Abstract
A benthic microfaunal record from the equatorial Atlantic Ocean over the past four glacial-interglacial cycles was investigated to understand temporal dynamics of deep-sea latitudinal species diversity gradients (LSDGs). The results demonstrate unexpected instability and high amplitude fluctuations of species diversity in the tropical deep ocean that are correlated with orbital-scale oscillations in global climate: Species diversity is low during glacial and high during interglacial periods. This implies that climate severely influences deep-sea diversity, even at tropical latitudes, and that deep-sea LSDGs, while generally present for the last 36 million years, were weakened or absent during glacial periods. Temporally dynamic LSDGs and unstable tropical diversity require reconsideration of current ecological hypotheses about the generation and maintenance of biodiversity as they apply to the deep sea, and underscore the potential vulnerability and conservation importance of tropical deep-sea ecosystems.
Keywords: deep-sea Ostracoda, global climate change, latitudinal species diversity gradients, macroecology, Quaternary paleoceanography
Latitudinal species diversity gradients (LSDGs), the patterns in which tropical regions contain more species than high latitudes, are one of the most basic ecological patterns on the earth (1). In the modern ocean, deep-sea bivalves, gastropods, isopods, cumaceans, and foraminifera all show strong LSDGs (2–6), and studies of benthic foraminifera assemblages indicate that the deep-sea gradients were established ≈36 million years ago (7). The environmental stability hypothesis holds that stability in tropical (1, 8), and deep-sea (9, 10) environments might enhance species diversity, but there is now evidence for highly fluctuating high-latitude deep-sea diversity during Quaternary climatic cycles (11–16). Surprisingly little attention has been given to understanding low-latitude deep-sea diversity and the temporal dynamics of the LSDGs. Although pollen records suggest a persistent latitudinal diversity gradient existed in terrestrial ecosystems over the last 13,000 years (17, 18), we know of no studies of species-level temporal dynamics of LSDGs based on fossil assemblages from marine environments, despite the sensitivity of marine ecosystems to climatic change (12, 13, 19–24).
The Ostracoda (Crustacea) are an important component of the deep-sea benthos (25–27), and the only commonly fossilized metazoan group in deep-sea sediments (12, 13, 28). Their various habitats and ecological preferences represent a wide range of deep-sea benthic niches, and their fossil record is considered representative of the benthic community (12, 13, 28). Furthermore, large (≈130 m) glacial-interglacial sea-level changes (29), which drastically altered shallow-marine environments, had negligible effects on deep-sea habitats (e.g., >1,000 m water depth). Here, we examine low-latitude Quaternary records of deep-sea ostracods and temporal changes in LSDGs in the North Atlantic Ocean during the last four glacial-interglacial climatic cycles.
Ocean Drilling Program (ODP) Site 925 was cored at the Ceara Rise in the western equatorial Atlantic (4° 12.2′ N, 43° 29.3′ W; 3040 m water depth; Fig. 1) (30, 31). The Ceara Rise is an intensively researched tropical region for Cenozoic paleoceanography (32–34). Continuous sedimentation, excellent chronology, and availability of climatic proxy records make Site 925 an ideal sediment core for Quaternary biodiversity research in the low latitude ocean. Although postmortem dissolution can affect fossil ostracod preservation below lysocline and carbonate compensation depth (35), Site 925 is located well above these depths (31, 36) and so this record is not seriously influenced by carbonate dissolution. This high-quality record of the tropical deep ocean shows that ostracod species diversity exhibits large amplitude fluctuations during the last 500 ka (thousands of years ago), and that the LSDGs in the deep ocean seem to have weakened or even collapsed during glacial periods.
Fig. 1.
Location of OSP site 925. This map was created using the Online Map Creation web site (www.aquarius.ifm-geomar.de).
Results and Discussion
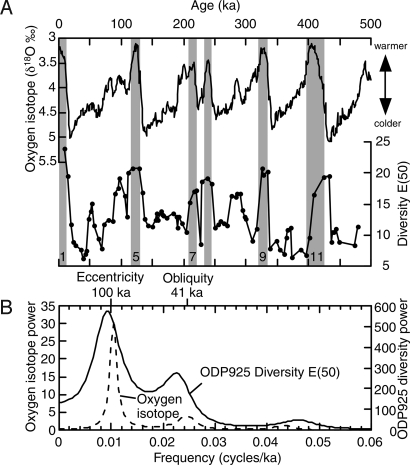
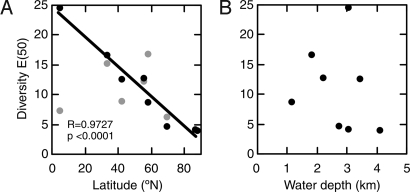
The ODP 925 record for the past four glacial-interglacial cycles shows diversities measured as the expected number of species in a sample of 50 individuals, E(50), that are high (up to ≈25) during interglacial and low (≈5 at minimum) during glacial periods (Fig. 2A). Diversity increases during glacial to interglacial transitions (Terminations 1–4) range from 2-fold to nearly 4-fold (Fig. 2A). These substantial oscillations span the present-day pole-to-equator diversity gradient in deep-sea ostracods (Figs. 2A and 3A). Data from ODP 925 produce a near-recent equatorial diversity estimate of E(50) approximately 25, whereas diversity in the Arctic Ocean is E(50) approximately 5, and values for midlatitude sites are intermediate (Fig. 3A and Table S1). During glacial intervals, tropical diversity is greatly depressed, but diversities at middle and high latitudes are much less affected (Fig. 3A). Consequently, the deep-sea LSDGs are weakened during glacial times, so much so that they appear to be completely absent, at least during the Last Glacial Maximum (≈20 ka).
Fig. 2.
Late Quaternary tropical deep-sea diversity changes. (A) Comparison between deep-sea oxygen isotope curve [LR04 global stack (37)] and ODP 925 ostracod species diversity E (50). The oxygen isotope curve represents global climate changes, and the lower isotope values indicate warmer (interglacial and interstadial) intervals. Major interglacial and interstadial peaks are highlighted by gray bars. Peak interglacial marine isotope stages are labeled. (B) Result of spectral analysis.
Fig. 3.
Modern and Last Glacial Maximum (≈20 ka) deep-sea diversity patterns. (A) Latitudinal patterns of modern coretop (black circles) and Last Glacial Maximum (gray circles) ostracod diversity. Regression line and r and P values for modern E(50). (B) Comparison between modern E(50) and water depth.
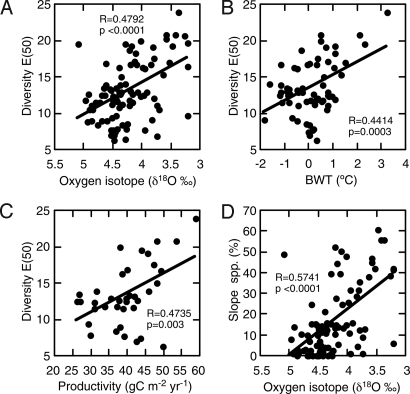
Spectral analysis shows that ODP 925 ostracod diversity fluctuated with periodicities that match those of 100 ka (eccentricity) and 41 ka (obliquity) Milankovitch climatic forcing (Fig. 2B). There is also a correlation between ODP 925 diversity and the deep-sea benthic foraminiferal oxygen isotope record (37), a proxy for global changes in temperature and ice volume (Figs. 2 and 4A). Diversity peaks correspond with the negative oxygen isotope excursions during interglacial and interstadial maxima (Fig. 2A). This pattern shows that global climate changes have strongly influenced tropical deep-sea diversity, similar to previously reported effects at mid to high latitudes (11, 13, 28). This result, coupled with the extremely dynamic diversity trajectory of the tropical ODP 925, suggests that the LSDGs in the deep ocean are not driven by a gradient of increasing environmental stability from poles to tropics.
Fig. 4.
Tropical deep-sea benthic species diversity and climatic and paleoecological factors. Relationships between ODP 925 diversity [E(50)] and (A) deep-sea oxygen isotope (37), (B) bottom-water temperature (BWT) (60), and (C) surface productivity (44). (D) Comparison between relative abundance of slope species and deep-sea oxygen isotope (37).
Both temperature and productivity have been considered important factors controlling deep-sea species diversity but their relative importance is uncertain (4, 13, 28, 38–41). Species diversity at ODP 925 is positively correlated with both bottom water temperature (P = 0.0003) and surface productivity oscillations (P = 0.003) (Fig. 4 B and C). However, temperature and surface productivity are themselves correlated at this site. When we perform a multiple regression to tease apart these relationships, we find that diversity is significantly and positively associated with temperature (P = 0.02) but not with productivity (P = 0.24). A positive correlation between temperature and species diversity has been reported before for deep-sea ostracods and foraminifera using late Quaternary and mid-Pliocene core records (11, 28, 38), and may reflect available energy (42) or perhaps physiological limits in which few taxa can tolerate very cold temperatures.
Although total productivity has been invoked as a determinant of deep-sea diversity (28, 40, 43), these analyses suggest a minor role, at least in this region and in this time scale. This result may partly reflect the lack of strong oscillations in productivity in this region during the late Quaternary [≈25–60 gC m−2 yr−1 (44), much smaller than the current range across the modern North Atlantic, ≈50–450 gC m−2 yr−1 (45)]. Nevertheless, although productivity was apparently not strongly seasonal at the Ceara Rise (36), no quantitative proxy for seasonality is currently available for ODP 925. Given that such seasonality has been shown to be an important determinant of modern deep-sea benthic foraminifera diversity (46), it is possible that this factor also plays a role here (3, 4, 28).
The multiple regression results indicate that downcore variations in species diversity at ODP 925 are predicted by temperature but not total productivity. While this temperature-diversity relationship may be causal, it is also possible that diversity is instead driven by some other environmental driver that, like temperature, tracks glacial-interglacial cycles. Such factors might be mediated by latitudinal shifts in the Intertropical Convergence Zone (ITCZ) and changes in North Brazil Current (33, 47), or changes in deep water characteristics reflecting the relative influence of North Atlantic Deep Water (NADW) versus Antarctic Bottom Water (AABW) (48, 49). NADW and AABW differ in temperature, nutrients contents, and salinity, although at present there is not much evidence that nutrients and salinity have much influence on deep-sea diversity.
Whatever the driver, these diversity fluctuations are not determined by species' originations or extinctions because the period covered in this study is much shorter than the durations of ostracod species, and few, if any, species originate or go extinct during this late Quaternary period. Instead, glacial-interglacial scale diversity changes must result from the shifting of species' distributions, either bathymetrically or laterally. Previous researches have hypothesized that the deep-sea diversity fluctuations involve the depth migrations of fauna during glacial-interglacial cycles (11, 28, 50). At ODP 925, the greater abundance of slope species during warmer periods when higher diversity prevailed (Fig. 4D) suggests the downward migration of slope species during these intervals. The rarity of slope taxa during glacial and stadial periods is consistent with shallowing of their ranges during these colder intervals. These range shifts might track temperature tolerances, or possibly some other aspect of the environment changing on Milankovitch time scales. Because slope species are much more diverse in tropical deep sea than in higher latitude oceans (Table S1), bathymetric shifts have greater diversity consequences at low latitudes, and thus modulate the deep-sea LSDGs. This idea is consistent with the deep-sea source-sink hypothesis suggesting that abyssal diversity of taxa having good dispersal ability is maintained by immigration from bathyal sources (51), a mechanism that may be applicable to organisms such as ostracods that lack swimming or dispersal larval stages (25).
Our results underscore the vulnerability and conservation importance of tropical deep-sea ecosystems, which may be an engine of global deep-sea biodiversity (52) and ecosystem functioning (53). Dramatic changes in the deep-sea LSDGs demonstrated here require reconsideration of view of persistent LSDGs in the deep sea, at least in the glacial-interglacial or shorter time scales. This dynamic nature seems to be consistent with recent discoveries of high ecosystem sensitivity to short time-scale climate changes (12, 20–22, 24).
Materials and Methods
The composite section of ODP Site 925 (30, 31) was sampled at approximately 20- cm intervals on average, yielding a sampling resolution of approximately 5 ka. The >150-μm-size fraction was examined for ostracod diversity. This size fraction is a standard for recent deep-sea ostracod research (54) and allows us to obtain all adults and juveniles of late molt stages from most deep-sea species. Although finer size fractions (63, 100, or 125 μm) are occasionally used in ostracod research, small ostracod species (e.g., Eucytherura spp., Pedicythere spp., Aratrocypris spp., Chejudocythere spp., Ruggieriella spp., and Swainocythere spp.) show low diversity and abundances, even when finer size fractions are used (55–57). Furthermore, even these small species mostly have >150-μm minor axis (i.e., height) of adult valve, and therefore will be recovered on a 150-μm sieve. Thus, our results are unlikely to be influenced by sieve mesh size. Body size does evolve, and ostracods tend to be larger during colder intervals (58). However, these evolutionary changes are much smaller than the differences between taxa, especially on these relatively short, glacial-interglacial time scales. Moreover, larger sizes during glacial periods would tend to increase observed diversity if the few smallest species became more likely to be retained on a sieve, and this effect therefore cannot explain low glacial diversity. The number of specimens refers to valves. More than 79 species were identified in total. The species identifications were initially conducted by M.Y. and H.O., but all were then confirmed by M.Y. This procedure assured consistent species concepts were applied to all samples. Ostracod carapaces have many morphological characters useful for species identification (59). Although juvenile valves in genera with smooth carapaces such as Krithe can be difficult, they still have characteristic morphological features (e.g., size and outline) specific to each species. We used E(Sn), the expected number of species in n individuals, for species diversity because it is widely used in deep-sea ecology. Other representative diversity measures show similar trends (Fig. S1). The ostracod species diversity calculation is based on three-point moving sums of the census dataset because of relatively small sample size (≈50 specimens per sample on average), but the trend is unchanged if the raw census dataset is used. Age control was established with correlation of the ODP 925 benthic foraminiferal δ18O record (31) to the LR04 global stack (37).
Published bottom-water temperature curve based on well-established Mg/Ca paleothermometry was available from tropical Atlantic deep-sea core M16772 (60), which was cored at similar latitude and water depth to Site 925 and so is ideal for comparison with the ODP 925 ostracod data. Published surface productivity curve estimated by the well established carbonate accumulation based method was available from the core GeoB 1523–1 (44), which was cored at almost the same location as ODP 925. It is known that carbonate accumulation can be affected by other factors than productivity (e.g., carbonate dissolution) as well as other productivity proxies (36). However, GeoB 1523–1 is located well above the lysocline and carbonate accumulation in this core is not seriously influenced by dissolution (36, 44). Furthermore, in oligotrophic regions dominated by calcite-secreting organisms (e.g., Ceara Rise), carbonate accumulation is known to be more reliable measure of productivity than organic carbon accumulation (36, 44). Oxygen isotope chronologies for these curves were updated using new global stack of the LR04 (37). The productivity values were recalculated based on this updated chronology because sedimentation rate enters into the productivity calculation. We smoothed temperature, LR04, and productivity curves using a cubic spline and used them to estimate the value for each of these variables for each faunal sample (Fig. 4) as described in Hunt et al. (38).
A maximum entropy spectral analysis was performed by using the software AnalySeries version 2.0.4.2 (61). ODP 925 diversity data were resampled every 1 ka before the analysis, which is equivalent to LR04 time resolution.
Modern coretop and Last Glacial Maximum ostracod diversities [E(50)] were calculated based on the census data of North Atlantic and Arctic deep-sea cores (11, 12, 62, 63) as shown in Table S1. All included data have robust chronology and similar taxonomy (i.e., most abundant genus Krithe and most of other major ostracod genera are identified to species level). A few samples were lumped for E(50) calculation if single samples included <50 specimens. Modern E(50) has no clear relationship with water depth for this selection of data (Fig. 3B).
In computing the relative abundance of slope species from Site 925, the following genera were considered typical slope inhabitants (56, 64): Bythocypris, Aratrocypris, Cytherella, Cytheropteron, Polycope, Pseudocythere, Eucytherura, Paracytherois, Paradoxostoma, Argilloecia, Zabythocypris, Ruggieriella, and Pedicythere.
Analyses other than spectral analysis were performed by using the statistical programming environment R (65). Ostracod data are available at the National Oceanic and Atmospheric Administration World Data Center for Paleoclimatology, www.ngdc.noaa.gov/paleo/paleo.html.
Supplementary Material
Acknowledgments.
We thank H. J. Dowsett, D. A. Willard, and two anonymous referees for constructively critical reviews, J. Dyszynski for sample processing, and W. B. Curry for ODP 925 isotope and composite data. This research used samples provided by the Integrated Ocean Drilling Program (IODP). This work was supported by a Smithsonian Postdoctoral Fellowship, a Smithsonian Marine Science Network Postdoctoral Fellowship, and Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad (to M.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. B.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910935106/DCSupplemental.
References
- 1.Willig MR, Kaufman DM, Stevens RD. Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Annu Rev Ecol Evol Syst. 2003;34:273–309. [Google Scholar]
- 2.Rex MA, et al. Global-scale latitudinal patterns of species diversity in the deep-sea benthos. Nature. 1993;365:636–639. [Google Scholar]
- 3.Rex MA, Stuart CT, Coyne G. Latitudinal gradients of species richness in the deep-sea benthos of the North Atlantic. Proc Natl Acad Sci USA. 2000;97:4082–4085. doi: 10.1073/pnas.050589497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Culver SJ, Buzas MA. Global latitudinal species diversity gradient in deep-sea benthic foraminifera. Deep-Sea Res I. 2000;47:259–275. [Google Scholar]
- 5.Gage JD, Lambshead PJD, Bishop JDD, Stuart CT, Jones NS. Large-scale biodiversity pattern of Cumacea (Peracarida: Crustacea) in the deep Atlantic. Mar Ecol Prog Ser. 2004;277:181–196. [Google Scholar]
- 6.McClain CR, Rex MA, Etter RJ. In: Marine Macroecology. Witman JD, Roy K, editors. Chicago: University of Chicago Press; 2009. pp. 65–100. [Google Scholar]
- 7.Thomas E, Gooday AJ. Cenozoic deep-sea benthic foraminifers: Tracers for changes in oceanic productivity? Geology. 1996;24:355–358. [Google Scholar]
- 8.Rohde K. Latitudinal gradients in species diversity: The search for the primary cause. Oikos. 1992;65:514–527. [Google Scholar]
- 9.Hessler RR, Sanders HL. Faunal diversity in the deep-sea. Deep-Sea Res. 1967;14:65–78. [Google Scholar]
- 10.Sanders HL. Marine benthic diversity: A comparative study. Am Nat. 1968;102:243–282. [Google Scholar]
- 11.Cronin TM, DeMartino DM, Dwyer GS, Rodriguez-Lazaro J. Deep-sea ostracode species diversity: Response to late Quaternary climate change. Mar Micropaleontol. 1999;37:231–249. [Google Scholar]
- 12.Yasuhara M, Cronin TM, deMenocal PB, Okahashi H, Linsley BK. Abrupt climate change and collapse of deep-sea ecosystems. Proc Natl Acad Sci USA. 2008;105:1556–1560. doi: 10.1073/pnas.0705486105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasuhara M, Cronin TM. Climatic influences on deep-sea ostracode (Crustacea) diversity for the last three million years. Ecology. 2008;89:S52–S65. doi: 10.1890/07-1021.1. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez Zarikian CA, Stepanova AY, Grützner J. Glacial-interglacial variability in deep sea ostracod assemblage composition at IODP Site U1314 in the subpolar North Atlantic. Mar Geol. 2009;258:69–87. [Google Scholar]
- 15.Didié C, Bauch HA, Helmke JP. Late Quaternary deep-sea ostracodes in the polar and subpolar North Atlantic: Paleoecological and paleoenvironmental implications. Palaeogeogr Palaeoclimatol Palaeoecol. 2002;184:195–212. [Google Scholar]
- 16.Wollenburg JE, Mackensen A, Kuhnt W. Benthic foraminiferal biodiversity response to a changing Arctic palaeoclimate in the last 24,000 years. Palaeogeogr Palaeoclimatol Palaeoecol. 2007;255:195–222. [Google Scholar]
- 17.Haskell JP. The latitudinal gradient of diversity through the Holocene as recorded by fossil pollen in Europe. Evol Ecol Res. 2001;3:345–360. [Google Scholar]
- 18.Silvertown J. History of a latitudinal diversity gradient: Woody plants in Europe 13,000–1,000 years B.P. J Biogeogr. 1985;12:519–525. [Google Scholar]
- 19.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 20.Ruhl HA, Ellena JA, Smith KL., Jr Connections between climate, food limitation, and carbon cycling in abyssal sediment communities. Proc Natl Acad Sci USA. 2008;105:17006–17011. doi: 10.1073/pnas.0803898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenseth NC, et al. Ecological effects of climate fluctuations. Science. 2002;297:1292–1296. doi: 10.1126/science.1071281. [DOI] [PubMed] [Google Scholar]
- 22.Walther G-R, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 23.Danovaro R, Dell'Anno A, Fabiano M, Pusceddu A, Tselepides A. Deep-sea ecosystem response to climate changes: The eastern Mediterranean case study. Trends Ecol Evol. 2001;16:505–510. [Google Scholar]
- 24.Brierley AS, Kingsford MJ. Impacts of climate change on marine organisms and ecosystems. Curr Biol. 2009;19:R602–R614. doi: 10.1016/j.cub.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 25.Brandt A, et al. First insights into the biodiversity and biogeography of the Southern Ocean deep sea. Nature. 2007;447:307–311. doi: 10.1038/nature05827. [DOI] [PubMed] [Google Scholar]
- 26.Yasuhara M, Okahashi H, Cronin TM. Taxonomy of Quaternary deep-sea ostracods from the western North Atlantic Ocean. Palaeontology. 2009;52:879–931. [Google Scholar]
- 27.Shimanaga M, Kitazato H, Shirayama Y. Seasonal patterns of vertical distribution between meiofaunal groups in relation to phytodetritus deposition in the bathyal Sagami Bay, central Japan. J Oceanogr. 2000;56:379–387. [Google Scholar]
- 28.Cronin TM, Raymo ME. Orbital forcing of deep-sea benthic species diversity. Nature. 1997;385:624–627. [Google Scholar]
- 29.Yokoyama Y, Lambeck K, De Deckker P, Johnston P, Fifield LK. Timing of the Last Glacial Maximum from observed sea-level minima. Nature. 2000;406:713–716. doi: 10.1038/35021035. [DOI] [PubMed] [Google Scholar]
- 30.Curry WB, et al. Proceedings of the Ocean Drilling Program, Initial Reports; College Station, TX: ODP; 1995. [Google Scholar]
- 31.Bickert T, Curry WB, Wefer G. Late Pliocene to Holocene (2.6–0 Ma) western equatorial Atlantic deep-water circulation: Inferences from benthic stable isotopes. Proc ODP, Sci Results. 1997;154:239–254. [Google Scholar]
- 32.Pälike H, Frazier J, Zachos JC. Extended orbitally forced palaeoclimatic records from the equatorial Atlantic Ceara Rise. Quat Sci Rev. 2006;25:3138–3149. [Google Scholar]
- 33.Rühlemann C, Diekmann B, Mulitza S, Frank M. Late Quaternary changes of western equatorial Atlantic surface circulation and Amazon lowland climate recorded in Ceará Rise deep-sea sediments. Paleoceanography. 2001;16:293–305. [Google Scholar]
- 34.Shackleton NJ, Curry WB, Richter C, Bralower TJ. Proceedings of the Ocean Drilling Program, Scientific Results; College Station, TX: ODP; 1997. [Google Scholar]
- 35.Yasuhara M, Cronin TM, Martínez Arbizu P. Abyssal ostracods from the South and Equatorial Atlantic Ocean: Biological and paleoceanographic implications. Deep-Sea Res I. 2008;55:490–497. [Google Scholar]
- 36.Rühlemann C, Müller PJ, Schneider RR. In: Use of Proxies in Paleoceanography: Examples from the South Atlantic. Fischer G, Wefer G, editors. Berlin: Springer-Verlag; 1999. pp. 315–344. [Google Scholar]
- 37.Lisiecki LE, Raymo ME. A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records. Paleoceanography. 2005 1010.1029/2004PA001071. [Google Scholar]
- 38.Hunt G, Cronin TM, Roy K. Species-energy relationship in the deep sea: A test using the Quaternary fossil record. Ecol Lett. 2005;8:739–747. [Google Scholar]
- 39.Smith CR, et al. Abyssal food limitation, ecosystem structure and climate change. Trends Ecol Evol. 2008;23:518–528. doi: 10.1016/j.tree.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Rex MA, Crame JA, Stuart CT, Clarke A. Large-scale biogeographic patterns in marine mollusks: A confluence of history and productivity? Ecology. 2005;86:2288–2297. [Google Scholar]
- 41.Danovaro R, Dell'Anno A, Pusceddu A. Biodiversity response to climate change in a warm deep sea. Ecol Lett. 2004;7:821–828. [Google Scholar]
- 42.Allen AP, Brown JH, Gillooly JF. Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science. 2002;297:1545–1548. doi: 10.1126/science.1072380. [DOI] [PubMed] [Google Scholar]
- 43.Levin LA, et al. Environmental influences on regional deep-sea species diversity. Annu Rev Ecol Syst. 2001;32:51–93. [Google Scholar]
- 44.Rühlemann C, et al. Late Quaternary productivity changes in the western equatorial Atlantic: Evidence from 230Th-normalized carbonate and organic carbon accumulation rates. Mar Geol. 1996;135:127–152. [Google Scholar]
- 45.Corliss BH, Sun X, Brown CW, Showers WJ. Influence of seasonal primary productivity on δ13C of North Atlantic deep-sea benthic foraminifera. Deep-Sea Res I. 2006;53:740–746. [Google Scholar]
- 46.Corliss BH, Brown CW, Sun X, Showers WJ. Deep-sea benthic diversity linked to seasonality of pelagic productivity. Deep-Sea Res I. 2009;56:835–841. [Google Scholar]
- 47.Harris SE, Mix AC. Pleistocene precipitation balance in the Amazon Basin recorded in deep sea sediments. Quat Res. 1999;51:14–26. [Google Scholar]
- 48.Lynch-Stieglitz J, et al. Atlantic meridional overturning circulation during the Last Glacial Maximum. Science. 2007;316:66–69. doi: 10.1126/science.1137127. [DOI] [PubMed] [Google Scholar]
- 49.Adkins JF, McIntyre K, Schrag DP. The salinity, temperature, and δ18O of the glacial deep ocean. Science. 2002;298:1769–1773. doi: 10.1126/science.1076252. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Lazaro J, Cronin TM. Quaternary glacial and deglacial Ostracoda in the thermocline of the Little Bahama Bank (NW Atlantic): Palaeoceanographic implications. Palaeogeogr Palaeoclimatol Palaeoecol. 1999;152:339–364. [Google Scholar]
- 51.Rex MA, et al. A source-sink hypothesis for abyssal biodiversity. Am Nat. 2005;165:163–178. doi: 10.1086/427226. [DOI] [PubMed] [Google Scholar]
- 52.Jablonski D, Roy K, Valentine JW. Out of the tropics: Evolutionary dynamics of the latitudinal diversity gradient. Science. 2006;314:102–106. doi: 10.1126/science.1130880. [DOI] [PubMed] [Google Scholar]
- 53.Danovaro R, et al. Exponential decline of deep-sea ecosystem functioning linked to benthic biodiversity loss. Curr Biol. 2008;18:1–8. doi: 10.1016/j.cub.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 54.Cronin TM, Dwyer GS. Deep sea ostracodes and climatic change. Paleontol Soc Pap. 2003;9:247–263. [Google Scholar]
- 55.Coles GP, Ainsworth NR, Whatley RC, Jones RW. Foraminifera and Ostracoda from Quaternary carbonate mounds associated with gas seepage in the Porcupine Basin, offshore western Ireland. Rev Esp Micropaleontl. 1996;28:113–151. [Google Scholar]
- 56.Corrége T. The relationship between water masses and benthic ostracod assemblages in the western Coral Sea, Southwest Pacific. Palaeogeogr Palaeoclimatol Palaeoecol. 1993;105:245–266. [Google Scholar]
- 57.Didié C, Bauch HA. Species composition and glacial-interglacial variations in the ostracode fauna of the northeast Atlantic during the past 200,000 years. Mar Micropaleontol. 2000;40:105–129. [Google Scholar]
- 58.Hunt G, Roy K. Climate change, body size evolution, and Cope's Rule in deep-sea ostracodes. Proc Natl Acad Sci USA. 2006;103:1347–1352. doi: 10.1073/pnas.0510550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horne DJ, Cohen A, Martens K. In: The Ostracoda: Applications in Quaternary Research. Holmes JA, Chivas AR, editors. Washington, DC: American Geophysical Union; 2002. pp. 5–36. [Google Scholar]
- 60.Martin PA, et al. Quaternary deep sea temperature histories derived from benthic foraminiferal Mg/Ca. Earth Planet Sci Lett. 2002;198:193–209. [Google Scholar]
- 61.Paillard D, Labeyrie L, Yiou P. Macintosh program performs time-series analysis. EOS. 1996;77:379. [Google Scholar]
- 62.Cronin TM, Boomer I, Dwyer GS, Rodriguez-Lazaro J. In: The Ostracoda: Applications in Quaternary Research. Holmes JA, Chivas AR, editors. Washington, DC: American Geophysical Union; 2002. pp. 99–119. [Google Scholar]
- 63.Cronin TM, et al. Late Quaternary paleoceanography of the Eurasian Basin, Arctic Ocean. Paleoceanography. 1995;10:259–281. [Google Scholar]
- 64.Cronin TM. Bathyal ostracodes from the Florida-Hatteras slope, the Straits of Florida, and the Blake Plateau. Mar Micropaleontol. 1983;8:89–119. [Google Scholar]
- 65.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.