Abstract
Emotional intelligence (EI) refers to a set of competencies that are essential features of human social life. Although the neural substrates of EI are virtually unknown, it is well established that the prefrontal cortex (PFC) plays a crucial role in human social-emotional behavior. We studied a unique sample of combat veterans from the Vietnam Head Injury Study, which is a prospective, long-term follow-up study of veterans with focal penetrating head injuries. We administered the Mayer-Salovey-Caruso Emotional Intelligence Test as a valid standardized psychometric measure of EI behavior to examine two key competencies of EI: (i) Strategic EI as the competency to understand emotional information and to apply it for the management of the self and of others and (ii) Experiential EI as the competency to perceive emotional information and to apply it for the integration into thinking. The results revealed that key competencies underlying EI depend on distinct neural PFC substrates. First, ventromedial PFC damage diminishes Strategic EI, and therefore, hinders the understanding and managing of emotional information. Second, dorsolateral PFC damage diminishes Experiential EI, and therefore, hinders the perception and integration of emotional information. In conclusion, EI should be viewed as complementary to cognitive intelligence and, when considered together, provide a more complete understanding of human intelligence.
Keywords: emotion, neuroeconomics, prefrontal cortex, social cognition, head injury
Emotional intelligence (EI) refers to a set of competencies that enable us to engage in sophisticated information processing about emotions and emotion-relevant stimuli and to use this information as a guide for thinking and behavior (1). Although emotional and cognitive intelligence form important components of general intelligence, there is a lively debate in diverse academic disciplines about whether EI should be considered as an instance of a standard intelligence and how EI can enrich the discussion of human capacities (2–4). For example, in behavioral economics, there is an ongoing debate about the distinctive contributions of cognitive intelligence and EI in serving adaptive functions that potentially benefit self- and other-regarding behavior. This controversy is captured in the two great works of Adam Smith. In his work Wealth of Nations (5), Smith essentially emphasized a cognitive intelligent view that unintentional benefits would stem from individuals' pursuit of their own wants and needs, and therefore, argued that a free market economy would be most productive and beneficial to society. In contrast, in his work Theory of Moral Sentiments (6), Smith essentially emphasized an emotional intelligent view that sympathy arising from an innate desire to identify with the emotions of others led people to strive to maintain good relations with their fellow human beings, and therefore provided the basis both for specific benevolent acts and for stabilizing the general social order.
Despite the pivotal role of EI in coping with the challenges of social daily life (7), remarkably little is known about the neural substrates of EI. However, it is well established that the prefrontal cortex (PFC), as the most recently evolved brain region (8), plays a crucial role in human social-emotional behavior (9–12). In particular, the ventromedial PFC (vmPFC) is hypothesized to mediate knowledge crucial to manage emotionally relevant information. For example, vmPFC damage results in social incompetence, problems in interpersonal interactions, and abnormal changes in mood and personality (13–16) as well as demonstration of poor decisions in laboratory tasks ranging from moral judgment to economic games (17–21). In contrast, the dorsolateral PFC (dlPFC) is hypothesized to support the perception of emotionally relevant information (11, 22). For example, recent evidence demonstrates the recruitment of the dlPFC for perceiving the permissibility or fairness of observed behavior ranging from economic games to judgment about appropriate forms of punishment in a legal and moral decision making (20, 23–27).
In this study, we examine two key competencies of EI by administering the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) (28), a valid standardized psychometric ability-measure of emotional intelligent behavior: (i) Strategic EI is the competency to understand (to realize the causes of emotions) and manage (to figure out effective strategies that apply emotions helping to achieve a goal) emotions; and (ii) Experiential EI is the competency to perceive (to correctly identify how people are feeling) and use (to integrate feelings into thinking) emotions (Fig. 1A). We tested a unique sample of brain-damaged veterans from the Vietnam Head Injury Study (29). This military population offers a number of advantages, including its size, relative uniformity, and preinjury variables for comparison with postinjury performance. We evaluated the performances of combat veterans (n = 67) and divided them into dlPFC (n = 17) and vmPFC (n = 21) lesion (experimental) groups and a non-head-injured (n = 29) group (control, NC) based upon the presence or absence of local penetrating head injuries (PHIs) due to low velocity shrapnel wounds. The experimental and control groups were matched with respect to age, level of education, handedness, and preinjury general intelligence. In addition, we administered standard neuropsychological tests to assess patients' cognitive functioning and intelligence. Our findings demonstrate that key components of EI are mediated by distinct PFC subregions.
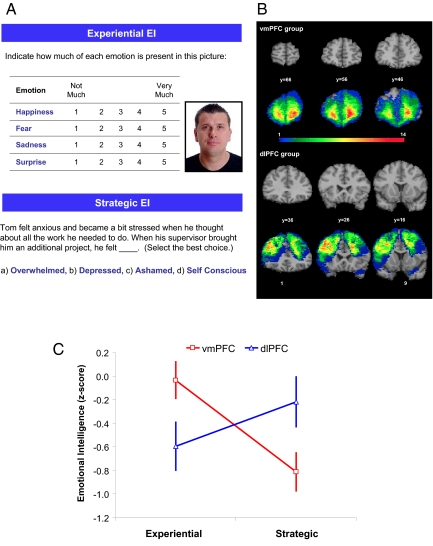
Fig. 1.
Neural substrates of EI. (A) Example items for Experiential and Strategic EI are shown. (B) Coronal views of a healthy adult brain (top and third row), vmPFC group lesion overlap (second row), and dlPFC group lesion overlap (bottom row) are shown in Montreal Neurological Institute space. In each coronal slice, the right hemisphere is on the reader's left. The color indicates the number of individuals with damage to a given voxel. (C) Normalized means (z-scores) and standard errors (SEM) for the Experiential and Strategic EI scores of the vmPFC and dlPFC lesion groups are presented. The individual that appears in A did not participate in the study. The picture is not originally taken from the MSCEIT E-IQ test.
Results
Damaged brain regions in our patient sample were outlined using computed tomography (CT) scans, and lesion locations were determined using the Analysis of Brain Lesions (ABLe) software (30, 31). The coronal views of lesion overlay maps for the damage in the dlPFC and vmPFC groups are displayed in Fig. 1B. The color indicates the number of individuals with damage to a given voxel.
The Experiential EI and Strategic EI scores of the dlPFC and vmPFC groups were normalized (z-transformation) in comparison to the performances of the NC group. A mixed two-way 2 (EI) × 2 (Group) repeated measures ANOVA was applied with EI (Experiential versus Strategic) as a within-subject factor and Group (dlPFC versus vmPFC) as a between-subject factor. The analysis showed no significant main effects for EI (F1,36 = 2.68, P = 0.110) and Group (F1,36 = 0.01, P = 0.938), but a significant interaction (EI × Group) effect (F1,36 = 22.41, P < 0.001), indicating a double dissociation in patients' EI performances (Fig. 1C). Planned followed-up independent-samples t tests revealed that the vmPFC group was significantly impaired in Strategic EI (t36 = −2.26; P < 0.030) and the dlPFC group was significantly impaired in Experiential EI (t36 = 2.29; P < 0.028). No EI performance differences were observed among lesion subgroups (left, right, and bilateral) (vmPFC: Experiential EI; χ2 = 0.92, P = 0.633; Strategic EI: χ2 = 0.76, P = 0.684; and dlPFC: Experiential EI: χ2 = 0.65, P = 0.724; Strategic EI: χ2 = 2.97, P = 0.226).
Despite the EI performance differences, the vmPFC and dlPFC did not differ on measures of cognitive intelligence [Wechsler Adult Intelligence Scale-III (WAIS-III): Full-scale IQ, t36 = 0.68; P = 0.501; verbal IQ, t36 = 0.58; P = 0.578; performance IQ, t36 = 0.75; P = 0.457], executive functioning [Delis Kaplan Executive Function System (D-KEFS): Tower Task, t36 = −0.64; P = 0.527; Trail Making, t36 = 0.76; P = 0.451], memory [Wechsler Memory Scale-III (WMS-III): General memory, t36 = −0.95, P = 0.348; working memory, t36 = −0.19, P = 0.949], verbal comprehension [Token Test (TT): t36 = −0.09; P = 0.932], and perception [Visual Object and Space Perception Battery (VOSP): t36 = 0.59; P = 0.555] (Table 1).
Table 1.
Description of neuropsychological tests (mean ± SD)
| Test/Group | vmPFC | dlPFC | NC |
|---|---|---|---|
| MSCEIT (Full Scale) | 91.3 ± 11.5 | 88.56 ± 12.4 | 95.8 ± 12.7 |
| MSCEIT (Experiential) | 103.6 ± 14.5 | 92.9 ± 14.0 | 104.4 ± 19.2 |
| Perceiving Emotion | 104.8 ± 13.8 | 96.6 ± 17.9 | 107.9 ± 23.3 |
| Using Emotion | 102.3 ± 15.3 | 96.21 ± 14.7 | 108.0 ± 13.9 |
| MSCEIT (Strategic) | 84.9 ± 7.9 | 92.1 ± 11.3 | 94.6 ± 11.9 |
| Understanding Emotion | 87.9 ± 9.8 | 93.3 ± 11.7 | 96.2 ± 10.9 |
| Managing Emotion | 83.0 ± 9.0 | 90.4 ± 11.6 | 91.3 ± 10.1 |
| WAIS-III (Full-Scale) | 104.9 ± 9.9 | 102.4 ± 13.6 | 110.0 ± 10.4 |
| WAIS-III (Verbal) | 106.7 ± 10.2 | 104.5 ± 13.3 | 110.0 ± 11.5 |
| WAIS-III (Performance) | 102.3 ± 11.7 | 99.0 ± 15.6 | 108.4 ± 12.4 |
| D-KEFS (Tower) | 10.3 ± 3.0 | 10.9 ± 3.3 | 11.4 ± 2.7 |
| D-KEFS (Trail Making) | 8.9 ± 3.1 | 8.1 ± 3.6 | 9.2 ± 3.5 |
| WMS-III (General Memory) | 96.2 ± 15.4 | 99.7 ± 12.3 | 105.5 ± 12.9 |
| WMS-III (Working Memory) | 99.8 ± 13.5 | 100.6 ± 13.1 | 109.2 ± 13.6 |
| TT (Total) | 98.1 ± 1.9 | 98.1 ± 2.9 | 98.9 ± 1.4 |
| VOSP (Total) | 19.9 ± 0.3 | 19.8 ± 0.5 | 19.6 ± 1.8 |
vmPFC, ventromedial PFC group; dlPFC, dorsolateral PFC group; NC, non-head-injured control group; MSCEIT, Mayer-Salovey-Caruso Emotional Intelligence Test for emotional intellectual ability, WAIS-III, Wechsler Adult Intelligence Scale-III for cognitive intellectual ability; D-KEFS, Delis Kaplan Executive Function System for executive functioning; WMS-III, Wechsler Memory Scale-III for general memory and working memory; TT, Token test for basic verbal comprehension; and VOSP, Visual Object and Space Perception Battery for object and space perception.
Discussion
The goal of this study was to investigate the neural bases of key competencies of EI. Based on the assumption that the PFC plays a crucial role in human social-emotional behavior, we administrated the MSCEIT in individuals with vmPFC and dlPFC brain damage. Our findings indicate a double dissociation in patients' EI performances and provide empirical evidence that key competencies underlying EI are mediated by distinct neural PFC substrates.
First, vmPFC damage diminishes Strategic EI, and therefore, hinders the understanding and managing of emotional information. The vmPFC is interconnected with limbic structures critical for long-term memory and the processing of internal states (affect and motivation) (32–34), which make it well suited for processing knowledge that is crucial for understanding and managing emotionally relevant information (4, 35–37). The neural system involved in Strategic EI overlaps with the neural system that subserves personal judgment and real-life decision-making. Building on convergent evidence, vmPFC damage results in social incompetence, diminished sensitivity to socially relevant stimuli and situational nuances, problems in interpersonal interactions, and abnormal changes in mood and personality (13–16). Moreover, patients with such lesions have a diminished capacity to respond to emotional value attributed to rewards and punishment (27, 38–40), demonstrate poor decisions in laboratory tasks ranging from moral judgment to economic games (17–21), and display poor judgment regarding their personal and occupational affairs (36, 41–43). Our finding for the Strategic EI component complements a previous lesion study that demonstrated an association between vmPFC damage and impaired EI measured by the Emotional Quotient Inventory (4). However, our study goes one step further by advancing our understanding of the underlying neural bases of EI and provides additional empirical evidence that different competencies underlying EI depend on separate neural PFC substrates.
Second, dlPFC damage diminishes Experiential EI and therefore hinders the perception and use of emotional information. The dlPFC is closely interconnected with the sensory neocortex receiving converging visual, somatosensory, and auditory information from the occipital, temporal, and parietal cortices (44–47), which make it well suited to perceive and use emotionally relevant information (48). The neural system involved in Experiential EI overlaps with the neural system that enable people to orchestrate their thoughts and actions in concert with their intentions to support goal-directed social-emotional behavior (49, 50). Accumulating evidence demonstrates the recruitment of the dlPFC for evaluating the permissibility or fairness of observed behavior ranging from economic games to judgment about appropriate forms of punishment in legal and moral decision making (20, 23–26). This finding sheds light on the role of the dlPFC, revealing a dimension of social cognition. It suggests that this region is central for emotional intelligent behavior as the competency to perceive emotional information and to integrate it into thinking.
Two important insights emerged from these findings. On the one hand, the present study reveals that competencies underlying EI have clear neural foundations and can be impaired despite otherwise normal basic intellectual functioning. Previous findings have demonstrated that the behavioral and emotional dysfunction associated with vmPFC damage cannot be explained by impaired cognitive intelligence measured by standard intelligence tests (e.g., WAIS/WAIS-R) (4, 13, 51, 52). Furthermore, although the dlPFC has been associated with cognitive intelligence (53–55), recent lesion evidence failed to support the hypothesis that dlPFC damage would disproportionately impair general measures of cognitive intelligence (e.g., verbal IQ, performance IQ, and full-scale IQ) (51). On the other hand, EI complements cognitive intelligence and permits the evaluation of individual differences in emotional and social processes—such processes are key factors in making the right versus wrong decisions in one's personal life and in influencing our choice about optimal situation-specific social and economic exchange strategies (7, 56).
Importantly, we stress that our findings highlight only two essential brain structures underlying key competencies of EI. Because vmPFC and dlPFC are higher-order association areas, it is likely that broadly distributed neural systems, incorporating subcortical limbic structures, such as the amygdala, which mediates emotional processes, and closely associated regions, such as the insula, cingulate cortex, and parietal cortices, which influence emotionally related behaviors (4, 37, 57, 58), will play important roles in subcomponents of EI. Future studies will need to examine each of these regions and their connectivity.
In conclusion, the reported findings broaden our understanding on how EI is mediated by the social brain and demonstrates that it can be dissociated from cognitive intelligence. In addition, the controversy over the coexistence and dual influences of cognitive and EI in behavioral economics embodied in the two great works of Adam Smith can be resolved if we recognize that social exchange is a fundamental distinguishing feature of humans and that it finds expression in both impersonal exchange through large-group markets and personal exchange in small-group social transactions (59). EI is a distinguishing feature of human social exchange and should be viewed as complementary to cognitive intelligence and, when considered together, will provide a more complete understanding of human behavior. Future studies have to address the precise relationship between cognitive (e.g., fluid and crystallized) and emotional (e.g., experiential and strategic) intelligence and to what extent different types of intelligence help to explain the observed deficits in patients with brain damage.
Materials and Methods
Subjects.
Participants were drawn from the W.F. Caveness Vietnam Head Injury Study registry, which is a prospective, long-term follow-up study of veterans with focal PHIs (29). We evaluated 67 combat veterans and divided them into dlPFC (n = 17) and vmPFC (n = 21) lesion (experimental) groups and a non-head-injured (n = 29) group (control, NC) based upon the presence or absence of local PHIs. The experimental and control groups were matched with respect to age (F2,66 = 1.25; P = 0.293), level of education (F2,66 = 0.09; P = 0.908), handedness (contingency coefficient = 0.24, P = 0.396), and preinjury general intelligence (F2,66 = 0.28; P = 0.758) (Table 2). All participants understood the study procedures and gave their written informed consent, which was approved by the Institutional Review Board at the National Naval Medical Center and the National Institute of Neurological Disorders and Stroke.
Table 2.
Description of demographic characteristics (mean ± SD)
| Group | vmPFC | dlPFC | NC |
|---|---|---|---|
| Age, y | 57.3 ± 1.9 | 57.9 ± 2.4 | 58.3 ± 2.1 |
| Education, y | 14.3 ± 2.5 | 14.6 ± 2.6 | 14.5 ± 2.5 |
| Handedness (R/A/L) | 18/0/3 | 17/0/0 | 24/1/4 |
| AFQT (Percentile) | 63.8 ± 23.8 | 64.9 ± 23.8 | 68.3 ± 21.1 |
vmPFC, ventromedial PFC group; dlPFC, dorsolateral PFC group; NC, non-head-injured control group; R, right-handed; A, ambidextrous; L, left-handed; AFQT, Armed Forces Qualification Test for pre-injury general intelligence administered to individuals upon entry into the military.
CT Acquisition and Analysis.
Axial CT scans without contrast were acquired on a GE Medical Systems Light Speed Plus CT scanner in helical mode at the Bethesda Naval Hospital, Bethesda, MD., Structural neuroimaging data were reconstructed with an in-plane voxel size of 0.4 × 0.4 mm, an overlapping slice thickness of 2.5 mm, and a 1-mm slice interval. Lesion location and volume from CT images were determined using the interactive ABLe software (30, 31), implemented in MEDx v3.44 (Medical Numerics) with enhancements to support the Automated Anatomical Labeling (AAL) atlas (60).
For the hypothesis about specific brain areas (vmPFC and dlPFC), regions of interests (ROIs) were defined in terms of AAL structures (60) and Talairach coordinates (61). As a part of this process, the CT image of each subject's brain was normalized to a CT template brain image in Montreal Neurological Institute (MNI) space. Afterward, the percentage of AAL structures that were intersected by the lesion was determined by analyzing the overlap of the spatially normalized lesion image with the AAL atlas. Lesion volume was calculated by manually tracing the lesion in all relevant slices of the CT image in native space and then summing the trace areas and multiplying by slice thickness. Manual tracing was performed by a trained psychiatrist (V.R.) with clinical experience of reading CT scans. It was then reviewed by an observer that was blind to the results of the clinical evaluation and neuropsychological testing (J.G.), enabling a consensus decision to be reached regarding the limits of each lesion.
The vmPFC ROI included portions of the following AAL structures: superior frontal gyrus (medial part), superior frontal gyrus (orbital part), superior frontal gyrus (medial orbital part), middle frontal gyrus (orbital part), inferior frontal gyrus (orbital part), gyrus rectus, olfactory cortex, anterior cingulate, and paracingulate gyri. The portions of these structures included in the vmPFC ROI were those areas that were inferior to the anterior commissure (z value less than zero) and between 0 and 20 mm left and right from the anterior commissure (left vmPFC: −20 < × <0; right vmPFC: 0 < × <20). These criteria outlined an area comprising the ventral portion of the medial prefrontal cortex (below the level of the genu of the corpus callosum) and the medial portion of the orbital surface (approximately the medial one-third of the orbitofrontal cortex in each hemisphere) as well as the subjacent white matter. Of the 21 vmPFC patients, seven had bilateral vmPFC lesions, nine had exclusively or predominantly left vmPFC lesions, and five had exclusively or predominantly right vmPFC lesions.
The dlPFC ROI included portions of the following AAL structures: superior frontal gyrus (dorsolateral part), middle frontal gyrus (lateral part), and inferior frontal gyrus (triangular part). The portions of these structures included in the dlPFC ROI were those areas that were inferior to the anterior commissure (z value more than zero) and between 0 and 10 mm left and right from the anterior commissure (left dlPFC: −10 < × <0; right dlPFC: 0 < × <10). These criteria outlined an area comprising the dorsal portion of the lateral prefrontal cortex and subjacent white matter. Of the 17 dlPFC patients, three had bilateral dlPFC lesions, six had exclusively or predominantly left dlPFC lesions, and eight had exclusively or predominantly right dlPFC lesions.
Neuropsychological Testing.
Participants were assessed from 2003 to 2006 at the National Naval Medical Center in Bethesda, MD, over a 5- to 7-day period with tests that measured a wide variety of neuropsychological functions including memory, language, executive functioning, and social cognition. For this study, we focused on the assessment of EI. We administered the MSCEIT V2.0, which is a valid standardized psychometric measure of emotionally intelligent behavior (28). It is acknowledged as a valid ability-measure of EI (62) and correlates with other self-report measures of EI such as the Bar-On Emotional Quotient Inventory (r = 0.46) (63).
The MSCEIT as a 141-item scale focuses on emotion-related competencies that can be assessed through performance-based standardized norms. Responses on the MSCEIT were scored with respect to their degree of correctness, as determined by their correspondence with the answers provided by a normative sample of the general population. Besides the Full-Scale EI, the MSCEIT yields two area scores each combining two branch scores: (i) Experiential EI as the competency of Perceiving Emotions (i.e., to perceive and identify emotions both in oneself and in others; for example, it includes the ability to accurately read facial expressions) and Using Emotions (i.e., to harness emotions to facilitate thinking; for example, anticipating another person's emotional reaction and using that knowledge to modify one's own behavior); and (ii) Strategic EI as the competency of Understanding Emotions (i.e., to realize the causes of emotions; for example, understanding the relationship between sadness and loss) and Managing Emotions (i.e., to figure out effective strategies that use emotions to help to achieve a goal; for example, conscious regulation of emotions both in oneself and in others). A more detailed discussion of the psychometric properties of the MSCEIT and how it was developed can be found in the MSCEIT user's manual (64) and elsewhere (1, 2).
In addition, participants were administered a set of neuropsychological tests including the WAIS-III (full-scale, performance, and verbal IQ) (65) for cognitive intellectual ability, D-KEFS (Tower Task and Trail Making) (66) for executive functioning, WMS-III (general memory index and working memory index) (67) for general and working memory, TT (Total score) (68) for basic verbal comprehension, and VOSP (Total score) (69) for object and space perception. Furthermore, preinjury general intelligence was assessed with the Armed Forces Qualification Test (AFQT-7A) administered to individuals upon entry into military. The AFQT is composed of four subtests (vocabulary knowledge, arithmetic word problems, object-function matching, and mental imagery) via multiple choice questions (70). It has been extensively standardized within the U.S. military and correlates highly with WAIS IQ scores (71).
Statistical Analysis.
Behavioral data analysis was carried out using SPSS 11.0 (www.spss.com; SPSS), and alpha was set to P < 0.05 (two-tailed) for all analyses. Data were tested for Gaussian distribution (Kolmogorov–Smirnov test) and variance homogeneity (Bartlett's test). Unless otherwise specified, data were normally distributed, and assumptions for analyses of variance were not violated. The Experiential and Strategic EI scores of the experimental groups were z-transformed in relation to normal group's performance. A mixed two-way 2 (EI) × 2 (group) repeated measures analysis of variance (ANOVA) was applied with EI (Experiential versus Strategic) as a within-subject factor and Group (dlPFC versus vmPFC) as a between subject factor. Planned follow-up independent-samples t tests were applied to compare performances between the experimental groups. Finally, nonparametric tests (Kruskal-Wallis test) were applied to compare EI performances among lesion (left, right, and bilateral) subgroups of each experimental group.
Acknowledgments.
We thank the National Naval Medical Center for their support and provision of their facilities and S. Bonifant, B. Cheon, C. Ngo, A. Greathouse, K. Reding, and G. Tasick for their invaluable help with the testing of participants and organization of this study. The work was supported by the United States National Institute of Neurological Disorders and Stroke intramural research program and a project grant from the United States Army Medical Research and Material Command administrated by the Henry M. Jackson Foundation (Vietnam Head Injury Study Phase III: a 30-year postinjury follow-up study, Grant DAMD17-01-1-0675). Note that the views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, nor the United States Government.
Footnotes
The authors declare no conflict of interest.
References
- 1.Mayer JD, Salovey P, Caruso DR. Emotional intelligence: New ability or eclectic traits? Am Psychol. 2008;63:503–517. doi: 10.1037/0003-066X.63.6.503. [DOI] [PubMed] [Google Scholar]
- 2.Mayer JD, Salovey P, Caruso DR, Sitarenios G. Emotional intelligence as a standard intelligence. Emotion. 2001;1:232–242. [PubMed] [Google Scholar]
- 3.Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. NeuroImage. 2004;22:1151–1156. doi: 10.1016/j.neuroimage.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;126:1790–1800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- 5.Smith A. Wealth of nations. In: Bullock CJ, editor. Harvard Classics. Vol 10. New York, NY: P.F. Colliert; 1909. [Google Scholar]
- 6.Smith A. The theory of moral sentiments. In: Raphael DD, Mactie AL, editors. Liberty Classics. Indianapolis, IN: Liberty Press; 1976. [Google Scholar]
- 7.Lopes PN, et al. Emotional intelligence and social interaction. Pers Soc Psychol Bull. 1004;30:1018–1034. doi: 10.1177/0146167204264762. [DOI] [PubMed] [Google Scholar]
- 8.Semendeferi K, Lu A, Schenker N, Damasio H. Humans and great apes share a large frontal cortex. Nat Neurosci. 2002;5:272–276. doi: 10.1038/nn814. [DOI] [PubMed] [Google Scholar]
- 9.Dunbar RIM. The social brain hypothesis. Evol Anthropol. 1998;6:178–190. [Google Scholar]
- 10.Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 11.Dalgleish T. The emotional brain. Nat Rev Neurosci. 2004;5:583–589. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- 12.Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- 13.Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: Patient EVR. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- 14.Grafman J, Vance SC, Weingartner H, Salazar AM, Amin D. The effects of lateralized frontal lesions on mood regulation. Brain. 1986;109:1127–1148. doi: 10.1093/brain/109.6.1127. [DOI] [PubMed] [Google Scholar]
- 15.Stuss DT, Gow CA, Hetherington CR. “No longer Gage:” Frontal lobe dysfunction and emotional changes. J Consult Clin Psychol. 1992;60:349–359. doi: 10.1037//0022-006x.60.3.349. [DOI] [PubMed] [Google Scholar]
- 16.Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: Insights from patients with orbitofrontal damage. J Pers Soc Psychol. 2003;85:594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- 17.Krajbich I, Adolphs R, Tranel D, Denburg NL, Camerer CF. Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. J Neurosci. 2009;29:2188–2192. doi: 10.1523/JNEUROSCI.5086-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: Evidence from the Ultimatum Game. J Neurosci. 2007;27:951–956. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koenigs M, et al. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446:908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krueger F, Grafman J, McCabe K. Review. Neural correlates of economic game playing. Philos Trans R Soc Lond B Biol Sci. 2008;363:3859–3874. doi: 10.1098/rstb.2008.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moll J, et al. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci USA. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuss DT, Benson DF. The Frontal Lobes. New York, NY: Raven Press; 1986. [Google Scholar]
- 23.Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- 24.Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314:829–832. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- 25.Buckholtz JW, et al. The neural correlates of third-party punishment. Neuron. 2008;60:930–940. doi: 10.1016/j.neuron.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44:389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 27.Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. Opinion: The neural basis of human moral cognition. Nat Rev Neurosci. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- 28.Mayer JD, Salovey P, Caruso DR, Sitarenios G. Measuring emotional intelligence with the MSCEIT V2.0. Emotion. 2003;3:97–105. doi: 10.1037/1528-3542.3.1.97. [DOI] [PubMed] [Google Scholar]
- 29.Raymont V, et al. Demographic, structural and genetic predictors of late cognitive decline after penetrating head injury. Brain. 2008;131:543–558. doi: 10.1093/brain/awm300. [DOI] [PubMed] [Google Scholar]
- 30.Makale M, et al. Quantification of brain lesions using interactive automated software. Behav Res Methods Instrum Comput. 2002;34:6–18. doi: 10.3758/bf03195419. [DOI] [PubMed] [Google Scholar]
- 31.Solomon J, Raymont V, Braun A, Butman JA, Grafman J. User-friendly software for the analysis of brain lesions (ABLe) Comput Methods Programs Biomed. 2007;86:245–254. doi: 10.1016/j.cmpb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 33.Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol. 1990;300:549–571. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- 34.Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol. 1981;198:121–136. doi: 10.1002/cne.901980111. [DOI] [PubMed] [Google Scholar]
- 35.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 36.Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38:589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- 37.Krueger F, Barbey AK, Grafman J. The medial prefrontal cortex mediates social event knowledge. Trends Cogn Sci. 2009;13:103–109. doi: 10.1016/j.tics.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 39.Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- 40.Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 41.Ackerly SS, Benton AL. Report of a case of bilateral frontal lobe defect. Research Publication of the Association of Research in Nervous and Mental Disease. 1948;27:479–504. [PubMed] [Google Scholar]
- 42.Adolphs R. Social cognition and the human brain. Trends Cogn Sci. 1999;3:469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- 43.Brickner RM. An interpretation of frontal lobe function based upon the study of a case of partial bilateral frontal lobectomy. Proceedings of the Association for Research of Nervous and Mental Disorders; 1934. pp. 259–351. [Google Scholar]
- 44.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 45.Goldman-Rakic PS, Schwartz ML. Interdigitation of contralateral and ipsilateral columnar projections to frontal association cortex in primates. Science. 1982;216:755–757. doi: 10.1126/science.6177037. [DOI] [PubMed] [Google Scholar]
- 46.Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- 47.Seltzer B, Pandya DN. Frontal lobe connections of the superior temporal sulcus in the rhesus monkey. J Comp Neurol. 1989;281:97–113. doi: 10.1002/cne.902810108. [DOI] [PubMed] [Google Scholar]
- 48.Mah L, Arnold MC, Grafman J. Impairment of social perception associated with lesions of the prefrontal cortex. Am J Psychiatry. 2004;161:1247–1255. doi: 10.1176/appi.ajp.161.7.1247. [DOI] [PubMed] [Google Scholar]
- 49.Lieberman MD. Social cognitive neuroscience: A review of core processes. Annu Rev Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- 50.Barbey AK, Krueger F, Grafman J. An evolutionarily adaptive neural architecture for social exchange. Trends Neurosci. 2009;32:603–610. doi: 10.1016/j.tins.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tranel D, Manzel K, Anderson SW. Is the prefrontal cortex important for fluid intelligence? A neuropsychological study using Matrix Reasoning. Clin Neuropsychol. 2008;22:242–261. doi: 10.1080/13854040701218410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blair RJ, Cipolotti L. Impaired social response reversal. A case of “acquired sociopathy”. Brain. 2000;123:1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- 53.Duncan J. Attention, intelligence, and the frontal lobes. In: Gazzaniga MS, editor. The Cognitive Neurosciences. Cambridge, MA: MIT Press; 1995. pp. 721–733. [Google Scholar]
- 54.Duncan J, et al. A neural basis for general intelligence. Science. 2000;289:457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- 55.Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behav Brain Sci. 2007;30:135–154. doi: 10.1017/S0140525X07001185. discussion 154–187. [DOI] [PubMed] [Google Scholar]
- 56.Grewal D, Davidson HA. Emotional intelligence and graduate medical education. JAMA. 2008;300:1200–1202. doi: 10.1001/jama.300.10.1200. [DOI] [PubMed] [Google Scholar]
- 57.Damasio AR. Toward a neurobiology of emotion and feeling: Operational concepts and hypotheses. Neuroscientist. 1995;1:19–25. [Google Scholar]
- 58.Bechara A, Tranel D, Damasio AR. Poor judgment in spite of high intellect: Neurological evidence for emotional intelligence. In: Bar-On R, Parker JDA, editors. The Handbook of Emotional Intelligence. San Francisco, CA: Jossey-Bass; 2000. pp. 192–214. [Google Scholar]
- 59.Smith V. The two faces of Adam Smith. South Econ J. 1998;65:1–19. [Google Scholar]
- 60.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 61.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
- 62.Mayer JD, Caruso DR, Salovey P. Emotional intelligence meets traditional standards for an intelligence. Intelligence. 1999;27:267–298. [Google Scholar]
- 63.Bar-On R. The Bar-On Emotional Quotient Inventory (EQ-i): A Test of Emotional Intelligence. Toronto, Canada: Multi-Health Systems; 1997. [Google Scholar]
- 64.Mayer JD, Salovey P, Caruso DR, Sitarenios G. Mayer-Salovey-Caruso Emotional Intelligence Test (Version 2.0) User's Manual. Toronto, Canada: Multi-Health Systmes; 2002. [Google Scholar]
- 65.Wechsler D. Wechsler Adult Intelligence Scale. 3rd Ed. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 66.Delis DC, Kaplan E, Kramer JH. Delis Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 67.Wechsler DA. Wechsler Memory Scale-III. New York, NY: Psychological Corporation; 1997. [Google Scholar]
- 68.McNeil MM, Prescott TE. Revised Token Test. Los Angeles, CA: Western Psychological Services; 1994. [Google Scholar]
- 69.Warrington EK, James M. Visual Object and Space Perception Battery. Oxford, UK: Pearson Assessment; 1991. [Google Scholar]
- 70.Anonymous. Armed Forces Qualification Test (AFQT-7A) Department of Defense Form 1293. 1960 [Google Scholar]
- 71.Grafman J, et al. Intellectual function following penetrating head injury in Vietnam veterans. Brain. 1988;111:169–184. doi: 10.1093/brain/111.1.169. [DOI] [PubMed] [Google Scholar]