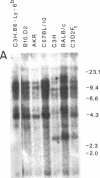
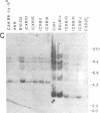
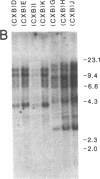
Abstract
The T-cell-activating protein TAP is a murine phosphatidylinositol-anchored glycoprotein whose expression is controlled by the Ly-6 locus. Previous studies have suggested an important role for this protein in physiological T-cell activation. Using oligonucleotide probes, we have now isolated a cDNA clone whose predicted sequence would encode a protein with an NH2-terminal sequence identical to that of the TAP molecule. Further analysis of the predicted protein sequence revealed a cysteine-rich protein with a hydrophobic domain at the COOH terminus and without N-linked glycosylation sites--all features consistent with our previous analysis of the TAP protein. In Southern blot analysis, the Ly-6.2 cDNA clone detects a multigene family and a restriction fragment length polymorphism that maps precisely to the Ly-6 locus. Expression of the cDNA clone in COS cells demonstrates that it codes for TAP and clarifies the relationship between the epitopes recognized by various alpha Ly-6 monoclonal antibodies. Finally, we have studied the expression of Ly-6 mRNA in a variety of cell lineages. Ly-6 transcripts were detected in all organs examined, including spleen, kidney, lung, brain, and heart. This demonstrates that the Ly-6 locus is transcriptionally active in a wide range of organs and suggests that the role of TAP or TAP-like proteins might extend to other tissues.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auchincloss H., Jr, Ozato K., Sachs D. H. Two distinct murine differentiation antigens determined by genes linked to the Ly-6 locus. J Immunol. 1981 Nov;127(5):1839–1843. [PubMed] [Google Scholar]
- Braun R. W., Reiser H. C. Replication of human cytomegalovirus in human peripheral blood T cells. J Virol. 1986 Oct;60(1):29–36. doi: 10.1128/jvi.60.1.29-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dembić Z., Haas W., Weiss S., McCubrey J., Kiefer H., von Boehmer H., Steinmetz M. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986 Mar 20;320(6059):232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Kimura S., Tada N., Liu-Lam Y., Hämmerling U. Studies of the mouse Ly-6 alloantigen system. II. Complexities of the Ly-6 region. Immunogenetics. 1984;20(1):47–56. doi: 10.1007/BF00373446. [DOI] [PubMed] [Google Scholar]
- Kuchel P. W., Campbell D. G., Barclay A. N., Williams A. F. Molecular weights of the Thy-1 glycoproteins from rat thymus and brain in the presence and absence of deoxycholate. Biochem J. 1978 Feb 1;169(2):411–417. doi: 10.1042/bj1690411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClair K. P., Palfree R. G., Flood P. M., Hammerling U., Bothwell A. Isolation of a murine Ly-6 cDNA reveals a new multigene family. EMBO J. 1986 Dec 1;5(12):3227–3234. doi: 10.1002/j.1460-2075.1986.tb04633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hussey R. E., Hodgdon J. C., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983 Jun 30;303(5920):808–810. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- Miller J., Germain R. N. Efficient cell surface expression of class II MHC molecules in the absence of associated invariant chain. J Exp Med. 1986 Nov 1;164(5):1478–1489. doi: 10.1084/jem.164.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Matsuki S., Ikehara M., Takahashi Y., Matsubara K. An alternative approach to deoxyoligonucleotides as hybridization probes by insertion of deoxyinosine at ambiguous codon positions. J Biol Chem. 1985 Mar 10;260(5):2605–2608. [PubMed] [Google Scholar]
- Palfree R. G., Dumont F. J., Hammerling U. Ly-6A.2 and Ly-6E.1 molecules are antithetical and identical to MALA-1. Immunogenetics. 1986;23(3):197–207. doi: 10.1007/BF00373821. [DOI] [PubMed] [Google Scholar]
- Palfree R. G., LeClair K. P., Bothwell A., Hämmerling U. cDNA characterization of an Ly-6.2 gene expressed in BW5147 tumor cells. Immunogenetics. 1987;26(6):389–391. doi: 10.1007/BF00343712. [DOI] [PubMed] [Google Scholar]
- Ralph W. W., Webster T., Smith T. F. A modified Chou and Fasman protein structure algorithm. Comput Appl Biosci. 1987 Sep;3(3):211–216. doi: 10.1093/bioinformatics/3.3.211. [DOI] [PubMed] [Google Scholar]
- Reiser H., Coligan J., Benacerraf B., Rock K. L. Biosynthesis, glycosylation, and partial N-terminal amino acid sequence of the T-cell-activating protein TAP. Proc Natl Acad Sci U S A. 1987 May;84(10):3370–3374. doi: 10.1073/pnas.84.10.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser H., Oettgen H., Yeh E. T., Terhorst C., Low M. G., Benacerraf B., Rock K. L. Structural characterization of the TAP molecule: a phosphatidylinositol-linked glycoprotein distinct from the T cell receptor/T3 complex and Thy-1. Cell. 1986 Nov 7;47(3):365–370. doi: 10.1016/0092-8674(86)90593-3. [DOI] [PubMed] [Google Scholar]
- Reiser H., Yeh E. T., Gramm C. F., Benacerraf B., Rock K. L. Gene encoding T-cell-activating protein TAP maps to the Ly-6 locus. Proc Natl Acad Sci U S A. 1986 May;83(9):2954–2958. doi: 10.1073/pnas.83.9.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Yeh E. T., Gramm C. F., Haber S. I., Reiser H., Benacerraf B. TAP, a novel T cell-activating protein involved in the stimulation of MHC-restricted T lymphocytes. J Exp Med. 1986 Feb 1;163(2):315–333. doi: 10.1084/jem.163.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. A., Huber B., Peña-Martinez J., Festenstein H. Genetic studies and effect on skin allograft survival of DBA-2 DAG, Ly, and M-locus antigens. Transplant Proc. 1973 Dec;5(4):1385–1387. [PubMed] [Google Scholar]
- Samelson L. E., Harford J. B., Klausner R. D. Identification of the components of the murine T cell antigen receptor complex. Cell. 1985 Nov;43(1):223–231. doi: 10.1016/0092-8674(85)90027-3. [DOI] [PubMed] [Google Scholar]
- Sandri-Goldin R. M., Goldin A. L., Levine M., Glorioso J. C. High-frequency transfer of cloned herpes simplex virus type 1 sequences to mammalian cells by protoplast fusion. Mol Cell Biol. 1981 Aug;1(8):743–752. doi: 10.1128/mcb.1.8.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Stiernberg J., Low M. G., Flaherty L., Kincade P. W. Removal of lymphocyte surface molecules with phosphatidylinositol-specific phospholipase C: effects on mitogen responses and evidence that ThB and certain Qa antigens are membrane-anchored via phosphatidylinositol. J Immunol. 1987 Jun 1;138(11):3877–3884. [PubMed] [Google Scholar]
- Takahashi Y., Kato K., Hayashizaki Y., Wakabayashi T., Ohtsuka E., Matsuki S., Ikehara M., Matsubara K. Molecular cloning of the human cholecystokinin gene by use of a synthetic probe containing deoxyinosine. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1931–1935. doi: 10.1073/pnas.82.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei F., Galfrè G., Alderson T., Lennox E. S., Milstein C. H 9/25 monoclonal antibody recognizes a new allospecificity of mouse lymphocyte subpopulations: strain and tissue distribution. Eur J Immunol. 1980 Apr;10(4):241–246. doi: 10.1002/eji.1830100404. [DOI] [PubMed] [Google Scholar]
- Yagüe J., White J., Coleclough C., Kappler J., Palmer E., Marrack P. The T cell receptor: the alpha and beta chains define idiotype, and antigen and MHC specificity. Cell. 1985 Aug;42(1):81–87. doi: 10.1016/s0092-8674(85)80103-3. [DOI] [PubMed] [Google Scholar]
- Yeh E. T., Reiser H., Bamezai A., Rock K. L. TAP transcription and phosphatidylinositol linkage mutants are defective in activation through the T cell receptor. Cell. 1988 Mar 11;52(5):665–674. doi: 10.1016/0092-8674(88)90404-7. [DOI] [PubMed] [Google Scholar]
- Yeh E. T., Reiser H., Benacerraf B., Rock K. L. Expression of T-cell-activating protein in peripheral lymphocyte subsets. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7424–7428. doi: 10.1073/pnas.83.19.7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E. T., Reiser H., Benacerraf B., Rock K. L. The expression, function, and ontogeny of a novel T cell-activating protein, TAP, in the thymus. J Immunol. 1986 Aug 15;137(4):1232–1238. [PubMed] [Google Scholar]