Abstract
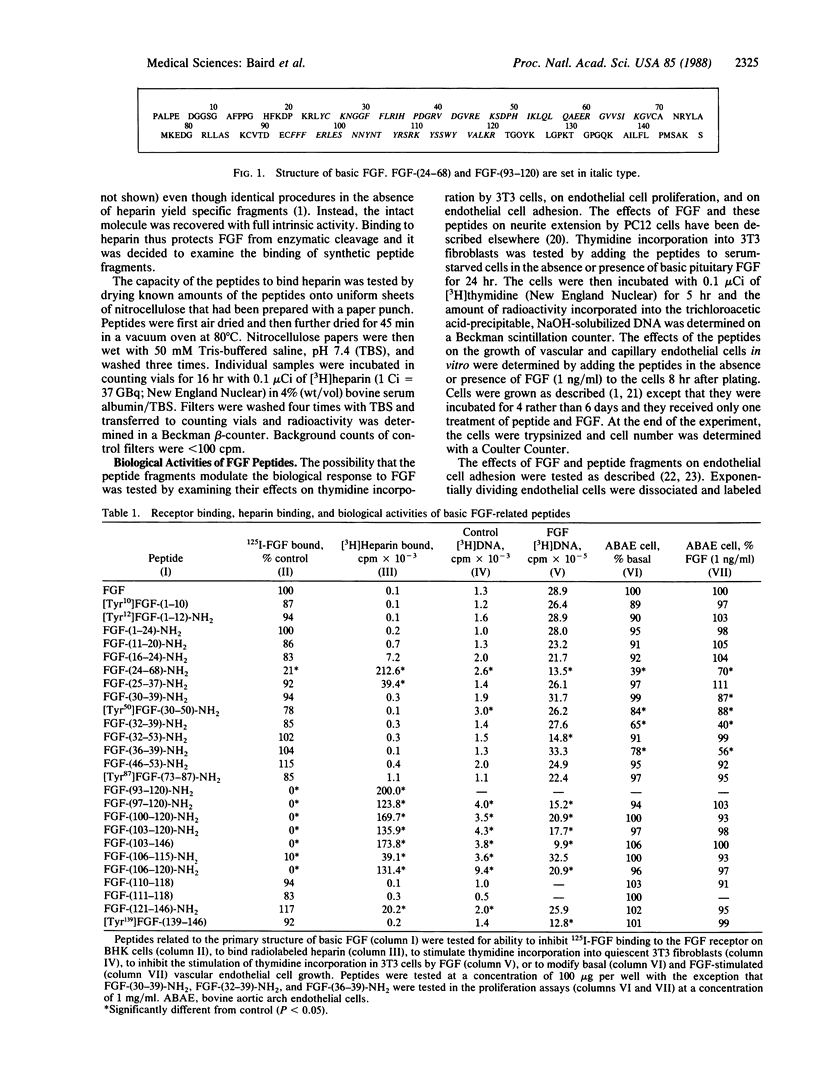
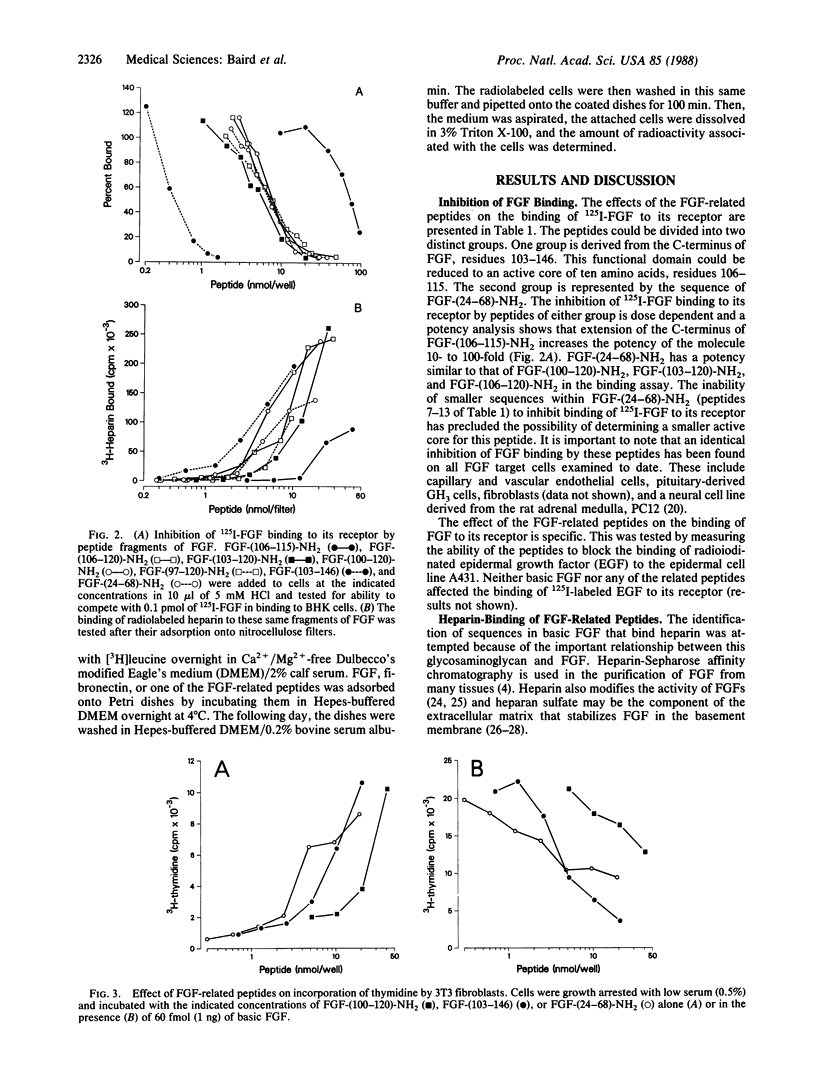
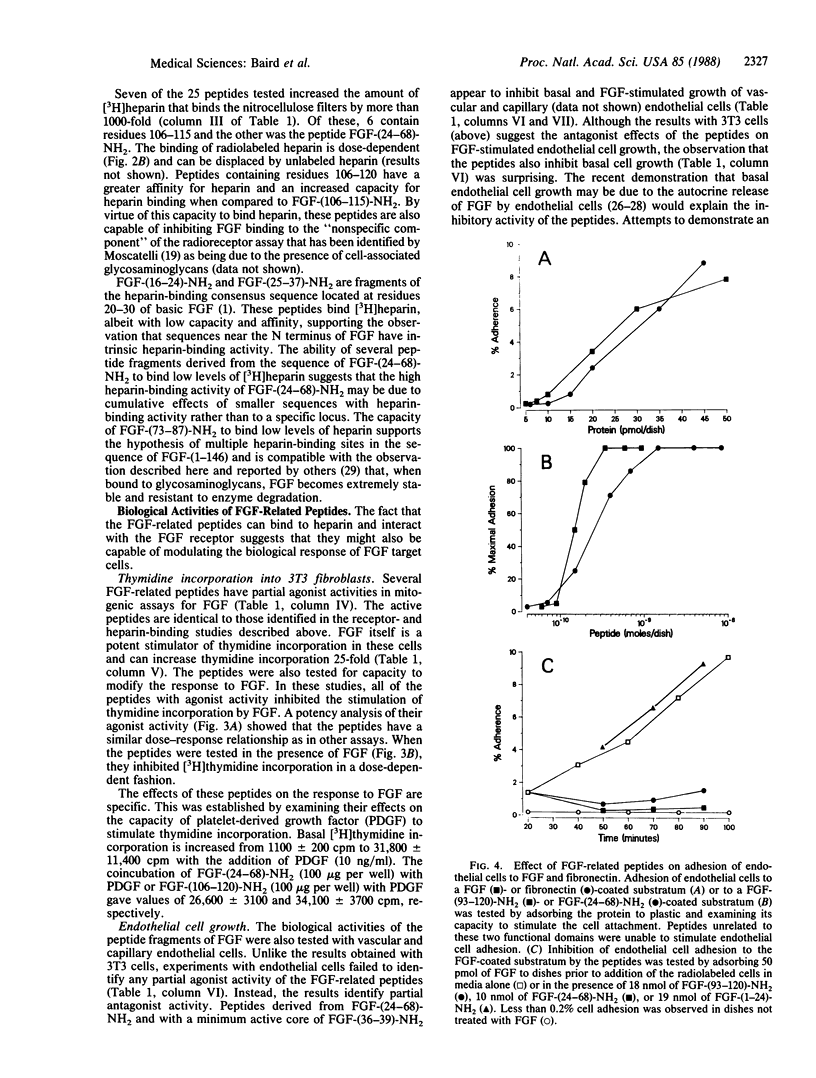
Two functional domains in the primary structure of basic fibroblast growth factor (FGF) have been identified on the basis of their ability to interact with the FGF receptor, bind radiolabeled heparin, and modulate the cellular response to FGF. Peptides derived from these two functional domains can act as partial agonists and antagonists in biological assays of FGF activity. Peptides related to the sequences of FGF-(24-68)-NH2 and FGF-(106-115)-NH2 inhibit thymidine incorporation into 3T3 fibroblasts when they are stimulated by FGF but have no effect when the cells are treated with either platelet-derived growth factor or epidermal growth factor. They also possess partial agonist activity and can stimulate DNA synthesis when tested in the absence of exogenous FGF. The active peptides have no effect on the binding of epidermal growth factor to its receptor on A431 cells and they can modulate the effects of FGF, but not fibronectin, on endothelial cell adhesion. The results suggest the possibility of designing specific analogs of FGF that are capable of inhibiting the biological effects of FGF.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Baird A., Ling N. Fibroblast growth factors are present in the extracellular matrix produced by endothelial cells in vitro: implications for a role of heparinase-like enzymes in the neovascular response. Biochem Biophys Res Commun. 1987 Jan 30;142(2):428–435. doi: 10.1016/0006-291x(87)90292-0. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Mehlman T., Marshak D. R., Fraser B. A., Maciag T. Structural evidence that endothelial cell growth factor beta is the precursor of both endothelial cell growth factor alpha and acidic fibroblast growth factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7216–7220. doi: 10.1073/pnas.83.19.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Dickson C., Peters G. Potential oncogene product related to growth factors. 1987 Apr 30-May 6Nature. 326(6116):833–833. doi: 10.1038/326833a0. [DOI] [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F., Ueno N., Baird A., Hill F., Denoroy L., Ling N., Gospodarowicz D., Guillemin R. Primary structure of bovine brain acidic fibroblast growth factor (FGF). Biochem Biophys Res Commun. 1985 Dec 17;133(2):554–562. doi: 10.1016/0006-291x(85)90942-8. [DOI] [PubMed] [Google Scholar]
- Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972 Mar;175(3):409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Gimenez-Gallego G., Rodkey J., Bennett C., Rios-Candelore M., DiSalvo J., Thomas K. Brain-derived acidic fibroblast growth factor: complete amino acid sequence and homologies. Science. 1985 Dec 20;230(4732):1385–1388. doi: 10.1126/science.4071057. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Bialecki H., Thakral T. K. The angiogenic activity of the fibroblast and epidermal growth factor. Exp Eye Res. 1979 May;28(5):501–514. doi: 10.1016/0014-4835(79)90038-1. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986 Sep;128(3):475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayek A., Culler F. L., Beattie G. M., Lopez A. D., Cuevas P., Baird A. An in vivo model for study of the angiogenic effects of basic fibroblast growth factor. Biochem Biophys Res Commun. 1987 Sep 15;147(2):876–880. doi: 10.1016/0006-291x(87)91011-4. [DOI] [PubMed] [Google Scholar]
- Heath W. F., Merrifield R. B. A synthetic approach to structure-function relationships in the murine epidermal growth factor molecule. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6367–6371. doi: 10.1073/pnas.83.17.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. J., Olden K., Yamada K. M. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986 Jul 25;233(4762):467–470. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
- Moscatelli D. High and low affinity binding sites for basic fibroblast growth factor on cultured cells: absence of a role for low affinity binding in the stimulation of plasminogen activator production by bovine capillary endothelial cells. J Cell Physiol. 1987 Apr;131(1):123–130. doi: 10.1002/jcp.1041310118. [DOI] [PubMed] [Google Scholar]
- Nestor J. J., Jr, Newman S. R., DeLustro B., Todaro G. J., Schreiber A. B. A synthetic fragment of rat transforming growth factor alpha with receptor binding and antigenic properties. Biochem Biophys Res Commun. 1985 May 31;129(1):226–232. doi: 10.1016/0006-291x(85)91426-3. [DOI] [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D., Dodge L., Fujii D. K. Heparin modulation of the neurotropic effects of acidic and basic fibroblast growth factors and nerve growth factor on PC12 cells. J Cell Physiol. 1987 Apr;131(1):131–140. doi: 10.1002/jcp.1041310119. [DOI] [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D. The identification and partial characterization of the fibroblast growth factor receptor of baby hamster kidney cells. J Biol Chem. 1985 Nov 5;260(25):13860–13868. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5985–5988. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M. Isolation of an adhesion-mediating protein from chick neural retina adherons. J Cell Biol. 1985 Sep;101(3):1071–1077. doi: 10.1083/jcb.101.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Klier F. G., Birdwell C. A role for adherons in neural retina cell adhesion. J Cell Biol. 1983 Apr;96(4):990–998. doi: 10.1083/jcb.96.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Ling N., Baird A. Multiple influences of a heparin-binding growth factor on neuronal development. J Cell Biol. 1987 Mar;104(3):635–643. doi: 10.1083/jcb.104.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Friedman J., Abraham J. A., Fiddes J. C., Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987 Jan 15;325(6101):257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Taira M., Yoshida T., Miyagawa K., Sakamoto H., Terada M., Sugimura T. cDNA sequence of human transforming gene hst and identification of the coding sequence required for transforming activity. Proc Natl Acad Sci U S A. 1987 May;84(9):2980–2984. doi: 10.1073/pnas.84.9.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno N., Baird A., Esch F., Ling N., Guillemin R. Isolation of an amino terminal extended form of basic fibroblast growth factor. Biochem Biophys Res Commun. 1986 Jul 31;138(2):580–588. doi: 10.1016/s0006-291x(86)80536-8. [DOI] [PubMed] [Google Scholar]
- Uhlrich S., Lagente O., Lenfant M., Courtois Y. Effect of heparin on the stimulation of non-vascular cells by human acidic and basic FGF. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1205–1213. doi: 10.1016/0006-291x(86)90353-0. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]


