (4.6) | 5.4 (6.1) | ||
| Insulin (μU/ml) | Control | 20.2 (13.3) | 23.2 (13.9) | 0.30 |
| LS | 23.7 (12.5) | 18.5 (18.8) |
Control group, N=10, LS group, N=20
Data expressed as mean (SD).
P-value compares the mean difference between the pre- and post-treatment changes in the variables between the two groups
Figure 2a.
Percent weight change (mean±SE) by group at 6 and 12 months. P<.001 for the comparison between lifestyle and control at month 6, and P = 0.003 for the comparison at month 12.
There was a non-significant trend for greater percent weight reduction in participants without underlying diabetes (n=16) compared to those with diabetes (n=14) (8.5 [9.5]% vs 3.8 [5.7]%, p=0.12), and in participants who were not on metformin (n=21) compared to those on metformin (n=9) (8.1 [8.4]% vs 2.1 [6.3]%, p=0.07). A subgroup analysis within the lifestyle intervention group, after correction for heterogeneity of variance, found greater percent weight reduction (p=.01) for both those without diabetes (13.6 [8.3]%) vs those with diabetes (5.1 [3.1]%), and also for those not using metformin (11.4 [7.9]%) vs those using metformin (4.4 [3.1]%).
There was no significant difference in the degree of weight loss among participants who had baseline overweight (BMI 25-29.9 kg/m2), class I (BMI 30-34.9 kg/m2) or class II obesity (BMI 35-40 kg/m2). Participants in the lifestyle intervention group who had baseline overweight, class I and class II obesity lost 8.7 (6.3)%, 11.5 (7.1)% and 6.9 (9.3)% of their body weight respectively (p=0.56).
The mean waist circumference change over the 48-week period was −7.4 cm (95% CI, −10.3 to −4.6) in the lifestyle intervention group as compared with +0.3 cm (95% CI, −3.2 to 3.8) in the control group (p=0.004).
Liver Histologic Findings (Table 3)
Table 3.
Change in Histologic parameters after 48 weeks of intervention*
| Variable | Group | Baseline | End of Study | P-value# |
|---|---|---|---|---|
| Fat (0-3) | Control | 1.9 (0.9) | 1.6 (1.0) | 0.02 |
| LS | 1.9 (0.7) | 0.8 (0.9) | ||
| Parenchymal Inflammation (0-3) |
Control | 1.7 (0.8) | 1.3 (0.8) | 0.18 |
| LS | 1.4 (0.6) | 0.9 (0.5) | ||
| Ballooning Injury (0-2) |
Control | 1.3 (0.5) | 0.6 (0.7) | 0.22 |
| LS | 1.2 (0.5) | 0.3 (0.6) | ||
| Fibrosis (0-4) | Control | 1.7 (0.8) | 1.4 (1.3) | 0.62 |
| LS | 1.4 (1.1) | 1.4 (1.0) | ||
| NAS (0-8) | Control | 4.9 (1.0) | 3.5 (1.8) | 0.05 |
| LS | 4.4 (1.1) | 2.0 (1.5) |
Control group, N=10, LS group, N=18
Data expressed as mean (SD)
P-value compares the mean difference between the pre- and post-treatment changes in the variables between the two groups.
The overall disease activity of nonalcoholic steatohepatitis (NAS [SD]) improved significantly in the lifestyle intervention group (−2.4 [1.6]) in comparison to the control group (−1.4 [2.1]) (p=0.05). Steatosis score also improved to a significantly greater degree in the lifestyle group as compared to the control group (−1.1 [0.8] vs −0.3 [0.8], p=0.02). Ballooning injury score improved in both groups while fibrosis score did not change in either group. The change in parenchymal inflammation, ballooning injury and fibrosis scores did not differ significantly between the two groups.
A greater proportion of participants in the lifestyle intervention group (11/18, 61%) had ≥3 points reduction in overall NASH activity score (NAS) from baseline than participants in the control group (2/10, 20%) (p=0.04). Similarly, at the end of the study period, a higher proportion of participants in the lifestyle intervention group had NAS ≤ 2 and no longer meet minimal histologic criteria for NASH as compared to the control group (67% vs 20%, p=0.02). Overall, 13/18 (72%) of participants in the lifestyle intervention group vs. 3/10 (30%) of participants in the control group had achieved the study end point (p=0.03).
The 3 subjects in the control group who achieved the study histologic end point had a variable degree of weight change (−6.0%, +0.9% and +9.8%). Two were diabetics (1 was on metformin and none were on TZDs). Two participants were obese (1 class I and 1 class II obesity), and 2 fulfilled criteria for the metabolic syndrome. One participant who lost 6% of body weight had normalization of transaminases.
Participants who achieved study weight loss goal (≥7%) had significant improvements in steatosis, parenchymal inflammation, ballooning injury and overall NASH activity scores (NAS) in comparison to those who did not achieve study weight loss goal (Table 4, all p <0.05). There was no improvement in fibrosis score in those who lost at least 7% compared to those who lost <7% of body weight (p=0.10).
Table 4.
Baseline Characteristics and Change (mean [SD]) in Histologic parameters according to study goal weight reduction (≥ 7%)
| Variable | Wt Loss < 7% (n=17) |
Wt Loss ≥ 7% (n=11) |
P-values |
|---|---|---|---|
| Baseline Characteristics | |||
| BMI Category, N (%) -overweight (25- 29.9 kg/m2) -obesity class I (30- 34.9 kg/m2) -obesity class II (35- 40 kg/m2) |
5 (26.3) 5 (26.3) 9 (47.4) |
3 (27.3) 6 (54.5) 2 (18.2) |
0.21 |
| Diabetes, N (%) | 10 (52.6) | 4 (36.4) | 0.39 |
| On metformin, N (%) |
7 (36.8) | 2 (18.2) | 0.28 |
| Histologic Parameters | |||
| Fat (0-3) |
−0.41 (0.80) | −1.36 (0.67) | <0.001 |
| Lobular inflammation (0-3) |
−0.24 (0.75) | −0.82 (0.75) | 0.03 |
| Ballooning Injury (0-2) |
−0.53 (0.80) | −1.27 (0.47) | 0.03 |
| Fibrosis (0-4) |
+ 0.06 (0.83) | −0.45 (0.93) | 0.10 |
| NAS (0-8) |
−1.18 (1.59) | −3.45 (1.21) | <0.001 |
| Participants with ≥3 points improvement in NAS from baseline, N (%) |
4 (23.5) | 9 (81.8) | 0.003 |
| Participants with NAS ≤2 at follow up, N (%) |
4 (23.5) | 10 (90.9) | <0.001 |
Liver Chemistry Tests
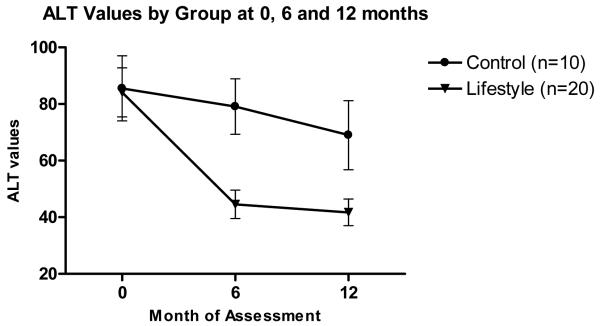
There was a significantly greater reduction in ALT levels in the lifestyle group in comparison to the control group. The mean reduction in ALT levels (SD) over the 48-week period were 42.4 (39.9) U/L (from 84 to 42 U/L) in the lifestyle group and 16.5 (36.6) (from 86 to 69 U/L) in the control group (p=0.01) (Table 2 and Figure 3) Normalization of ALT occurred in 12/20 (60%) of the participants in the lifestyle group and 3/10 (30%) in the control group (p=0.12).
Figure 3.
Serial mean alanine aminotransferase (ALT) values (mean±SE) by group at 0, 6 and 12 months. P= <.001 for the comparison between lifestyle and control at month 6, and P = 0.01 for the comparison at month 12.
AST levels decreased in both groups over the study period (20.2 [22.8] U/L in the lifestyle group and 18.0 [44.3] U/L in the control group). There was no statistical difference in AST reduction between the two groups (p=0.11).
Correlation analyses
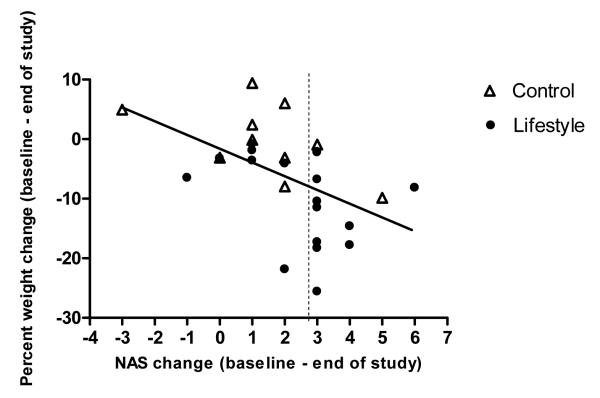
Percent weight reduction from baseline correlated significantly with improved liver chemistry (ALT values) (r=0.496, p=0.005), improvements in the degree of hepatic steatosis (r=0.616, p<0.001) and the overall NASH disease activity (r=0.497, p=0.007). (Figure 4)
Figure 4.
Scatterplot with slope line depicting change in participant NASH Activity Score (NAS) as a function of their percent weight change. The slope line shows the trend (r=−.497, p=0.007) for participants who lost more weight to change more NAS points. Each point on the graph represents one study participant. Solid circle indicates Lifestyle intervention group. Triangle with no fill indicates Control group. Points to the right of the dashed vertical line indicate those who achieved the study end point of a 3 point reduction in NAS (study end point)
Adverse Events
There were no adverse events related to the weight loss interventions. Two participants had abdominal pain after liver biopsy but none had internal bleeding or perforation of visceral organ.
Discussion
A major problem in the management of NASH is the lack of effective therapy(5). Weight reduction through diet and exercise has been promoted as initial therapy for NASH but there is very little evidence to support the effectiveness of this approach(12, 13). Our study is the first randomized controlled trial using lifestyle modification as active treatment intervention in adult patients with well characterized NASH. Our data suggest that overweight or obese patients with NASH can successfully achieve a weight reduction of 7-10% of initial body weight and maintain it through one year of study participation. In the current study, participants in the lifestyle intervention group lost an average of 9.3% from baseline weight as compared to 0.2% in the control group. Importantly, the results from this study suggest that lifestyle modifications focusing on diet, exercise and behavioral changes can successfully lead to improvements in overall NASH histologic activity, degree of steatosis and liver chemistry.
Published studies on weight reduction as a treatment for NASH have several major limitations(30, 31). Most notably, there has yet to be a rigorously conducted randomized controlled trial to address the efficacy of weight reduction in adult patients with NASH. Most published studies to date were either small retrospective or prospective case series without inclusion of a comparison group(32, 33). Many studies did not stratify patients according to histologic criteria(34), and thus may have included not only patients with NASH but also patients with simple steatosis who have a different natural history and clinical outcomes. In addition, these studies used primary outcomes that presently are not well accepted such as serum aminotransferases or sonographic findings(35-38). Another important shortcoming of earlier studies is that they used weight reduction strategies such as prolonged fasting(39) or very low calorie dieting(40) that can not be sustained over a long period of time. Several recent pharmaceutical trials for NASH have included dietary intervention for comparison (8, 41). Although the effects of nutritional counseling in these studies appeared to be inferior to the investigational drugs, these dietary interventions produced minimal or no weight loss and thus can not address the question of whether weight loss leads to improvements in NASH.
This study had a number of strengths, including the selection of patients with well characterized NASH both clinically and histologically, the randomized design, the high completion rate (97%, only one drop out) and the use of the current histologic scoring criteria by NASH CRN. In addition, our study used a standardized, protocol based lifestyle intervention similar to the programs implemented in the Diabetes Prevention Program (15) and Look AHEAD, an ongoing study with overweight individuals with type 2 diabetes (18). The effect sizes for overall NASH disease activity (cohen's d=0.82) and steatosis (cohen's d=0.97) were medium to large, and thus differences between lifestyle and control were statistically significant even with the relatively small sample size.
Our results suggest that the lifestyle intervention can successfully produce a 10% weight reduction on average in NASH patients and that this degree of weight reduction is successful in achieving resolution of NASH in a significant proportion of patients. In our study, 67% of participants in the lifestyle group had their post intervention biopsy score (NAS) below 3 and no longer meet minimal histologic criteria for NASH as compared to only 20% of participants in the control group (p=0.02). Moreover, magnitude of weight loss correlated strongly with improvements in disease markers of NASH including ALT level, grade of steatosis and overall histologic NASH activity. It appears that at least 7% weight reduction would be required to improve NASH histologic activity. Participants who achieved the study weight loss goal of 7% had significantly greater improvements in all aspects of NASH histologic activity including steatosis, lobular inflammation, ballooning injury.
We did not observe significant change in the degree of hepatic fibrosis after one year of study intervention. This may indicate that the effect of weight loss on fibrosis is smaller than the effect on steatosis or overall activity, and thus could not be detected with our sample size, or that longer than one year is needed to demonstrate changes in fibrosis score. In addition, participants in our study had a relatively low fibrosis score at baseline (mean [SD] = 1.52 [0.96]). Sixteen % of participants had bridging fibrosis (stage 3) and none had cirrhosis (stage 4) which makes it more difficult to demonstrate changes. Future clinical trials in NASH should consider patient enrollment scheme to include subjects with a full spectrum of NASH severity.
Another observation from this study is that serum ALT levels and histologic parameters of NASH activity (steatosis, parenchymal inflammation and ballooning injury) improved although to a lesser extent even in those who had minimal weight loss or those assigned to the control group. This finding was observed in other pharmaceutical trials as well where subjects in the control group who received nutritional counseling may have had improvements in serum ALT levels and NASH histologic parameters despite non-significant weight reduction(8, 42). The reason for this observation is not entirely clear but may be related to changes in eating habits or dietary components, or changes in physical activity that are difficult to quantify. In addition, the improvement in serum ALT could be partly due to the phenomenon of regression to the mean. This finding underscores the limitation of our current tools in the assessment of NASH disease activity. It has important implication for designing future clinical trials in NASH.
In conclusion, an intensive lifestyle intervention program can successfully produce a 7-10% weight reduction in patients with NASH. This degree of weight reduction can lead to significant improvements in liver chemistry and histologic activity of NASH. Given the additional benefit of weight loss on cardiovascular risk factors, lifestyle intervention with the goal of at least 7% weight reduction represents an attractive therapy for this increasingly common disease.
Supplementary Material
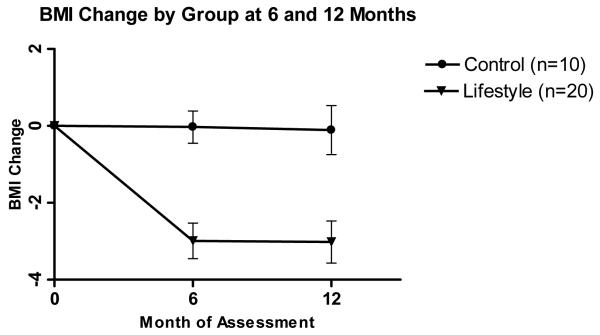
Figure 2b.
Body Mass Index (BMI) change (mean±SE) by group at 6 and 12 months. P<.001 for the comparison between lifestyle and control at month 6, and P = 0.004 for the comparison at month 12.
Acknowledgment
We thank Janice Clark RN for project coordination. We also thank Dr Janus Ong for Data and Safety Monitoring of this trial.
Funding/Support: National Institutes of Health, 5R03DK67263-2. This was also supported in part by the Intramural Research Program of the National Cancer Institute, NIH (D.E.K)
Abbreviations
- NASH
nonalcoholic steatohepatitis
- NAFLD
nonalcoholic fatty liver disease
- BMI
body mass index
- LS
lifestyle intervention
- NAS
NAFLD activity score
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- HOMA
homeostasis model assessment of insulin resistance
Footnotes
Trial Registration: clinicaltrials.gov, NCT00266019
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 3.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 4.McCullough AJ. Thiazolidinediones for nonalcoholic steatohepatitis--promising but not ready for prime time. N Engl J Med. 2006;355:2361–2363. doi: 10.1056/NEJMe068232. [DOI] [PubMed] [Google Scholar]
- 5.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 6.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008–1017. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 7.Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 8.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 9.Lutchman G, Modi A, Kleiner DE, Promrat K, Heller T, Ghany M, Borg B, et al. The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology. 2007;46:424–429. doi: 10.1002/hep.21661. [DOI] [PubMed] [Google Scholar]
- 10.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 11.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 12.Wang RT, Koretz RL, Yee HF., Jr. Is weight reduction an effective therapy for nonalcoholic fatty liver? A systematic review. Am J Med. 2003;115:554–559. doi: 10.1016/s0002-9343(03)00449-2. [DOI] [PubMed] [Google Scholar]
- 13.Clark JM. Weight loss as a treatment for nonalcoholic fatty liver disease. J Clin Gastroenterol. 2006;40(Suppl 1):S39–43. doi: 10.1097/01.mcg.0000168641.31321.fa. [DOI] [PubMed] [Google Scholar]
- 14.Wing RR. Behavioral approaches to the treatment of obesity. Marcel Dekker, Inc; New York: 1998. pp. 855–873. [PubMed] [Google Scholar]
- 15.The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 19.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 20.Flechtner-Mors M, Ditschuneit HH, Johnson TD, Suchard MA, Adler G. Metabolic and weight loss effects of long-term dietary intervention in obese patients: four-year results. Obes Res. 2000;8:399–402. doi: 10.1038/oby.2000.48. [DOI] [PubMed] [Google Scholar]
- 21.Jeffery RW, Wing RR. Long-term effects of interventions for weight loss using food provision and monetary incentives. J Consult Clin Psychol. 1995;63:793–796. doi: 10.1037//0022-006x.63.5.793. [DOI] [PubMed] [Google Scholar]
- 22.Wadden TA, Vogt RA, Andersen RE, Bartlett SJ, Foster GD, Kuehnel RH, Wilk J, et al. Exercise in the treatment of obesity: effects of four interventions on body composition, resting energy expenditure, appetite, and mood. J Consult Clin Psychol. 1997;65:269–277. doi: 10.1037//0022-006x.65.2.269. [DOI] [PubMed] [Google Scholar]
- 23.Wing RR, Jeffery RW, Burton LR, Thorson C, Nissinoff KS, Baxter JE. Food provision vs structured meal plans in the behavioral treatment of obesity. Int J Obes Relat Metab Disord. 1996;20:56–62. [PubMed] [Google Scholar]
- 24.Ditschuneit HH, Flechtner-Mors M, Johnson TD, Adler G. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clin Nutr. 1999;69:198–204. doi: 10.1093/ajcn/69.2.198. [DOI] [PubMed] [Google Scholar]
- 25.P-S FX. The effects of increased physical activity on food intake, metabolic rate, and health risks in obese individuals. The Guilford Press; New York: 1992. pp. 190–210. [Google Scholar]
- 26.Jakicic JM, Winters C, Lang W, Wing RR. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: a randomized trial. Jama. 1999;282:1554–1560. doi: 10.1001/jama.282.16.1554. [DOI] [PubMed] [Google Scholar]
- 27.D'Zurilla TJ, Goldfried MR. Problem solving and behavior modification. J Abnorm Psychol. 1971;78:107–126. doi: 10.1037/h0031360. [DOI] [PubMed] [Google Scholar]
- 28.Marlatt GA, GJ . Relapse prevention: Maintenance strategies in addictive behavior change. Guilford; New York: 1985. [Google Scholar]
- 29.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 30.Harrison SA, Day CP. Benefits of lifestyle modification in NAFLD. Gut. 2007;56:1760–1769. doi: 10.1136/gut.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellentani S, Dalle Grave R, Suppini A, Marchesini G. Behavior therapy for nonalcoholic fatty liver disease: The need for a multidisciplinary approach. Hepatology. 2008;47:746–754. doi: 10.1002/hep.22009. [DOI] [PubMed] [Google Scholar]
- 32.Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, Emick D, et al. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 33.Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, Powell EE. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413–419. doi: 10.1136/gut.2003.027581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas EL, Brynes AE, Hamilton G, Patel N, Spong A, Goldin RD, Frost G, et al. Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:5813–5819. doi: 10.3748/wjg.v12.i36.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki A, Lindor K, Saver J, Lymp J, Mendes F, Muto A, Okada T, et al. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol. 2005;43:1060–1066. doi: 10.1016/j.jhep.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–419. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- 37.Sreenivasa Baba C, Alexander G, Kalyani B, Pandey R, Rastogi S, Pandey A, Choudhuri G. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J Gastroenterol Hepatol. 2006;21:191–198. doi: 10.1111/j.1440-1746.2005.04233.x. [DOI] [PubMed] [Google Scholar]
- 38.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drenick EJ, Simmons F, Murphy JF. Effect on hepatic morphology of treatment of obesity by fasting, reducing diets and small-bowel bypass. N Engl J Med. 1970;282:829–834. doi: 10.1056/NEJM197004092821502. [DOI] [PubMed] [Google Scholar]
- 40.Palmer M, Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology. 1990;99:1408–1413. doi: 10.1016/0016-5085(90)91169-7. [DOI] [PubMed] [Google Scholar]
- 41.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, et al. Randomized, Placebo-Controlled Trial of Pioglitazone in Nondiabetic Subjects With Nonalcoholic Steatohepatitis. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 42.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.