Abstract
Cyclic AMP plays a critical role in adipocyte differentiation and maturation. However, it is not clear which of the two intracellular cAMP receptors, exchange protein directly activated by cAMP/cAMP-regulated guanine nucleotide exchange factor or protein kinase A/cAMP-dependent protein kinase, is essential for cAMP-mediated adipocyte differentiation. In this study, we utilized a well-defined adipose differentiation model system, the murine preadipocyte line 3T3-L1, to address this issue. We showed that knocking down Epac expression in 3T3-L1 cells using lentiviral based small hairpin RNAs down-regulated peroxisome proliferator-activated receptor gamma expression and dramatically inhibited adipogenic conversion of 3T3-L1 cells while inhibiting PKA catalytic subunit activity by two mechanistically distinct inhibitors, heat stable protein kinase inhibitor and H89, had no effect on 3T3-L1 adipocyte differentiation. Moreover, cAMP analog selectively activating Epac was not able to stimulate adipogenic conversion. Our study demonstrated that while PKA catalytic activity is dispensable, activation of Epac is necessary but not sufficient for adipogenic conversion of 3T3-L1 cells.
Keywords: Cyclic AMP, Epac, Exchange protein directly activated by cAMP, PKA (Protein kinase A), PPARγ, Peroxisome proliferator-activated receptor γ, 3T3-L1, Adipogenesis, Adipocyte, Differentiation
2. INTRODUCTION
Adipocyte differentiation from its precursors is rigorously controlled by defined molecular events (1). The precise mechanism that governs adipogenesis remains elusive. The immortalized mouse 3T3-L1 preadipocytes have been widely used as an ex vivo model system to study the molecular events involved in the differentiation of adipose tissue. The conversion of 3T3-L1 preadipocytes into adipocytes resembles the in vivo development of the mammalian adipose tissue in many aspects. Confluent and growth-arrested 3T3-L1 cells undergo spontaneous differentiation and convert into mature lipid droplet accumulating adipocytes with certain frequency (2-4). Under the induction of a prodifferentiative mixture, which include insulin, dexamethasone (Dex), and a cAMP-elevating agent, 3-isobutyl-1-methylxanthine (IBMX), 3T3-L1 cells go through one or two rounds of mitotic division and increase expression of CCAAT/enhancer binding protein (C/EBP) β and δ (1,5,6). These early events are followed by induction of C/EBPα and peroxisome proliferator-activated receptor γ (PPARγ) (7,8). PPARγ and C/EBPα are considered as the master transcriptional regulators of adipogenesis and drive adipocyte-specific gene expression (4,9).
cAMP is generally considered to be essential for the induction of adipocyte differentiation. Increase of intracellular cAMP levels activates the cAMP-responsive element-binding protein (CREB), a critical transcriptional activator for adipocyte differentiation (10,11). CREB promotes adipogenesis by inducing the expression of C/EBPβ (11) and PPARγ (12). Increase in intracellular cAMP has also been shown to induce the production of a putative endogenous PPARγ ligand during early adipogenesis (13). In addition, activation of CREB by cAMP is also known to regulate other important players involved in the early adipocyte differentiation process such as, the gene expression of stearoyl-CoA desaturase gene 1, Wnt10b, cyclin D1, cyclic nucleotide phosphodiesterase 3B and the regulator of G protein signaling 2 (RGS2), a member of the RGS protein superfamily (14-18).
The effects of cAMP are mediated by two intracellular cAMP receptors, the classic cAMP-dependent protein kinase (PKA) and newly discovered exchange protein directly activated by cAMP (Epac) (19). PKA is a serine-threonine kinase that mediates the effects of cAMP by phosphorylating down-stream targets (20). The PKA holoenzyme contains two catalytic (C) subunits and two regulatory (R) subunits. There are two major isoforms of PKA, designated as PKA(I) and PKA(II), due exclusively to differences in the R subunits, RI and RII (20). While in mature adipocytes, activation of PKA is known to promote lipolysis by phosphorylating hormone-sensitive lipases and perilipin (21-23), the definitive role that PKA play in adipogenesis is not clear. Since the discovery of the Epac family of guanine exchange proteins, extensive studies so far have established that Epac proteins are increasingly involved in a host of cAMP-related cellular functions (24). To investigate the underlying mechanism by which cAMP uses to regulate preadipocyte diffentiation, we examined the roles that Epac and PKA play in cAMP-mediated adipocyte differentiation in 3T3-L1 adipogenic cells. Here we report that while Epac is essential for cAMP-mediated adipogenesis, kinase activity of the PKA catalytic subunit is not required for 3T3-L1 adipocyte differentiation.
3. MATERIALS AND METHODS
3.1. Reagents
Insulin, Dexamethesome, IBMX, lentiviral-based shRNAs, and myristoylated PKI(14-22) (mPKI), a membrane-permeable PKA-specific inhibitor, were purchased from Sigma-Aldrich (St. Louis, MO). H-89 and forskolin was purchased from Alexis Biochemicals (San Diego, CA). 8-pCPT-2’-O-Me-cAMP was purchased from BIOLOG Life Science Institute (Bremen, Germany).
3.2. Cell culture and adipocyte differentiation in vitro
3T3-L1 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum in humidified atmosphere of 5% CO2/95% air at 37 °C to confluence. Two days later, the induction of adipocyte differentiation was initiated by treatment of the cells with the differentiation medium containing 1 μM insulin, 1 μM Dex, and 0.5 mM IBMX for 2 days, followed by 2 days of treatment with the medium containing 1 μM insulin alone. Medium was replaced every 2 days for the following 8 days. Differentiation of preadipocytes to mature adipocytes was confirmed by observation using microscope and by Oil Red O staining of lipid vesicles. In the experiments to assess the effects of the reagents to be tested, they were added into the differentiation medium and kept in the medium throughout the initial 2 days of induction. The reagents to be tested were used at the following concentrations; PKI (50 μM), H-89 (5 μM).
3.3. GPDH activity assay
8−9 days after differentiation induction, 3T3-L1 cells were rinsed once in cold PBS and resuspended in a lysis buffer containing 25 mM Tris (pH 7.5), 130 mM NaCl, 10 mM MgCl2, 1 mM EDTA, 1 mM DTT, 0.1 mM phenylmethylsulfonyl fluoride, 1% Triton-X 100, and 1 μg/ml aprotinin, leupeptin, and pepstatin. The cell lysate was centrifuged at 15,000 g for 15 min, after which the supernatant solution was removed and centrifuged at 100,000 g for 1 h. Protein concentration of 100,000 g supernatant was determined with the Bio-Rad protein assay reagent. GPDH activity was measured at room temperature with equal amount of total proteins in a Hitachi U2000 spectrometer. The standard reaction mixture contained 50 mM Tris, pK 7.5, 5 mM EDTA, 0.16 mM NADH, and 0.8 mM dihydroxyacetone phosphate. The reaction was initiated with by the addition of dihydroxyacetone phosphate and recorded as a function of time.
3.4. Oil Red O staining
Seven to ten days after the induction of differentiation, cells were rinsed twice with phosphate-buffered saline (PBS), fixed with 10% formalin for 1 h, and washed three times with PBS. Cells were stained with filtered Oil Red O (0.3%) working solution for 20 min, and then stained cells were washed three times with distilled water. Images were collected using a Nikon-inverted microscope with a Nikon D100 digital camera.
3.5. Stable shRNA transfection of 3T3-L1 cells by lentiviral infection
Stable shRNA cell lines were established as described previously (25). Briefly, lentiviral shRNA supernatant was produced by transient transfection of HEK293T cells (ATCC, Manassas, VA) using Lipofectamine (Invitrogen Life Technologies, Inc., Gaithersburg, MD), according to the manufacturer's instructions. Plasmids used were the short hairpin RNAs in pLKO.1-puro vector (Sigma-Aldrich, St. Louis, MO). The viral-containing supernatants were harvested 48 h after transfection, filtered through a 0.45-μm filter unit. To transduce 3T3-L1 cells with lentivirus shRNAs, logarithmically growing 3T3-L1 cells were seeded at a density of 2×105 cells per well in 6-well plates. 0.5 ml of lentivirus suspension and 8 μg/ml of polybrene were added to RPMI medium containing 5% FBS in a total volume of 1ml. Cells were allowed to incubate at 37°C for 12 h before removing the medium and replacing with 2 ml of fresh RPMI for expansions of the transductants. 24 h after lentiviral infection, Cells were selected with puromycin at 2.5μg/ml for another 5 days before further experiments.
3.6. Reverse transcriptase polymerase chain reaction (RT-PCR) analysis
Cells (5 × 106) were lysed in Trizol and RNA extraction was performed according to a standard protocol supplied by the manufacturer (Invitrogen, Inc., Carlsbad, CA, USA) and pellets were resuspended in RNAase free water. cDNAs were then transcribed with a high-capacity reverse transcription system from Applied Biosystems. Quantitative real-time PCR analysis was performed transcribed cDNAs as previously described (26).
3.7. Immunoblotting analysis
Equal amounts of protein (5−30μg) were loaded onto 10% SDS polyacrylamide mini-gels (Bio-Rad), transferred to PVDF membranes. After being blocked overnight in 5% milk in TBS-Tween, blots were incubated with Epac1-specific antibody as described (27) for 1.5 hrs, followed by HRP-conjugated secondary antibody (1:4,000) for 45 min. Antigen-antibody complexes were detected by enhanced chemiluminescence.
4. RESULTS
4.1. Activation of PKA catalytic subunit is not necessary for 3T3-L1 preadipocyte differentiation
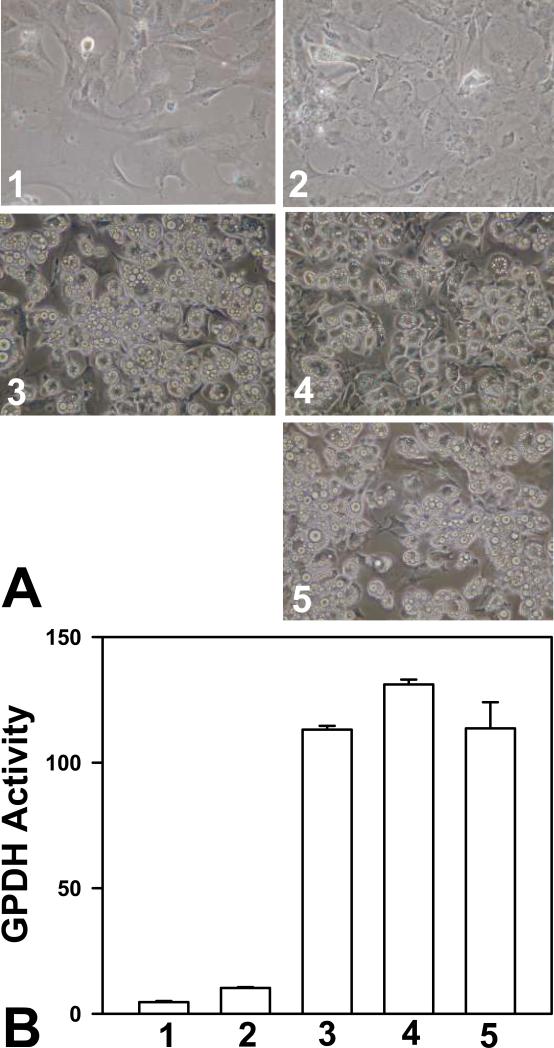
In addition to hormonal factors, insulin and glucocorticoid, adipogenic conversion of 3T3-L1 preadipocytes in culture requires cAMP elevating agent IBMX or forskolin. To determine if catalytic activity of PKA is necessary for cAMP-mediated 3T3-L1 preadipocyte differentiation, we tested the effects of two mechanistically distinct PKA-specific inhibitors, H89 and a myristoylated, membrane permeable form of heat-stable protein kinase inhibitor (mPKI), on 3T3-L1 preadipocyte differentiation. As shown in Figure 1A, cocktail mixture of insulin, Dex, and IBMX led to robust formation of adipocytes rich in lipid droplets while insulin and Dex without IBMX were not effective in inducing adipocyte differentiation, suggesting that cAMP is required for 3T3-L1 preadipocyte differentiation. Surprisingly, both PKA-specific inhibitors, H89 and mPKI, failed to suppress cAMP-mediated 3T3-L1 preadipocyte differentiation. Similar results were obtained when the activity of glycerophosphate dehydrogenase (GPDH), an adipocyte specific lipogenic enzyme, was used as readout for adipocyte differentiation (Figure 1B). While insulin, Dex and IBMX significantly induced GPDH activity, H89 and mPKI again were ineffective in suppressing insulin/Dex/IBMX mediated GPDH activation. To ensure that the apparent non-effect of H89 and mPKI is not due to a lack of efficacy of the reagents, we confirmed the inhibition of PKA activity in 3T3-L1 cells treated with H89 and mPKI in the presence of insulin/Dex/IBMX (data not shown). Taken together, our results suggest that PKA kinase activity is not required for the differentiation of 3T3-L1 preadipocytes.
Figure 1.
PKA catalytic activity is not required for the differentiation of 3T3-L1 cells. 3T3-L1 cells, two days post-confluence, were treated with various reagents as described below and induced to differentiate. Effects of treatments were documented either by cell imaging using a digital camera under a phase-contrast microscope (A) or by monitoring the cellular glycerophosphate dehydrogenase (B). Treatments include: 1, vehicles only; 2, 1 μM insulin and 1 μM Dex; 3, 1 μM insulin, 1 μM Dex, and 0.5 mM IBMX; 4, 1 μM insulin, 1 μM Dex, 0.5 mM IBMX and 5 μM H-89; and 5, 1 μM insulin, 1 μM Dex, 0.5 mM IBMX and 50 μM mPKI.
4.2. Epac1 is required for 3T3-L1 differentiation
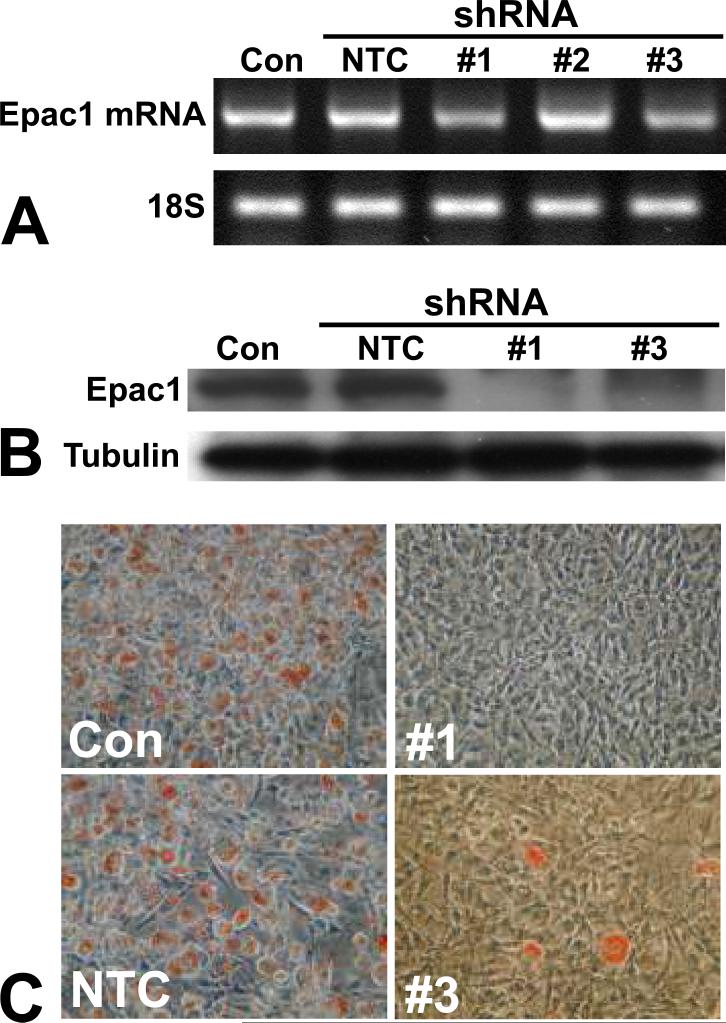
To determine if Epac1 is essential for 3T3-L1 differentiation, we suppressed the Epac1 expression in 3T3-L1 cells using lentivirus-mediated shRNAs specific for Epac1. 3T3-L1 differentiation was examined thereafter. To eliminate the potential off-target effects, three independent Epac1-specific shRNAs, as well as a non-targeting control (NTC) shRNA construct were used. While Epac1 shRNA 1 and 3 were capable of suppressing the expression of Epac1 at mRNA level mRNA as monitored by RT-PCR, shRNA construct 2 was ineffective and excluded from subsequent studies (Figure 2A). Knockdown of Epac1 expression by Epac1 shRNA constructs 1 and 3 was further confirmed at the protein level by Western blot using Epac-specific antibodies (Figure 2B). Down-regulation of Epac1 expression led to a dramatic reduction of insulin/Dex/IBMX induced adipose differentiation in 3T3-L1 cells transduced with either Epac1 shRNA as demonstrated by Oil Red O staining when compared to control cells transduced with NTC shRNA (Figure 2C). These results suggest that Epac1 is essential for 3T3-L1 preadipocyte differentiation.
Figure 2.
Epac1 expression is required for 3T3-L1 adipogenic differentiation. (A) mRNA expression levels of Epac1 after lentiviral shRNA silencing as measured by RT-PCR. (B) Protein levels of Epac1 after lentiviral shRNA silencing as measured by Western blot. (C) Effects of Epac1 gene silencing by lentiviral shRNAs on adipocyte differentiation efficiency of 3T3-L1 cells as determined by Oil Red O staining. NTC: non-targeting control shRNA.
4.3. Epac1 knockdown suppresses PPARγ
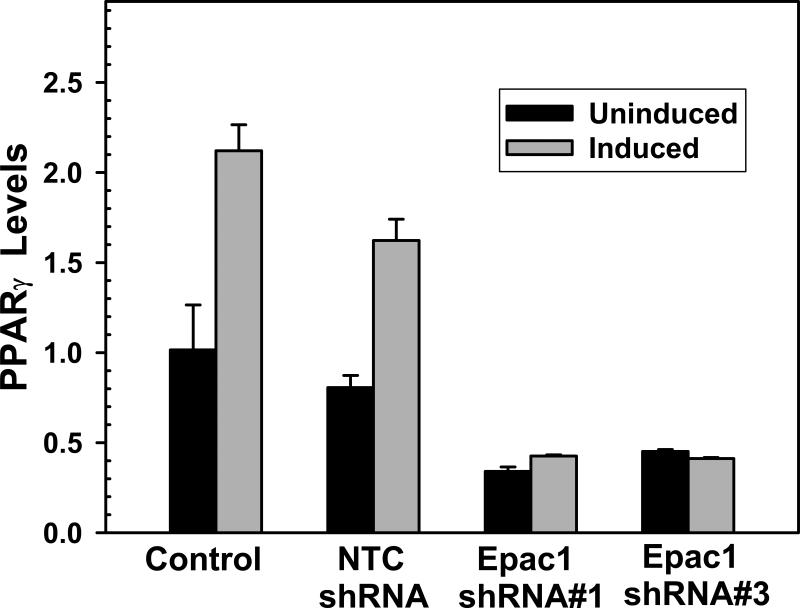
To determine the mechanism of inhibition of 3T3-L1 differentiation mediated by Epac1 silencing; we determined the levels of PPARγ, a master regulator of adipocyte differentiation, during adipocyte differentiation in 3T3-L1 cells transduced with either Epac1 or non-targeting shRNAs. As shown in Figure 3, in 3T3-L1 cells transduced with the non-targeting shRNA, the levels of PPARγ as monitored by real-time PCR increased significantly in response to insulin/Dex/IBMX induction. This is consistent with the fact that PPARγ is critical for the adipocyte differentiation program. In contrast, suppressing of Epac1 by either shRNA 1 or 3 led to an abridged basal PPARγ level in 3T3-L1 cells. Furthermore, this reduced basal level of 3T3-L1 cells remained low after insulin/Dex/IBMX treatment. These results suggest that Epac1 contributes to cAMP-mediated adipocyte differentiation in 3T3-L1 cells in part by regulating the expression level of PPARγ.
Figure 3.
Silencing of Epac1 expression suppresses PPARγ expression in 3T3-L1 cells. Confluent 3T3-L1 cells treated with or without Epac1-specific or non-targeting control lentiviral shRNAs were incubated with 1 μM insulin, 1 μM Dex, and 0.5 mM IBMX (induced) or vehicle (uninduced) for two days. Total RNAs were then isolated and the levels of PPARγ expression were measured by real time PCR.
4.4. Activation of Epac is not sufficient for cAMP-mediated 3T3-L1 differentiation
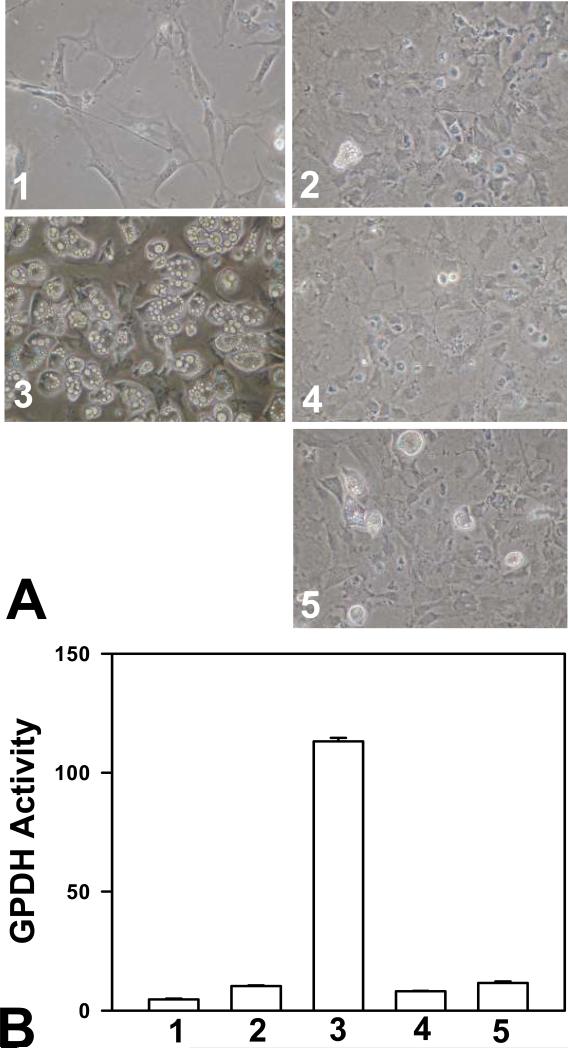
To investigate if activation of Epac alone is sufficient to mediate cAMP-induced 3T3-L1 differentiation, we used a selective Epac activator, 8-pCPT-2’-O-Me-cAMP, to substitute IBMX in the differentiation assay. To our surprise, even at a high concentration of 200 μM, Epac specific agonist 8-pCPT-2’-O-Me-cAMP did not induce significant adipocyte differentiation over insulin/Dex treatment (Figure 4A), and was not able to stimulate GPDH expression as IBMX (Figure 4B). This apparent non-effect of 8-pCPT-2’-O-Me-cAMP was not due to the lack of compound efficacy as it was able to significantly activate Rap1, a down-stream effector of Epac (data not shown). These observations are also consistent with a recent report by Petersen et al. showing that 8-pCPT-2’-O-Me-cAMP could not mimic the adipogenic effect of an increase in the endogenous level of cAMP in 3T3-L1 cells treated with Dex and insulin (28).
Figure 4.
Activation of Epac is not sufficient for cAMP-stimulated differentiation of 3T3-L1 cells. 3T3-L1 cells, two days post-confluent, were treated with various reagents as described below and induced to differentiate. Effects of treatments were documented either by cell imaging using a digital camera under a phase-contrast microscope (A) or by monitoring the cellular glycerophosphate dehydrogenase (B). Treatments include: 1, vehicles only; 2, 1 μM insulin and 1 μM Dex; 3, 1 μM insulin, 1 μM Dex, and 0.5 mM IBMX; and 4 and 5, 1 μM insulin, 1 μM Dex, and 100 or 200 μM 8-pCPT-2’-O-Me-cAMP, respectively.
5. DISCUSSION
cAMP elevating agents, when combined with insulin and Dex, induce differentiation of preadipocytes to mature adipocytes. The precise mechanism for cAMP-mediated proadipogenic effect is still elusive. Before the discovery of Epac, cellular functions of cAMP were largely attributed to PKA, which was once the only known intracellular receptor for cAMP. The identification of Epac proteins opens up a new chapter of cAMP signaling research as Epac and PKA have been shown to exert distinct or even opposite functions in various tissues and cells (19,29,30). In the present study, we showed that two selective PKA C subunit inhibitors, H89 and mPKI, failed to block the proadipogenic effect of cAMP. In fact, H89 enhanced adipocyte differentiation of 3T3-L1 cells. These observations are in agreement with several recent reports showing H89's proadipogenic effect (31-33). Since both H89 and mPKI potently inhibited the cellular PKA kinase activities under our experimental conditions, the incapacity of H89 and mPKI to block the proadipogenic effect of cAMP is not due to the lack of efficacy of either H89 or mPKI. In addition, we can also exclude the possibility of experimental artifacts as H89 and mPKI are two structurally and mechanistically distinct inhibitors. While H89 is a small synthetic compound that competitively binds to the ATP pocket on the PKA C subunits (34), PKI is an endogenous peptide that exclusively inhibits the cellular activities of all PKA catalytic subunits (35). Taken together, our results suggest that PKA's kinase activity is not required for cAMP's proadipogenic effect in 3T3-L1 cells.
Unlike PKA catalytic activity, Epac activity is required for 3T3-L1 adipocyte differentiation as knocking down of Epac1 expression by Epac1-specific shRNAs led to substantial inhibition of insulin/Dex/IBMX induced adipocytes formation. While majority of earlier studies exclusively rely on Epac-selective cAMP analogs or so called dominant negative Epac mutants defective in binding cAMP to probe the cellular function of Epac, these approaches have significant limitations. For example, it has been revealed recently that Epac-selective agonists, including 8-pCPT-2’-O-Me-cAMP, have undesired off-target effect of either acting as a substrate or inhibitor for various phosphodiesterases (36). Likewise, the dominant negative effect of Epac mutants defective in cAMP binding has never been convincingly documented. On the other hand, the use of two independent Epac1-specific shRNAs allows us to demonstrate unambiguously that Epac plays an essential role in 3T3-L1 preadipocyte differentiation. In addition, our study showed that Epac1 silencing by shRNAs down-regulated PPARγ. PPARγ, a master regulator of adipocyte differentiation, is both necessary and sufficient for adipogenesis (37,38). These results suggest that Epac mediates the effect of cAMP during adipocyte differentiation in part by regulating PPARγ.
Interestingly, while Epac is essential for 3T3-L1 adipocyte differentiation, substitution of IBMX with Epac specific cAMP analogs failed to promoter adipocyte formation, suggesting additional cAMP signaling components are required. It has been reported that there are significant increases in the expression levels of PKA regulatory subunits RI and RII accompanied by a concomitant dramatic decrease in PKA catalytic activity during the progression of adipogenesis (31). One speculation is that this shift in balance of PKA R and C subunit expressions during adipocyte differentiation is important for adipogenesis and that activation of the PKA R subunits, but not the C subunits, in addition to Epac activation, is required for the differentiation of 3T3-L1 cells. Although the mechanism of this potential kinase-independent PKA function in adipogenesis remains to be uncovered, the ability of PKA R subunits to function outside the context of the PKA catalytic subunit is not new and has been well documented (39). For example, it has been reported that RIIβ can bind to cAMP-responsive element and activate transcription in a cAMP-dependent manner (40) whereas RIα is capable of interacting with the activated epidermal growth factor receptor (41). While one recent study suggests that cAMP-mediated stimulation of adipocyte differentiation requires the synergistic action of both PKA and Epac (28), other reports including our current study show that PKA is not required for 3T3-L1 adipocyte differentiation (31,42). This discrepancy could be potentially reconciled by the differential requirement of PKA catalytic and regulatory subunits for adipocyte differentiation. Our study points to the possibility that the proadipogenic effect of PKA may be contributed by the PKA regulatory subunits independent of the catalytic subunits or kinase activity. How the cAMP receptors collaborate with each other in promoting adipogenesis is under further investigation.
6. ACKNOWLEDGEMENTS
Xiaodong Cheng is supported by a Public Health Service grant (GM066170) from the National Institute of General Medical Sciences and a Grant-in-Aid from the American Heart Association (0755049Y). This publication is made possible in part by a Center Grant ES006676 from the National Institute of Environmental Health Sciences.
Abbreviations
- C/EBP
CCAAT/enhancer binding protein
- CREB
cAMP-responsive element-binding protein
- Epac/cAMP-GEF
exchange protein directly activated by cAMP/cAMP-regulated guanine nucleotide exchange factor
- GPDH
glycerophosphate dehydrogenase
- PKA/cAPK
protein kinase A/cAMP-dependent protein kinase
- PKI
heat stable protein kinase inhibitor
- PPARγ
peroxisome proliferator-activated receptor γ
- shRNA
small hairpin RNA
Footnotes
Publisher's Disclaimer: This is an, un-copyedited, author manuscript that has been accepted for publication in the Frontiers in Bioscience”. Cite this article as appearing in the Journal of Frontiers in Bioscience. Full citation can be found by searching the Frontiers in Bioscience (http://bioscience.org/search/authors/htm/search.htm) following publication and at PubMed (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=pubmed) following indexing. This article may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright holder, the Frontiers in Bioscience. From the time of acceptance following peer review, the full final copy edited article of this manuscript will be made available at http://www.bioscience.org/. The Frontiers in Bioscience disclaims any responsibility or liability for errors or omissions in this version of the un-copyedited manuscript or in any version derived from it by the National Institutes of Health or other parties.
7. REFERENCES
- 1.Cowherd RM, Lyle RE, McGehee RE., Jr Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol. 1999;10:3–10. doi: 10.1006/scdb.1998.0276. [DOI] [PubMed] [Google Scholar]
- 2.Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 3.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 4.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 5.Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 6.MacDougald OA, Lane MD. Transcriptional regulation of gene expression during adipocyte differentiation. Annu Rev Biochem. 1995;64:345–373. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- 7.Lin FT, Lane MD. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc Natl Acad Sci U S A. 1994;91:8757–8761. doi: 10.1073/pnas.91.19.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milestone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 9.Bernlohr DA, Bolanowski MA, Kelly TJ, Jr., Lane MD. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1985;260:5563–5567. [PubMed] [Google Scholar]
- 10.Reusch JE, Colton LA, Klemm DJ. CREB activation induces adipogenesis in 3T3-L1 cells. Mol Cell Biol. 2000;20:1008–1020. doi: 10.1128/mcb.20.3.1008-1020.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang JW, Klemm DJ, Vinson C, Lane MD. Role of CREB in transcriptional regulation of CCAAT/enhancer-binding protein beta gene during adipogenesis. J Biol Chem. 2004;279:4471–4478. doi: 10.1074/jbc.M311327200. [DOI] [PubMed] [Google Scholar]
- 12.Fox KE, Fankell DM, Erickson PF, Majka SM, Crossno JT, Jr., Klemm DJ. Depletion of cAMP-response element-binding protein/ATF1 inhibits adipogenic conversion of 3T3-L1 cells ectopically expressing CCAAT/enhancer-binding protein (C/EBP) alpha, C/EBP beta, or PPAR gamma 2. J Biol Chem. 2006;281:40341–40353. doi: 10.1074/jbc.M605077200. [DOI] [PubMed] [Google Scholar]
- 13.Tzameli I, Fang H, Ollero M, Shi H, Hamm JK, Kievit P, Hollenberg AN, Flier JS. Regulated production of a peroxisome proliferator-activated receptor-gamma ligand during an early phase of adipocyte differentiation in 3T3-L1 adipocytes. J Biol Chem. 2004;279:36093–36102. doi: 10.1074/jbc.M405346200. [DOI] [PubMed] [Google Scholar]
- 14.Casimir DA, Ntambi JM. cAMP activates the expression of stearoyl-CoA desaturase gene 1 during early preadipocyte differentiation. J Biol Chem. 1996;271:29847–29853. doi: 10.1074/jbc.271.47.29847. [DOI] [PubMed] [Google Scholar]
- 15.Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 16.Fox KE, Colton LA, Erickson PF, Friedman JE, Cha HC, Kelly P, MacDougald OA, Klemm DJ. Regulation of Cyclin D1 and Wnt10b Gene Expression by cAMP-responsive Element-binding Protein during Early Adipogenesis Involves Differential Promoter Methylation. J Biol Chem. 2008;283:35096–35105. doi: 10.1074/jbc.M806423200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Tang JR, Choi YH, Napolitano M, Hockman S, Taira M, Degerman E, Manganiello VC. Importance of cAMP-response element-binding protein in regulation of expression of the murine cyclic nucleotide phosphodiesterase 3B (Pde3b) gene in differentiating 3T3-L1 preadipocytes. J Biol Chem. 2006;281:21096–21113. doi: 10.1074/jbc.M601307200. [DOI] [PubMed] [Google Scholar]
- 18.Cheng YS, Lee TS, Hsu HC, Kou YR, Wu YL. Characterization of the transcriptional regulation of the regulator of G protein signaling 2 (RGS2) gene during 3T3-L1 preadipocyte differentiation. J Cell Biochem. 2008;105:922–930. doi: 10.1002/jcb.21893. [DOI] [PubMed] [Google Scholar]
- 19.Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 2008;40:651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- 21.Langin D, Ekholm D, Ridderstrale M, Lafontan M, Belfrage P. cAMP-dependent protein kinase activation mediated by beta 3-adrenergic receptors parallels lipolysis in rat adipocytes. Biochim Biophys Acta. 1992;1135:349–352. doi: 10.1016/0167-4889(92)90242-4. [DOI] [PubMed] [Google Scholar]
- 22.Fricke K, Heitland A, Maronde E. Cooperative activation of lipolysis by protein kinase A and protein kinase C pathways in 3T3-L1 adipocytes. Endocrinology. 2004;145:4940–4947. doi: 10.1210/en.2004-0803. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Hupfeld CJ, Taylor SS, Olefsky JM, Tsien RY. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature. 2005;437:569–573. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
- 24.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Ji Z, Mei FC, Miller AL, Thompson EB, Cheng X. Protein kinase A (PKA) isoform RIIbeta mediates the synergistic killing effect of cAMP and glucocorticoid in acute lymphoblastic leukemia cells. J Biol Chem. 2008;283:21920–21925. doi: 10.1074/jbc.M803193200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji Z, Mei FC, Xie J, Cheng X. Oncogenic KRAS activates hedgehog signaling pathway in pancreatic cancer cells. J Biol Chem. 2007;282:14048–14055. doi: 10.1074/jbc.M611089200. [DOI] [PubMed] [Google Scholar]
- 27.Qiao J, Mei FC, Popov VL, Vergara LA, Cheng X. Cell Cycle-dependent Subcellular Localization of Exchange Factor Directly Activated by cAMP. J Biol Chem. 2002;277:26581–26586. doi: 10.1074/jbc.M203571200. [DOI] [PubMed] [Google Scholar]
- 28.Petersen RK, Madsen L, Pedersen LM, Hallenborg P, Hagland H, Viste K, Doskeland SO, Kristiansen K. cAMP-mediated stimulation of adipocyte differentiation requires the synergistic action of Epac- and PKA-dependent processes. Mol Cell Biol. 2008;28:3804–3816. doi: 10.1128/MCB.00709-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem. 2002;277:11497–11504. doi: 10.1074/jbc.M110856200. [DOI] [PubMed] [Google Scholar]
- 30.Ji Z, Mei FC, Johnson BH, Thompson EB, Cheng X. Protein kinase A, not Epac, suppresses hedgehog activity and regulates glucocorticoid sensitivity in acute lymphoblastic leukemia cells. J Biol Chem. 2007;282:37370–37377. doi: 10.1074/jbc.M703697200. [DOI] [PubMed] [Google Scholar]
- 31.Li F, Wang D, Zhou Y, Zhou B, Yang Y, Chen H, Song J. Protein kinase A suppresses the differentiation of 3T3-L1 preadipocytes. Cell Res. 2008;18:311–323. doi: 10.1038/cr.2008.12. [DOI] [PubMed] [Google Scholar]
- 32.Madsen L, Pedersen LM, Liaset B, Ma T, Petersen RK, van den Berg S, Pan J, Muller-Decker K, Dulsner ED, Kleemann R, Kooistra T, Doskeland SO, Kristiansen K. cAMP-dependent signaling regulates the adipogenic effect of n-6 polyunsaturated fatty acids. J Biol Chem. 2008;283:7196–7205. doi: 10.1074/jbc.M707775200. [DOI] [PubMed] [Google Scholar]
- 33.Petersen RK, Jorgensen C, Rustan AC, Froyland L, Muller-Decker K, Furstenberger G, Berge RK, Kristiansen K, Madsen L. Arachidonic acid-dependent inhibition of adipocyte differentiation requires PKA activity and is associated with sustained expression of cyclooxygenases. J Lipid Res. 2003;44:2320–2330. doi: 10.1194/jlr.M300192-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Engh RA, Girod A, Kinzel V, Huber R, Bossemeyer D. Crystal structures of catalytic subunit of cAMP-dependent protein kinase in complex with isoquinolinesulfonyl protein kinase inhibitors H7, H8, and H89. Structural implications for selectivity. J Biol Chem. 1996;271:26157–26164. doi: 10.1074/jbc.271.42.26157. [DOI] [PubMed] [Google Scholar]
- 35.Walsh DA, Ashby CD, Gonzalez C, Calkins D, Fischer EH. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971;246:1977–1985. [PubMed] [Google Scholar]
- 36.Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, Schwede F, Genieser HG, Bos JL, Doskeland SO, Beavo JA, Butt E. Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods. 2008;5:277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- 37.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 38.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 39.Chin KV, Yang WL, Ravatn R, Kita T, Reitman E, Vettori D, Cvijic ME, Shin M, Iacono L. Reinventing the wheel of cyclic AMP: novel mechanisms of cAMP signaling. Ann N Y Acad Sci. 2002;968:49–64. doi: 10.1111/j.1749-6632.2002.tb04326.x. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava RK, Lee YN, Noguchi K, Park YG, Ellis MJ, Jeong JS, Kim SN, Cho-Chung YS. The RIIbeta regulatory subunit of protein kinase A binds to cAMP response element: an alternative cAMP signaling pathway. Proc Natl Acad Sci U S A. 1998;95:6687–6692. doi: 10.1073/pnas.95.12.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tortora G, Damiano V, Bianco C, Baldassarre G, Bianco AR, Lanfrancone L, Pelicci PG, Ciardiello F. The RIalpha subunit of protein kinase A (PKA) binds to Grb2 and allows PKA interaction with the activated EGF-receptor. Oncogene. 1997;14:923–928. doi: 10.1038/sj.onc.1200906. [DOI] [PubMed] [Google Scholar]
- 42.Martini CN, Plaza MV, Vila MC. PKA-dependent and independent cAMP signaling in 3T3-L1 fibroblasts differentiation. Mol Cell Endocrinol. 2009;298:42–47. doi: 10.1016/j.mce.2008.10.023. [DOI] [PubMed] [Google Scholar]