Abstract
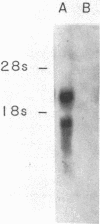
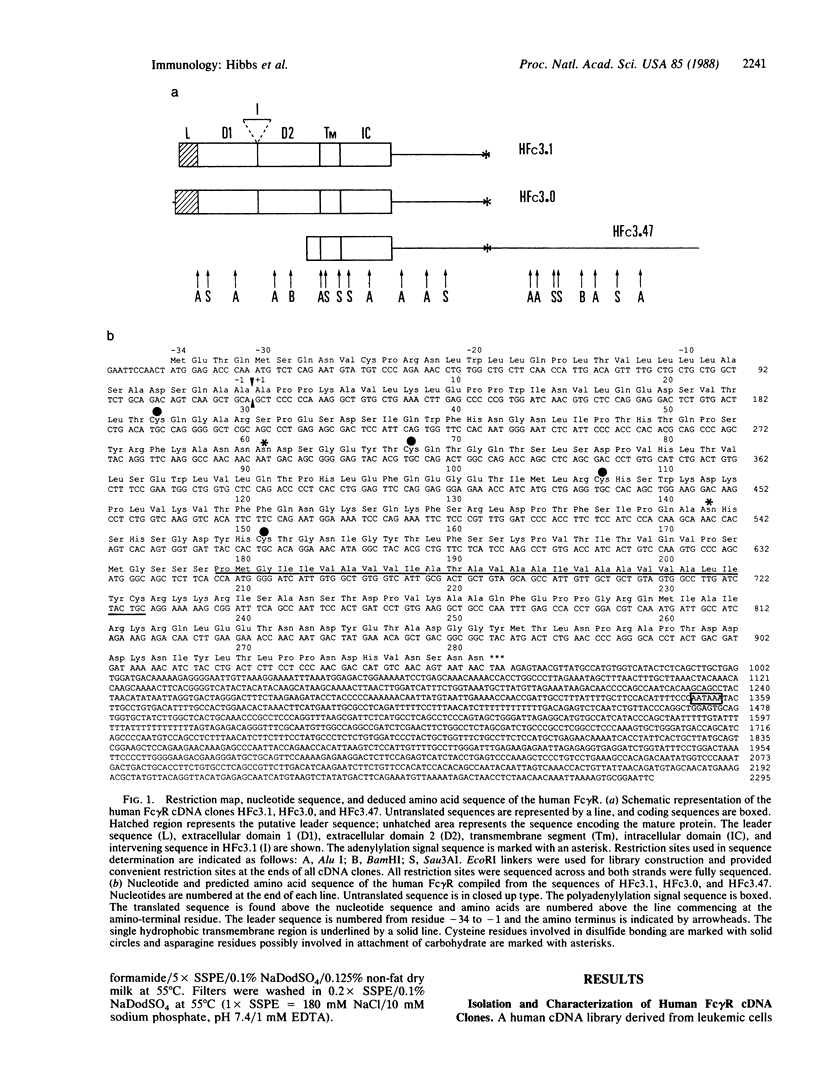
Human IgG Fc receptor (Fc gamma R) cDNA clones were isolated by cross-species hybridization by probing cDNA libraries with the low-affinity Fc gamma R beta 1 cDNA clone from mouse as well as a pool of oligonucleotides constructed from the nucleotide sequence of this Fc gamma R. Three cDNA clones were isolated and analysis of the predicted amino acid sequence indicated that the human Fc gamma R protein is synthesized with a 34-amino acid leader and the mature protein is composed of 281 amino acids. The extracellular region of this Fc gamma R was divided into two domains, which were very similar to each other and to the corresponding regions of both mouse alpha and beta Fc gamma Rs and showed a clear relationship to immunoglobulin variable regions. One possible N-linked glycosylation site was found in each of the extracellular domains. The human Fc gamma R leader sequence was shown to be similar to the mouse alpha Fc gamma R leader sequence, but the transmembrane region was most similar to the mouse beta 1 Fc gamma R. The intracellular domain of the human Fc gamma R was surprisingly different from both mouse Fc gamma Rs. RNA blot analysis of human cells demonstrated two transcripts (2.5 and 1.5 kilobases) that arise by use of different adenylylation signals. The cellular expression of these transcripts suggest that they encode the low-affinity p40 Fc gamma R protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. L. Isolation of the receptor for IgG from a human monocyte cell line (U937) and from human peripheral blood monocytes. J Exp Med. 1982 Dec 1;156(6):1794–1806. doi: 10.1084/jem.156.6.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M. M., Hamburger M. I., Lawley T. J., Kimberly R. P., Plotz P. H. Defective reticuloendothelial system Fc-receptor function in systemic lupus erythematosus. N Engl J Med. 1979 Mar 8;300(10):518–523. doi: 10.1056/NEJM197903083001002. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Metcalf D., Gough J., Grail D., Dunn A. R. Structure and expression of the mRNA for murine granulocyte-macrophage colony stimulating factor. EMBO J. 1985 Mar;4(3):645–653. doi: 10.1002/j.1460-2075.1985.tb03678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs M. L., Walker I. D., Kirszbaum L., Pietersz G. A., Deacon N. J., Chambers G. W., McKenzie I. F., Hogarth P. M. The murine Fc receptor for immunoglobulin: purification, partial amino acid sequence, and isolation of cDNA clones. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6980–6984. doi: 10.1073/pnas.83.18.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth P. M., Hibbs M. L., Bonadonna L., Scott B. M., Witort E., Pietersz G. A., McKenzie I. F. The mouse Fc receptor for IgG (Ly-17): molecular cloning and specificity. Immunogenetics. 1987;26(3):161–168. doi: 10.1007/BF00365906. [DOI] [PubMed] [Google Scholar]
- Huang H. J., Jones N. H., Strominger J. L., Herzenberg L. A. Molecular cloning of Ly-1, a membrane glycoprotein of mouse T lymphocytes and a subset of B cells: molecular homology to its human counterpart Leu-1/T1 (CD5). Proc Natl Acad Sci U S A. 1987 Jan;84(1):204–208. doi: 10.1073/pnas.84.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P., Williams A. F. Striking similarities between antigen receptor J pieces and sequence in the second chain of the murine CD8 antigen. Nature. 1986 Sep 4;323(6083):74–76. doi: 10.1038/323074a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lewis V. A., Koch T., Plutner H., Mellman I. A complementary DNA clone for a macrophage-lymphocyte Fc receptor. 1986 Nov 27-Dec 3Nature. 324(6095):372–375. doi: 10.1038/324372a0. [DOI] [PubMed] [Google Scholar]
- Looney R. J., Abraham G. N., Anderson C. L. Human monocytes and U937 cells bear two distinct Fc receptors for IgG. J Immunol. 1986 Mar 1;136(5):1641–1647. [PubMed] [Google Scholar]
- Nakauchi H., Nolan G. P., Hsu C., Huang H. S., Kavathas P., Herzenberg L. A. Molecular cloning of Lyt-2, a membrane glycoprotein marking a subset of mouse T lymphocytes: molecular homology to its human counterpart, Leu-2/T8, and to immunoglobulin variable regions. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5126–5130. doi: 10.1073/pnas.82.15.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph S. J., Thomas M. L., Morton C. C., Trowbridge I. S. Structural variants of human T200 glycoprotein (leukocyte-common antigen). EMBO J. 1987 May;6(5):1251–1257. doi: 10.1002/j.1460-2075.1987.tb02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Luster A. D., Weinshank R., Kochan J., Pavlovec A., Portnoy D. A., Hulmes J., Pan Y. C., Unkeless J. C. Structural heterogeneity and functional domains of murine immunoglobulin G Fc receptors. Science. 1986 Nov 7;234(4777):718–725. doi: 10.1126/science.2946078. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourvieille B., Gorman S. D., Field E. H., Hunkapiller T., Parnes J. R. Isolation and sequence of L3T4 complementary DNA clones: expression in T cells and brain. Science. 1986 Oct 31;234(4776):610–614. doi: 10.1126/science.3094146. [DOI] [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Fleit H., Mellman I. S. Structural Aspects and Heterogeneity of Immunoglobulin Fc Receptors. Adv Immunol. 1981;31:247–270. doi: 10.1016/s0065-2776(08)60922-0. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N., Clark M. J., Gagnon J. Cell surface glycoproteins and the origins of immunity. Ann Inst Pasteur Immunol. 1985 Mar-Apr;136C(2):283–294. doi: 10.1016/s0769-2625(85)80060-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]