Abstract
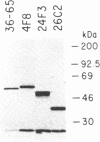
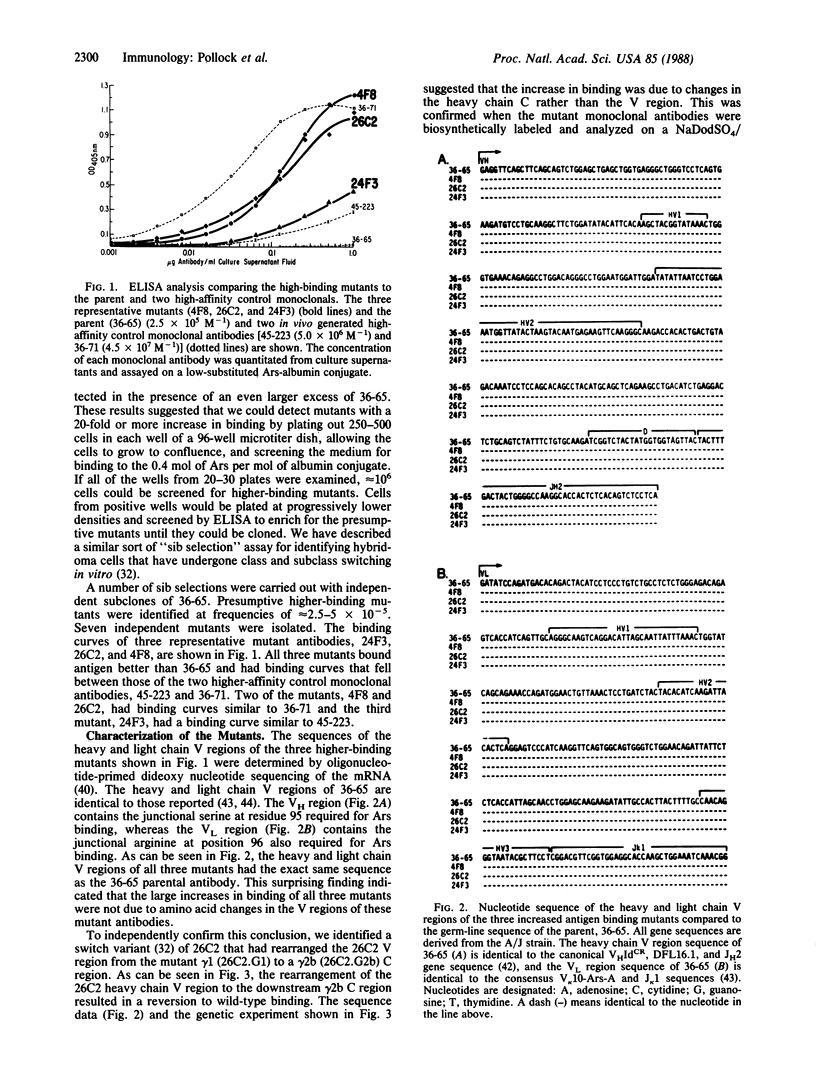
Sib selection and an ELISA have been used to isolate hybridoma subclones producing mutant antibodies that bind antigen better than the parental monoclonal antibody. Such mutants arise spontaneously in culture at frequencies of 2.5-5 X 10(-5). The sequences of the heavy and light chain variable regions of the mutant antibodies are identical to that of the parent and the Ka values of the mutants and the parent are the same. The increase in binding is associated with abnormalities of the constant region polypeptide and probably reflect changes in avidity of these antibodies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D., Cumano A., Dildrop R., Kocks C., Rajewsky K., Rajewsky N., Roes J., Sablitzky F., Siekevitz M. Timing, genetic requirements and functional consequences of somatic hypermutation during B-cell development. Immunol Rev. 1987 Apr;96:5–22. doi: 10.1111/j.1600-065x.1987.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Baumann B., Potash M. J., Köhler G. Consequences of frameshift mutations at the immunoglobulin heavy chain locus of the mouse. EMBO J. 1985 Feb;4(2):351–359. doi: 10.1002/j.1460-2075.1985.tb03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek C., Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev. 1987 Apr;96:23–41. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Brüggemann M., Radbruch A., Rajewsky K. Immunoglobulin V region variants in hybridoma cells. I. Isolation of a variant with altered idiotypic and antigen binding specificity. EMBO J. 1982;1(5):629–634. doi: 10.1002/j.1460-2075.1982.tb01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clarke S. H., Huppi K., Ruezinsky D., Staudt L., Gerhard W., Weigert M. Inter- and intraclonal diversity in the antibody response to influenza hemagglutinin. J Exp Med. 1985 Apr 1;161(4):687–704. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Rudikoff S., Giusti A. M., Scharff M. D. Somatic mutation in a cultured mouse myeloma cell affects antigen binding. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1240–1244. doi: 10.1073/pnas.79.4.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Scharff M. D. Antigen-binding mutants of mouse myeloma cells. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5687–5691. doi: 10.1073/pnas.74.12.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumano A., Rajewsky K. Clonal recruitment and somatic mutation in the generation of immunological memory to the hapten NP. EMBO J. 1986 Oct;5(10):2459–2468. doi: 10.1002/j.1460-2075.1986.tb04522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart P. J., Bogenhagen D. F. Clusters of point mutations are found exclusively around rearranged antibody variable genes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3439–3443. doi: 10.1073/pnas.80.11.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Melvold R. W., Nathenson S. G. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA sequence analysis: Kbm9 and Kbm6. Proc Natl Acad Sci U S A. 1986 May;83(10):3371–3375. doi: 10.1073/pnas.83.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti A. M., Chien N. C., Zack D. J., Shin S. U., Scharff M. D. Somatic diversification of S107 from an antiphosphocholine to an anti-DNA autoantibody is due to a single base change in its heavy chain variable region. Proc Natl Acad Sci U S A. 1987 May;84(9):2926–2930. doi: 10.1073/pnas.84.9.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan N. S., Monafo W. J., Davie J. M. Interaction of IgG3 anti-streptococcal group A carbohydrate (GAC) antibody with streptococcal group A vaccine: enhancing and inhibiting effects of anti-GAC, anti-isotypic, and anti-idiotypic antibodies. J Immunol. 1987 Jan 1;138(1):285–292. [PubMed] [Google Scholar]
- Griffiths G. M., Berek C., Kaartinen M., Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984 Nov 15;312(5991):271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- Hartman A. B., Rudikoff S. VH genes encoding the immune response to beta-(1,6)-galactan: somatic mutation in IgM molecules. EMBO J. 1984 Dec 1;3(12):3023–3030. doi: 10.1002/j.1460-2075.1984.tb02249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Black S. J., Tokuhisa T., Herzenberg L. A. Memory B cells at successive stages of differentiation. Affinity maturation and the role of IgD receptors. J Exp Med. 1980 May 1;151(5):1071–1087. doi: 10.1084/jem.151.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäck H. M., Wabl M. High rates of deletions in the constant region segment of the immunoglobulin mu gene. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4934–4938. doi: 10.1073/pnas.84.14.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Davis M., Sinn E., Patten P., Hood L. Antibody diversity: somatic hypermutation of rearranged VH genes. Cell. 1981 Dec;27(3 Pt 2):573–581. doi: 10.1016/0092-8674(81)90399-8. [DOI] [PubMed] [Google Scholar]
- Manser T., Wysocki L. J., Margolies M. N., Gefter M. L. Evolution of antibody variable region structure during the immune response. Immunol Rev. 1987 Apr;96:141–162. doi: 10.1111/j.1600-065x.1987.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Siekevitz M., Margolies M. N., Mudgett-Hunter M., Gefter M. L. Hybridoma proteins expressing the predominant idiotype of the antiazophenylarsonate response of A/J mice. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1120–1124. doi: 10.1073/pnas.77.2.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. L., Scharff M. D. Mutational events in mouse myeloma cells. Crit Rev Immunol. 1981 Sep;3(1):1–22. [PubMed] [Google Scholar]
- Oi V. T., Vuong T. M., Hardy R., Reidler J., Dangle J., Herzenberg L. A., Stryer L. Correlation between segmental flexibility and effector function of antibodies. Nature. 1984 Jan 12;307(5947):136–140. doi: 10.1038/307136a0. [DOI] [PubMed] [Google Scholar]
- Rao A., Faas S. J., Cantor H. Activation specificity of arsonate-reactive T cell clones. Structural requirements for hapten recognition and comparison with monoclonal antibodies. J Exp Med. 1984 Feb 1;159(2):479–494. doi: 10.1084/jem.159.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell J. D., Gearhart P. J., Karush F. Restriction in IgM expression. IV. Affinity analysis of monoclonal anti-phosphorylcholine antibodies. J Immunol. 1983 Jan;130(1):313–316. [PubMed] [Google Scholar]
- Rothstein T. L., Gefter M. L. Affinity analysis of idiotype-positive and idiotype-negative Ars-binding hybridoma proteins and Ars-immune sera. Mol Immunol. 1983 Feb;20(2):161–168. doi: 10.1016/0161-5890(83)90127-x. [DOI] [PubMed] [Google Scholar]
- Rudikoff S., Giusti A. M., Cook W. D., Scharff M. D. Single amino acid substitution altering antigen-binding specificity. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1979–1983. doi: 10.1073/pnas.79.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz I., Capra J. D. V kappa and J kappa gene segments of A/J Ars-A antibodies: somatic recombination generates the essential arginine at the junction of the variable and joining regions. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1085–1089. doi: 10.1073/pnas.84.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M. J., Nemazee D. A., Sato V. L., Van Snick J., Carson D. A., Weigert M. G. Variable region sequences of murine IgM anti-IgG monoclonal autoantibodies (rheumatoid factors). A structural explanation for the high frequency of IgM anti-IgG B cells. J Exp Med. 1986 Aug 1;164(2):407–427. doi: 10.1084/jem.164.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Huang S. Y., Gefter M. L. The genetic basis of antibody production: a single heavy chain variable region gene encodes all molecules bearing the dominant anti-arsonate idiotype in the strain A mouse. Eur J Immunol. 1983 Feb;13(2):123–132. doi: 10.1002/eji.1830130207. [DOI] [PubMed] [Google Scholar]
- Siskind G. W., Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- Slaughter C. A., Capra J. D. Amino acid sequence diversity within the family of antibodies bearing the major antiarsonate cross-reactive idiotype of the A strain mouse. J Exp Med. 1983 Nov 1;158(5):1615–1634. doi: 10.1084/jem.158.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira G., Bargellesi A., Teillaud J. L., Scharff M. D. The identification of monoclonal class switch variants by sib selection and an ELISA assay. J Immunol Methods. 1984 Nov 30;74(2):307–315. doi: 10.1016/0022-1759(84)90298-9. [DOI] [PubMed] [Google Scholar]
- Trimble W. S., Baker M. D., Boulianne G. L., Murialdo H., Hozumi N., Shulman M. J. Analysis of hybridoma mutants defective in synthesis of immunoglobulin M. Somat Cell Mol Genet. 1986 Sep;12(5):467–477. doi: 10.1007/BF01539918. [DOI] [PubMed] [Google Scholar]
- Wabl M., Burrows P. D., von Gabain A., Steinberg C. Hypermutation at the immunoglobulin heavy chain locus in a pre-B-cell line. Proc Natl Acad Sci U S A. 1985 Jan;82(2):479–482. doi: 10.1073/pnas.82.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert M. G., Cesari I. M., Yonkovich S. J., Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970 Dec 12;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Wysocki L., Manser T., Gefter M. L. Somatic evolution of variable region structures during an immune response. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1847–1851. doi: 10.1073/pnas.83.6.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton D. E., Desaymard C., Scharff M. D. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1981;1(1):5–11. doi: 10.1089/hyb.1.1981.1.5. [DOI] [PubMed] [Google Scholar]
- Yelton D. E., Scharff M. D. Mutant monoclonal antibodies with alterations in biological functions. J Exp Med. 1982 Oct 1;156(4):1131–1148. doi: 10.1084/jem.156.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack D. J., Morrison S. L., Cook W. D., Dackowski W., Scharff M. D. Somatically generated mouse myeloma variants synthesizing IgA half-molecules. J Exp Med. 1981 Nov 1;154(5):1554–1569. doi: 10.1084/jem.154.5.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]