Abstract
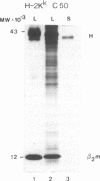
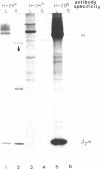
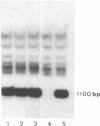
Transfection of cells with the H-2Kk gene lacking the transmembrane and cytoplasmic segments resulted in secretion of the H-2Kk protein, as determined by immunoprecipitation with monoclonal anti-H-2Kk antibodies. Transgenic (H-2b X H-2d)F1 mice were established carrying integrated copies of the modified H-2Kk gene. Expression of the soluble H-2Kk antigen in the transgenic mice was demonstrated in cell supernatants of biosynthetically labeled splenic and thymic Con A blasts as well as bone marrow-derived macrophages. Soluble H-2Kk molecules were also present in the sera of the transgenic animals. No cell-surface expression of the H-2Kk antigen could be observed. In spite of the presence of the soluble H-2Kk molecules in the transgenic mice, the animals were able to generate H-2Kk-specific cytolytic T cells as well as antibody responses when stimulated with cell-surface-bound H-2Kk antigens. These responses were indistinguishable from those of the nontransgenic littermates. Possible explanations for the observed lack of tolerance are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold B., Burgert H. G., Archibald A. L., Kvist S. Complete nucleotide sequence of the murine H-2Kk gene. Comparison of three H-2K locus alleles. Nucleic Acids Res. 1984 Dec 21;12(24):9473–9487. doi: 10.1093/nar/12.24.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold B., Burgert H. G., Hamann U., Hämmerling G., Kees U., Kvist S. Cytolytic T cells recognize the two amino-terminal domains of H-2 K antigens in tandem in influenza A infected cells. Cell. 1984 Aug;38(1):79–87. doi: 10.1016/0092-8674(84)90528-2. [DOI] [PubMed] [Google Scholar]
- Arnold B., Horstmann U., Kuon W., Burgert H. G., Hämmerling G. J., Kvist S. Alloreactive cytolytic T-cell clones preferentially recognize conformational determinants on histocompatibility antigens: analysis with genetically engineered hybrid antigens. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7030–7034. doi: 10.1073/pnas.82.20.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Actively acquired tolerance of foreign cells. Nature. 1953 Oct 3;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Bevan M. J. Killer cells reactive to altered-self antigens can also be alloreactive. Proc Natl Acad Sci U S A. 1977 May;74(5):2094–2098. doi: 10.1073/pnas.74.5.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami R. S., Paterson P. Y., Day E. D., Varitek V. A. Myelin basic protein serum factor. An endogenous neuroantigen influencing development of experimental allergic encephalomyelitis in Lewis rats. J Exp Med. 1978 Dec 1;148(6):1716–1721. doi: 10.1084/jem.148.6.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Construction of influenza haemagglutinin genes that code for intracellular and secreted forms of the protein. Nature. 1982 Dec 16;300(5893):598–603. doi: 10.1038/300598a0. [DOI] [PubMed] [Google Scholar]
- Golding H., Singer A. Role of accessory cell processing and presentation of shed H-2 alloantigens in allospecific cytotoxic T lymphocyte responses. J Immunol. 1984 Aug;133(2):597–605. [PubMed] [Google Scholar]
- Gordon J. W., Ruddle F. H. Gene transfer into mouse embryos: production of transgenic mice by pronuclear injection. Methods Enzymol. 1983;101:411–433. doi: 10.1016/0076-6879(83)01031-9. [DOI] [PubMed] [Google Scholar]
- Goronzy J., Schaefer U., Eichmann K., Simon M. M. Quantitative studies on T cell diversity. II. Determination of the frequencies and Lyt phenotypes of two types of precursor cells for alloreactive cytotoxic T cells in polyclonally and specifically activated splenic T cells. J Exp Med. 1981 Apr 1;153(4):857–870. doi: 10.1084/jem.153.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. E., Cairns L., Rosen F. S., Borel Y. A natural model of immunologic tolerance. Tolerance to murine C5 is mediated by T cells, and antigen is required to maintain unresponsiveness. J Exp Med. 1982 Aug 1;156(2):567–584. doi: 10.1084/jem.156.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S., Koch N., Robinson P., Hämmerling G. Comparison of allogeneic and xenogeneic determinants on the H-2Kk molecule. Transplantation. 1983 Aug;36(2):177–180. doi: 10.1097/00007890-198308000-00013. [DOI] [PubMed] [Google Scholar]
- Machy P., Arnold B., Aliño S., Leserman L. D. Interferon sensitive and insensitive MHC variants of a murine thymoma differentially resistant to methotrexate-containing antibody-directed liposomes and immunotoxin. J Immunol. 1986 Apr 15;136(8):3110–3115. [PubMed] [Google Scholar]
- Mann D. W., Stroynowski I., Hood L., Forman J. Cytotoxic T lymphocytes from mice with soluble class I Q10 molecules in their serum are not tolerant to membrane-bound Q10. J Immunol. 1987 Jan 1;138(1):240–245. [PubMed] [Google Scholar]
- Margulies D. H., Ramsey A. L., Boyd L. F., McCluskey J. Genetic engineering of an H-2Dd/Q10b chimeric histocompatibility antigen: purification of soluble protein from transformant cell supernatants. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5252–5256. doi: 10.1073/pnas.83.14.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyada C. G., Klofelt C., Reyes A. A., McLaughlin-Taylor E., Wallace R. B. Evidence that polymorphism in the murine major histocompatibility complex may be generated by the assortment of subgene sequences. Proc Natl Acad Sci U S A. 1985 May;82(9):2890–2894. doi: 10.1073/pnas.82.9.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R., Seidman J. G. Structure of wild-type and mutant mouse beta 2-microglobulin genes. Cell. 1982 Jun;29(2):661–669. doi: 10.1016/0092-8674(82)90182-9. [DOI] [PubMed] [Google Scholar]
- Pilarski L. M. A requirement for antigen-specific helper T cells in the generation of cytotoxic T cells from thymocyte precursors. J Exp Med. 1977 Mar 1;145(3):709–725. doi: 10.1084/jem.145.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUOSLAHTI E., Pihko H., Becker M., Mäkelä O. Rabbit alpha-fetoprotein: normal levels and breakage tolerance with haptenated homologous alpha-fetoprotein. Eur J Immunol. 1975 Jan;5(1):7–10. doi: 10.1002/eji.1830050103. [DOI] [PubMed] [Google Scholar]
- Robinson P. J., Graf L. Dynamic aspects of beta 2-microglobulin replacement on the surface of mouse lymphoma cells. Transplant Proc. 1983 Dec;15(4):2051–2053. [PubMed] [Google Scholar]
- Speck N. A., Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1987 Mar;7(3):1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Voller A., Bidwell D., Huldt G., Engvall E. A microplate method of enzyme-linked immunosorbent assay and its application to malaria. Bull World Health Organ. 1974;51(2):209–211. [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]