Abstract
Little is known about the signaling that occurs in an antigen presenting cell (APC) during contact with a T cell. Here we report the concentration of the signaling lipid, PI(4,5)P2, at the APC side of the immunological synapse. In both human and mouse cells, a PI(4,5)P2-specific fluorescent reporter, PH-GFP, detected an antigen-dependent enrichment of PI(4,5)P2 at the synapse between antigen-specific T cells and APC. When PIP(4,5)P2 was sequestered by a high concentration of PH-GFP reporter, cells were less susceptible to CTL-mediated lysis than control cells. These findings suggest a new regulatory target for modulating immune function that may be exploited for immune escape by pathogens and tumors.
Keywords: Cytotoxicity, T cells Cytotoxic, MHC, Antigen Presentation
Introduction
Engagement of T cells and antigen presenting cells is highly orchestrated. Cognate cells must find each other, in an antigen-specific manner, and upon recognition form conjugates that remain stable for minutes to hours. In vitro, these contacts have been shown to involve rearrangements of receptors and ligands into a highly-organized immunological synapse (IS) (1). Many molecules that localize to the synapse play important signaling roles for activation and effector function (2-4).
Despite a wealth of study into the effector cell side of the immunological synapse, there has been little progress in understanding changes in organization and signaling on the APC side upon T cell engagement. APC molecules that localize to the synapse are T cell ligands; their redistribution into central, and proximal supramolecular complexes (5) seems to be driven and regulated by the T cell (6). A few studies demonstrate a role for actin organization in the APC side of the synapse (7,8). One recent paper showed a scaffolding protein of neuronal synapses localizing to the immunological synapse (9). Beyond these findings, little more is known about how the APC side of the synapse is organized and about what, if any, signaling occurs there.
This report shows that phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), an actin-modulating and signaling lipid (10), concentrates at the immunological synapse in APCs. Through its interactions with many regulators of actin polymerization including cdc42, NWASP and gelsolin (11), it maintains cell shape and membrane integrity (12). In addition, PI(4,5)P2 regulates dynamic processes such as cell movement, cell reshaping, phagocytosis and vesicle traffic (13-15). PI(4,5)P2 anchors cytosolic factors to the membrane; its own localization within the membrane has been shown to be cholesterol-dependent (12, 16). Upon cleavage by phospholipases, PI(4,5)P2 cleavage-products can activate calcium channel and other signaling pathways (17).
To our knowledge, this is the first report of a signaling lipid being concentrated at the IS on the APC side. Our data show that this concentration of PI(4,5)P2 plays a role in function and suggest that other signaling pathways may be triggered at the synapse in the APC.
Materials and Methods
Cell Lines, Constructs, Treatments
JY HLA-A2,B7 Human B-cells and T2-Kb cells are described elsewhere (18). T2-Kb were pulsed with SIY(SIYRYYGL) peptide for 1-2 hours at 37°C. Allogenic, HLA-A2-negative T cells were provided the JHMI Laboratory of Immunogenetics. Naïve alloreactive T cells were activated by co-culture with irradiated JY stimulator cells for 4 days (18). 2C T cells (19) were provided by J Schneck (Dept of Pathology, Johns Hopkins Medical Institutions) and activated by irradiated splenocytes from Balb/c mice. Expression constructs for PH-GFP and PH-GFP R40L were provided by Tomas Balla (20). T2 cells were transfected, transiently or stably, by electroporation with ∼10ug DNA. Stable cell lines were generated by drug selection (300μg/ml G418) and flow cytometric sorting.
Functional Assays
Death of APCs was measured in terms of Annexin V, and 7-AAD labeling. T2-Kb cells, pulsed with SIY peptide (19), were mixed with activated 2C T cells at an E/T=5 for 2 hours followed by Annexin V staining for apoptosis. FACS analysis was conducted on a BD FACSCalibur. Anti-mouse CD8 (BD), anti-mouse H2-Kb 20.8.4s (21), and anti-HLA Ke2 were used for staining (22).
For MHC crosslinking experiments, ionized glass coverslips were coated with mABs anti-Kb,Y3 clone (23), or anti-CD59, MEM43 clone (24). T2-Kb APCs were allowed to settle and adhere to the coverslip, and fixed after 30 minutes with 4% PFA. Alternatively, anti-MHC beads were constructed using 5μm magnetic Protein A beads (Dynal, Oslo Norway) conjugated with mAb anti-Kb (20.8.4s). For MHC capping experiments, cells were labeled with 20.8.4-Cy3 at 37°C for 15 minutes, then washed, and fixed as before.
Microscopy
All imaging was done on a Zeiss LSM 510-meta Confocal microscope using appropriate filter settings and laser lines. For fixed conjugates, T2-Kb APCs and 2C activated T cells or beads were incubated for 30 minutes and then fixed in 4% PFA. Cells were washed and mounted in microslides (Vitrotubes). For live cell imaging, alloreactive T cells were mixed with JY B cells, lightly centrifuged, and mounted in PBS + 1% FBS in microslides. The stage was warmed to 37°C. Images were analyzed using Image Examiner (Zeiss) and ImageJ. Quantification and plots were generated using Excel (Microsoft) and Prism 4.0 (GraphPad).
Results
Two APC cell lines, JY cells (HLA-A2) and T2-Kb (H2-Kb) were used to investigate the response of APCs to T cell engagement. The APCs were transfected with PH-GFP, a fusion protein which binds PI(4,5)P2 and reports its cellular localization. In APCs expressing PH-GFP, a characteristic uniform membrane localization was observed (Figure 1A) as previous reports have shown (25). Cells expressing the mutant variant, PH-GFP R40L, showed a diffuse cytoplasmic distribution (Figure 1B). When effector T cells were mixed with antigen-specific APCs, of PI(4,5)P2 concentrated at the interface between APC and T cell (Figure 1C,D). There was a 30-50% increase in intensity at the interface as compared to the rest of the APC cell membrane (Figure 1E,F). This enrichment was seen for both live and fixed cell conjugates. PH-GFP R40L did not concentrate at the IS. Since these sites of contact have been shown to be single membrane thickness by electron microscopy (26), it is unlikely that membrane ruffling could explain the increase in signal, but rather argues for an active recruitment of PI(4,5)2.
Figure 1. PI(4,5)P2 localization polarizes in APCs after T cell contact in an MHC-independent manner.
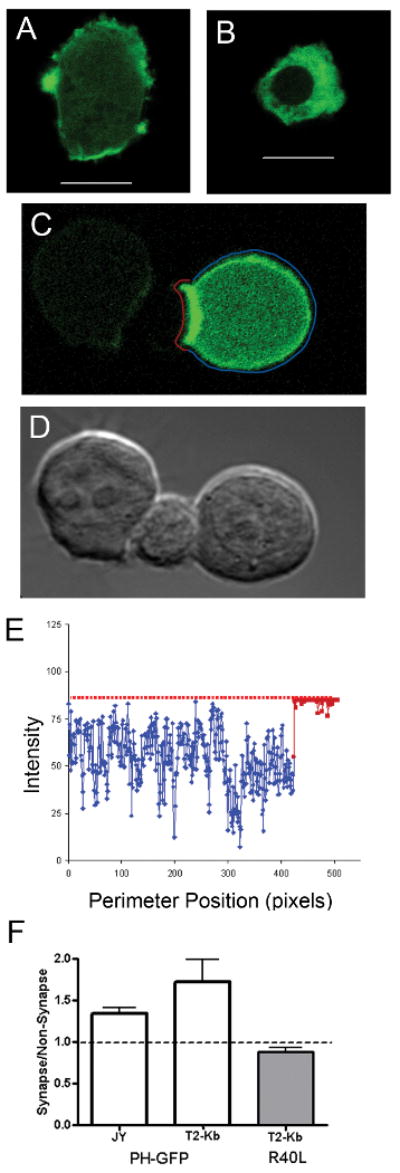
(A) PH-GFP distribution reports on PI(4,5)P2 localization in JY B-cells; R40L point mutant (B) no longer localizes to PI(4,5)P2 pools. PI(4,5)P2 localization polarizes upon contact with effector T cells, shown in (C) with DIC image in (D). The ratio of PH-GFP intensity at the contact site, highlighted in red, to the intensity in the remainder of the cell perimeter (highlighted in blue, on the cell (C)) is plotted in (E). The average ratios for APC:T cell conjugates of JY+alloreactive human T cells, or T2-Kb (loaded with SIY peptide)+activated 2C T cells are shown in F. 10 μm scale bars. Error bars are SD from 3 pooled independent experiments.
To see if MHC engagement alone was sufficient to induce PIP2 redistribution, coverslips were coated with either anti-MHC antibodies or control antibody (anti-CD59) and allowed to conjugate with APCs. We did not observe a change in PH-GFP surface distribution when MHC I molecules or CD59 molecules (controls) were bound by antibody on coverslips or beads (Supplemental Figure 1). Capping MHC with soluble antibody also did not induce a consistent redistribution of PH-GFP.
The accumulation of PI(4,5)P2 at the immunological synapse suggested a functional response by APCs to T cell engagement. Live cell imaging was used to observe the dynamics of PI(4,5)P2 lipids and cell lysis. During live cell imaging of CTL-APC conjugates, in some cases, T cells were in contact with multiple target cells, each expressing PH-GFP at a different level. Time-lapse imaging of these aggregates showed that while T cells scanned APCs without bias, they preferentially lysed cells expressing lower levels of PH-GFP; APCs that expressed higher levels of PH-GFP were more resistant to T cell lysis. Two sets of time-lapse images are shown in Figure 2 (and in Supplemental movie 1).
Figure 2. APCs require PI(4,5)P2 at the IS for T cell mediated lysis.
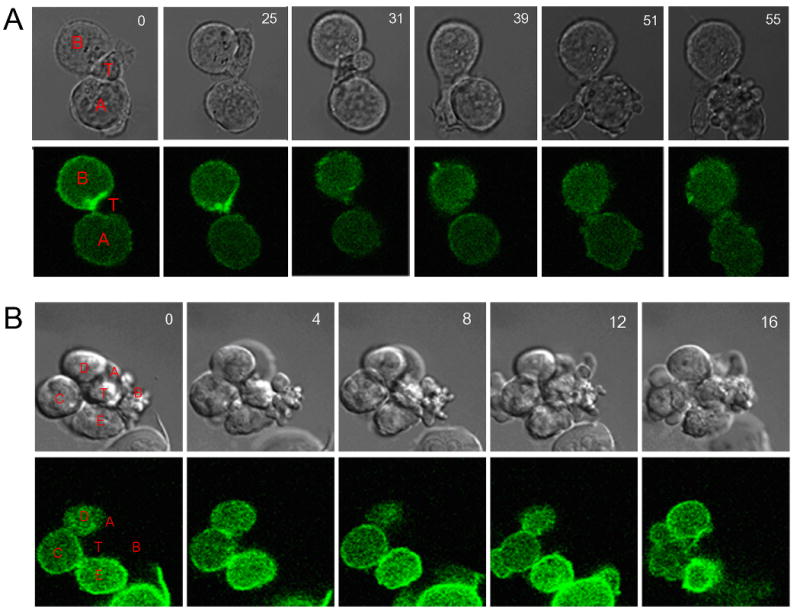
Two (A&B) time lapse movies were taken as z-stacks, with the time indicated for each set of DIC, PH-GFP images. In (2A), two B-cells, expressing low (cell A) and high (cell B) levels of PH-GFP are in contact with a T cell. During the time course, the T cell samples cell B and then cell A, with no bias towards either cell. After 50 minutes of imaging, only cell A shows membrane blebbing and apoptosis. In (2B), the T cell (labeled T) is in contact with the surrounding APCs (labeled A-E, in order of increasing PH-GFP expression). Cells A and B show membrane blebbing in the DIC images at the start of imaging. After 16 minutes, cell C also begins blebbing its membrane, indicating apoptosis.
PH-GFP is frequently used as a reporter for PI(4,5)P2 localization, but at high expression levels it will compete with endogenous molecules that bind PI(4,5)P2 (25) resulting in a PI(4,5)P2-hypomorphic cell. This suggested that the resistance of PH-GFP high cells is due to sequestering of PI(4,5)P2. To test this idea, we measured the ability of 2C CTLs to kill peptide-loaded T2Kb targets expressing PH-GFP or the R40L variant. Apoptosis was markedly reduced in PH-GFP expressing APCs as compared to cells expressing the PH-GFP R40L (Figure 3). These results confirmed that blocking PI(4,5)P2 in APCs inhibited effector T cell function. A similar reduction was found for allo-reactive T cell mediated lysis of JY targets expressing PH-GFP, as measured by a chromium release assay (data not shown).
Figure 3. Reduction of APC PI(4,5)P2 affects their lysis by activated CTLs.
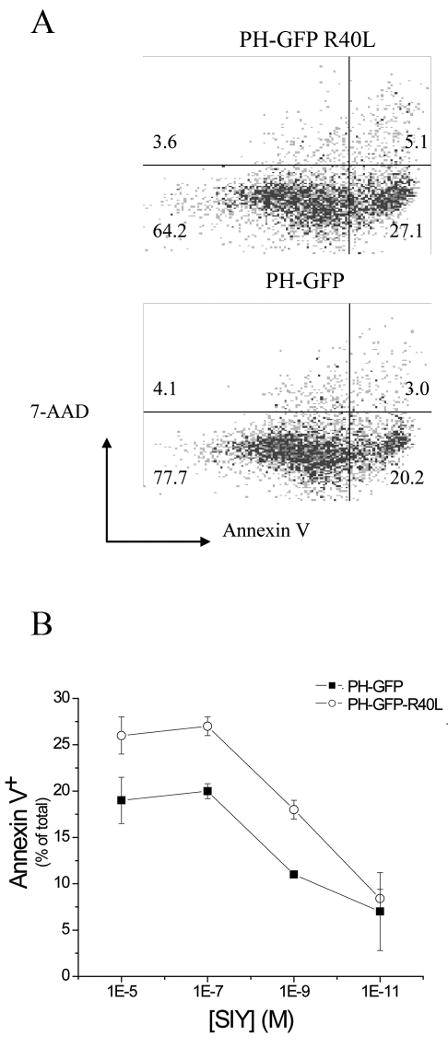
(A) Sample dot plots of Annexin V and 7-AAD staining of T2-Kb cells stably transfected with PH-GFP or PH-GFP-R40L cells. Cells are gated on GFP positive cells. (B) Activated CTL lysis of T2-Kb - PH-GFP or PH-GFP-R40L cells from a single experiment representative of 3 separate measurements. Values are average ± S.E. for Effector:Target ratio 5 over a range of SIY concentration.
Sequestering PI(4,5)P2 by expression of PH-GFP did not reduce surface MHC class I levels (data not shown). MHC surface stability, measured in terms of MHC surface half-life after Brefeldin-A treatment, was also unaffected. This indicates that sequestering PI(4,5)P2 in APCs affects a pathway to cell death that is downstream of MHC display.
Discussion
APCs concentrate PI(4,5)P2 in their contacts with T cells. Crosslinking MHC on the plasma membrane was insufficient to induce this concentration of PI(4,5)P2. This suggests a more complex engagement is needed to generate signals from PI(4,5)P2. It is known that capping MHC molecules can induce calcium signals (27-28) but only after several hours. Crosslinking of MHC did not induce local concentration of PIP2 in the same way as T-cell engagement.
Since PIP(4,5)P2 diffuses rapidly, it is likely that local synthesis is not be sufficient to maintain its localization; rather exchange with PIP2 binding proteins could retain these lipids at the synapse (29). Recruitment of PIP2 binding proteins may play a role in MHC I-dependent signal transduction but further work will be required to elucidate their identity.
It is unclear how blocking MHC I signaling with PH-GFP affects CTL-mediated apoptosis. It is possible that PI(4,5)P2 sequestration affects T cell degranulation or susceptibility of target cells to lysis. The accumulation of PI(4,5)P2 at the IS may be required for uptake of cytolytic granules since PI(4,5)P2 plays an important role in various pathways of endocytosis and phagocytosis at the plasma membrane (30-31). It is thought that granzyme B function requires internalization and acidification prior to activation (32). It may be that PH-GFP expression inhibits a PI(4,5)P2-dependent endocytic pathway, such as clathrin-coated trafficking pathway. By blocking granule uptake, the cells are protected from apoptosis.
APC responses to CTL contact are poorly defined. Various viral mechanisms exist to evade MHC class I presentation (33). PI(4,5)P2 inhibition represents a novel form of CTL-mediated inhibition. This strategy inhibits lysis without compromising class I presentation, which might activate NK-cell recognition. A similar mechanism may be employed by intracellular pathogens, seeking to prevent apoptosis of their host cells. Furthermore, it may be fruitful to investigate the role of natural and synthetic PI-inhibitors, used to inhibit tumor growth (34), to see if they have unexpected effects on cell-mediated immunity.
Supplementary Material
T2-Kb cells expressing PH-GFP (A-D) or PH-GFP R40L (E-F) were treated with high-affinity anti-Kb (HB-11) tagged with Cy-3 at 37°C to allow crosslinking of MHC molecules. Cells were fixed and mounted on poly-lysine coverslips, and imaged by Confocal microscopy. Z-stack images show capping of MHC-class I on the surface. Some cells showed no specific recruitment of PH-GFP (examples in A&B), while others showed some inconsistent colocalization (C&D). In E& F, T2-Kb cells expressing PH-GFP were adhered to coverslips pre-coated with antibodies against H2-Kb, (examples in E) or against CD-59 control antigen (examples in F). After 10 minutes at 37°C, cells were fixed and imaged by Confocal microscopy. Imaging the contact surface did not show any specific recruitment or punctate distribution of PH-GFP at the interface. All scale bars represent 10μm.
Acknowledgments
We wish to thank Jonathan Schneck and his laboratory for 2C T cells, reagents and critical discussions of the work.
This work was funded by National Institutes of Health Grant AI-14584 (ME) and National Institutes of Health Training Grant 5T32 AI-07247 (SRS).
Footnotes
Disclosures
none to report.
References
- 1.Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 2.Davis DM, Dustin ML. What is the importance of the immunological synapse? Trends Immunol. 2004;25:323–327. doi: 10.1016/j.it.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Dustin ML. Impact of the immunological synapse on T cell signaling. Results Probl Cell Differ. 2006;43:175–198. doi: 10.1007/400_019. [DOI] [PubMed] [Google Scholar]
- 4.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol. 2002;20:371–394. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 5.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groves JT, Dustin ML. Supported planar bilayers in studies on immune cell adhesion and communication. J Immunol Methods. 2003;278:19–32. doi: 10.1016/s0022-1759(03)00193-5. [DOI] [PubMed] [Google Scholar]
- 7.Gordy C, Mishra S, Rodgers W. Visualization of antigen presentation by actin-mediated targeting of glycolipid-enriched membrane domains to the immune synapse of B cell APCs. J Immunol. 2004;172:2030–2038. doi: 10.4049/jimmunol.172.4.2030. [DOI] [PubMed] [Google Scholar]
- 8.Setterblad N, Becart S, Charron D, Mooney N. B cell lipid rafts regulate both peptide-dependent and peptide-independent APC-T cell interaction. J Immunol. 2004;173:1876–1886. doi: 10.4049/jimmunol.173.3.1876. [DOI] [PubMed] [Google Scholar]
- 9.Bloom O, Unternaehrer JJ, Jiang A, Shin JS, Delamarre L, Allen P, Mellman I. Spinophilin participates in information transfer at immunological synapses. J Cell Biol. 2008;181:203–211. doi: 10.1083/jcb.200711149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downes CP, Gray A, Lucocq JM. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol. 2005;15:259–268. doi: 10.1016/j.tcb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Sechi AS, Wehland J. The actin cytoskeleton and plasma membrane connection: PtdIns(4,5)P(2) influences cytoskeletal protein activity at the plasma membrane. J Cell Sci. 2000;113(21):3685–3695. doi: 10.1242/jcs.113.21.3685. [DOI] [PubMed] [Google Scholar]
- 12.Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci U S A. 2003;100:13964–13969. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rozelle AL, Machesky LM, Yamamoto M, Driessens MH, Insall RH, Roth MG, Luby-Phelps K, Marriott G, Hall A, Yin HL. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr Biol. 2000;10:311–320. doi: 10.1016/s0960-9822(00)00384-5. [DOI] [PubMed] [Google Scholar]
- 14.De Matteis MA, Godi A. PI-loting membrane traffic. Nat Cell Biol. 2004;6:487–492. doi: 10.1038/ncb0604-487. [DOI] [PubMed] [Google Scholar]
- 15.Martin TF. PI(4,5)P(2) regulation of surface membrane traffic. Curr Opin Cell Biol. 2001;13:493–499. doi: 10.1016/s0955-0674(00)00241-6. [DOI] [PubMed] [Google Scholar]
- 16.Pike LJ, Miller JM. Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J Biol Chem. 1998;273:22298–22304. doi: 10.1074/jbc.273.35.22298. [DOI] [PubMed] [Google Scholar]
- 17.Krauss M, Haucke V. Phosphoinositides: Regulators of membrane traffic and protein function. FEBS Lett. 2007 doi: 10.1016/j.febslet.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 18.Fooksman DR, Gronvall GK, Tang Q, Edidin M. Clustering class I MHC modulates sensitivity of T cell recognition. J Immunol. 2006;176:6673–6680. doi: 10.4049/jimmunol.176.11.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sykulev Y, Brunmark A, Tsomides TJ, Kageyama S, Jackson M, Peterson PA, Eisen HN. High-affinity reactions between antigen-specific T cell receptors and peptides associated with allogeneic and syngeneic major histocompatibility complex class I proteins. Proc Natl Acad Sci U S A. 1994;91:11487–11491. doi: 10.1073/pnas.91.24.11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozato K, Sachs DH. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981;126:317–321. [PubMed] [Google Scholar]
- 22.Schreiber AB, Schlessinger J, Edidin M. Interaction between major histocompatibility complex antigens and epidermal growth factor receptors on human cells. J Cell Biol. 1984;98:725–731. doi: 10.1083/jcb.98.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammerling GJ, Rusch E, Tada N, Kimura S, Hammerling U. Localization of allodeterminants on H-2K b antigens determined with monoclonal antibodies and H-2 mutant mice. Proc Natl Acad Sci USA. 1982;79:4737–4741. doi: 10.1073/pnas.79.15.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawada R, Ohashi K, Anaguchi H, Okazaki H, Hattori M, Minato N, Naruto M. Isolation and expression of the full-length cDNA encoding CD59 antigen of human lymphocytes. DNA Cell Biol. 1990;9:213–220. doi: 10.1089/dna.1990.9.213. [DOI] [PubMed] [Google Scholar]
- 25.Balla T, Varnai P. Visualizing cellular phosphoinositide pools with GFP-fused protein- modules. Sci STKE. 2002;2002:L3. doi: 10.1126/stke.2002.125.pl3. [DOI] [PubMed] [Google Scholar]
- 26.Pinkoski MJ, Hobman M, Heibein JA, Tomaselli K, Li F, Seth P, Froelich CJ, Bleackley RC. Entry and trafficking of granzyme B in target cells during granzyme B-perforin-mediated apoptosis. Blood. 1998;92:1044–1054. [PubMed] [Google Scholar]
- 27.Geppert TD, Wacholtz MC, Patel SS, Lightfoot E, Lipsky PE. Activation of human T cell clones and Jurkat cells by cross-linking class I MHC molecules. J Immunol. 1989;142:3763–3772. [PubMed] [Google Scholar]
- 28.Gur H, Geppert TD, Wacholtz MC, Lipsky PE. The cytoplasmic and the transmembrane domains are not sufficient for class I MHC signal transduction. Cell Immunol. 1999;191:105–116. doi: 10.1006/cimm.1998.1417. [DOI] [PubMed] [Google Scholar]
- 29.Golebiewska U, Nyako M, Woturski W, Zaitseva I, McLaughlin S. Diffusion coefficient of fluorescent phosphatidylinositol 4,5-bisphosphate in the plasma membrane of cells. Mol Biol Cell. 2008;19(4):1663–9. doi: 10.1091/mbc.E07-12-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doughman RL, Firestone AJ, Anderson RA. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J Membr Biol. 2003;194:77–89. doi: 10.1007/s00232-003-2027-7. [DOI] [PubMed] [Google Scholar]
- 31.Coppolino MG, Dierckman R, Loijens J, Collins RF, Pouladi M, Jongstra-Bilen J, Schreiber AD, Trimble WS, Anderson R, Grinstein S. Inhibition of phosphatidylinositol-4-phosphate 5-kinase Ialpha impairs localized actin remodeling and suppresses phagocytosis. J Biol Chem. 2002;277:43849–43857. doi: 10.1074/jbc.M209046200. [DOI] [PubMed] [Google Scholar]
- 32.Griffiths GM. Endocytosing the death sentence. J Cell Biol. 2003;160:155–156. doi: 10.1083/jcb.200212143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keckler MS. Dodging the CTL response: viral evasion of Fas and granzyme induced apoptosis. Front Biosci. 2007;12:725–732. doi: 10.2741/2096. [DOI] [PubMed] [Google Scholar]
- 34.Weber G. Down-regulation of increased signal transduction capacity in human cancer cells. Adv Enzyme Regul. 2005;45:37–51. doi: 10.1016/j.advenzreg.2005.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
T2-Kb cells expressing PH-GFP (A-D) or PH-GFP R40L (E-F) were treated with high-affinity anti-Kb (HB-11) tagged with Cy-3 at 37°C to allow crosslinking of MHC molecules. Cells were fixed and mounted on poly-lysine coverslips, and imaged by Confocal microscopy. Z-stack images show capping of MHC-class I on the surface. Some cells showed no specific recruitment of PH-GFP (examples in A&B), while others showed some inconsistent colocalization (C&D). In E& F, T2-Kb cells expressing PH-GFP were adhered to coverslips pre-coated with antibodies against H2-Kb, (examples in E) or against CD-59 control antigen (examples in F). After 10 minutes at 37°C, cells were fixed and imaged by Confocal microscopy. Imaging the contact surface did not show any specific recruitment or punctate distribution of PH-GFP at the interface. All scale bars represent 10μm.


