Abstract
We propose a mechanism for tumor growth emphasizing the role of homeostatic regulation and tissue stability. We show that competition between surface and bulk effects leads to the existence of a critical size that must be overcome by metastases to reach macroscopic sizes. This property can qualitatively explain the observed size distributions of metastases, while size-independent growth rates cannot account for clinical and experimental data. In addition, it potentially explains the observed preferential growth of metastases on tissue surfaces and membranes such as the pleural and peritoneal layers, suggests a mechanism underlying the seed and soil hypothesis introduced by Stephen Paget in 1889, and yields realistic values for metastatic inefficiency. We propose a number of key experiments to test these concepts. The homeostatic pressure as introduced in this work could constitute a quantitative, experimentally accessible measure for the metastatic potential of early malignant growths.
The progression of cancer is a multi-step process. Over 80% of malignant tumors are carcinomas that originate in epithelial tissues from where they invade through the basal membrane into the connective tissue. At some point, subpopulations of cells may detach from the primary tumor and spread via the bloodstream and the lymphatic system. Some of them give rise to metastases in distant organs. Metastases account for the majority of patients’ deaths due to cancer, and thus understanding the metastatic process is of critical importance. The metastatic cascade is a very inefficient process, as only one in about a thousand cells that leave the primary tumor goes on to form a macroscopic secondary tumor. This property is referred to as “metastatic inefficiency” (Chambers et al., 2002; Sahai, 2007). Recent experimental results have shown, however, that cell extravasation is highly efficient, namely that over 80% of the metastatic cells that are present in the bloodstream manage to enter a distant organ (Luzzi et al., 1998; Cameron et al., 2000; Zijlstra et al., 2002). Thus, the main contribution to metastatic inefficiency arises from the failure of cancerous cells to grow inside invaded organs. Metastatic tumors also show preferential growth in different organs with a distribution that cannot be explained by blood flow patterns alone. Hence, the efficiency of the metastatic process depends on specific interactions between the invading cancer cells and the local organ tissues (Fidler et al., 2003). This concept, referred to as the “seed and soil hypothesis,” was introduced by Stephen Paget as early as 1889 (Weinberg, 2007): “the seed”—the metastatic cell—needs to be compatible with “the soil”—the host tissue—for successful growth to occur (Fidler, 2003; Couzin, 2003). Despite this early observation, the nature of the interactions controlling both the efficiency of the metastatic process and its tissue specificity remains a poorly understood aspect of cancer progression even today.
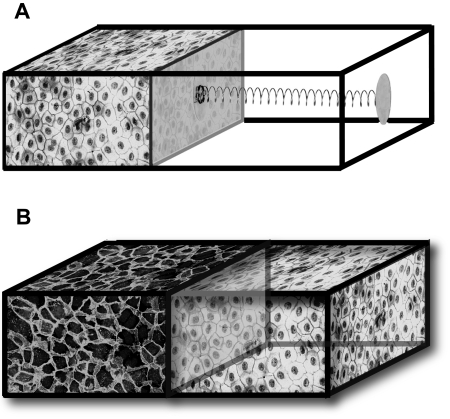
In this work, we introduce the notion of homeostatic pressure and propose that it is an important property for describing the competition between different tissues grown in a finite volume. The concept of homeostatic pressure is best defined from the following experiment: consider a chamber in which cells can be cultured with a setup that enables successful proliferation, allowing in particular for water, oxygen, nutrients, and growth factors to diffuse through the compartment walls, keeping the cells’ biochemical environment constant. The compartment is closed on one side by a piston connected to a rigid wall with a spring [Fig. 1A]. As the growing tissue fills the available space and gradually compresses the spring, the pressure rises until a steady state is reached in which division balances apoptosis and the piston stops moving. The spring position is stable, since further growth increases the pressure above this value and favors apoptosis, whereas recession of the piston decreases the pressure and favors division. This steady state is characterized by a well-defined pressure exerted on the spring and a well-defined density of cells, which we refer to as the homeostatic pressure and density of the tissue in this particular biochemical environment. Note that the homeostatic pressure is different from the hydrostatic pressure since the lateral walls of the chamber allow for fluid transport. Instead, it resembles more an osmotic pressure but originates from the forces driving tissue expansion.
Figure 1. (A) Schematic representation of a measurement apparatus for the homeostatic pressure.
As the tissue proliferates, the piston compresses the spring and the pressure exerted on the tissue increases. Once a steady state is reached, cell division and apoptosis balance. The cell density and the pressure exerted on the spring at this point define the homeostatic mechanical state of the tissue in a given biochemical environment. (B) Schematic representation of a tissue-competition experiment. The two tissues are in mechanical contact through a freely moving, impermeable piston. The tissue with the lower homeostatic pressure is compressed to a cell density above its homeostatic point and initiates apoptosis. The other tissue proliferates and expands until the opposing tissue has disappeared.
We now show that the ability of one tissue to replace another one in a competition for space depends on the relative values of their homeostatic pressures. Let us consider a similar chamber, but in which the piston separates two tissue compartments H and T, establishing mechanical contact[see Fig. 1B]. Suppose that tissue H has a homeostatic pressure pH,h smaller than that of tissue T, pT,h. As cells divide, the pressure rises and first reaches the homeostatic pressure pH,h. At this point, tissue H stops growing while tissue T continues to proliferate and drives the pressure above pH,h. As a result, the apoptosis rate of H becomes larger than its division rate, resulting in its recession. The process continues until tissue H completely disappears. The winning compartment always corresponds to the tissue with the larger homeostatic pressure.
It is interesting to consider the effect of biochemical signaling or immunological interactions between the two tissues. In particular, consider the case where H resists the expansion of T by locally decreasing the homeostatic pressure of T. If this decrease is large enough, tissue T shrinks and the result of the competition will be reversed as compared to the case without signaling. However, if we consider a sufficiently large compartment T, the region close to H has a negligible contribution to the overall compartment growth, and T expands as in the absence of signaling. There is a particular size of compartment T for which, at the homeostatic pressure pH,h, its average growth vanishes: the excess division away from the piston exactly balances the excess death close to it. A steady state is possible for this particular size of compartment T, but it is unstable. This introduces the second important concept of this paper: the existence of a critical size beyond which a tumor tends to grow and below which it tends to shrink.
A critical size can also exist due to interfacial tension in higher-dimensional geometries, such as the two-dimensional organization of a monolayered epithelium or the three-dimensional configuration of a secondary tumor within the bulk of a host tissue. The concept of tissue interfacial tension has already been used to explain cell sorting of tissues with different adhesive properties (Duguay et al., 2003), and quantified for several tissues (Foty, 1996). Tissue interfacial tension can also originate from the mechanical contraction of cytoskeletal elements at the interface (Lecuit and Lenne, 2007; Schötz et al., 2008). In a spheroid of tissue T located within the bulk of tissue H, the excess pressure in T is given by Laplace’s law: Δp=2γ∕r, where γ is the interfacial tension between H and T and r is the radius of the spheroid. As a result, for small enough radii, the pressure in T is larger than pT,h, and T recedes. For large radii, however, the excess pressure as given by Laplace’s law vanishes and we recover the previous one-dimensional case where T grows. There is again an unstable critical radius rc for which a steady state exists.
So far, we have considered cell growth and death processes as entirely deterministic, in which case only tumors larger than the critical size can grow. However, single cells give rise to tumors and metastases (Talmadge and Fidler, 1982; Talmadge and Zbar, 1987; Chambers and Wilson, 1988). This is possible because cell growth and death are stochastic processes. In this paper, we calculate the probability for a single cell to give rise to a macroscopic tumor and obtain results that are compatible with experimental data on metastatic inefficiency (Luzzi et al., 1998; Cameron et al., 2000; Zijlstra et al., 2002). The concepts we use here are similar to those used to describe the statistics of nucleation processes as they occur in first-order phase transitions. It is well known that nucleation is easier on surfaces or foreign bodies than in the bulk of a system. The same holds true for tumor growth: we show that it is more likely for tumors to reach the critical size at an interface than in the bulk of a tissue, in agreement with experimental and clinical observations (Cameron et al., 2000; Weiss, 1985). Hence, in this paper, we argue that an unstable critical size for tumor growth exists, which is responsible for the inefficiency of the metastatic cascade and could account for the preferred growth of metastases on surfaces and interfaces. We treat only the early stages of tumor and metastatic growth, where the heterogeneity of tumors—due to effects such as the diffusion of nutrients and growth factors or genetic mutations—can be neglected. These effects play an important role for larger tumor sizes only (Hanahan and Weinberg, 2000).
RESULTS
Tissue rheology and homeostasis
While the notion of homeostatic pressure and density is model independent, the details of the tissue dynamics are not. Here, we employ a continuous description that we expect to be valid for systems large compared to the cell size and time scales large compared to the characteristic times of individual cellular processes. The local density of cells ρ obeys the continuity equation:
| (1) |
where v denotes the local velocity of the tissue and ∇⋅(ρv) the divergence of the cell flux. The right hand side corresponds to source and sink terms that describe the local production and destruction of cells due to cell division (kd) and apoptosis (ka). In addition, tissues must also satisfy momentum conservation, which, for systems where inertia plays a negligible role, reduces to force-balance:
| (2) |
Here, ∂α denotes the partial derivative with respect to the coordinate α (α=x,y,z), and summation over repeated indices is implicit; σαβ denotes the total stress tensor that we split into a velocity-independent part and a dynamic part . For an isotropic tissue, the velocity-independent part reads −pδαβ, where p is the tissue pressure discussed above. The dynamic part, however, encodes the rheological properties of the tissue in a constitutive equation that relates it to the velocity-gradient tensor ∂αvβ. Tissues are complex media with a rheological behavior intermediate between those of liquids and solids (Foty et al., 1994; Schötz et al., 2008). On time scales short compared to their viscoelastic relaxation time, tissues have a finite shear modulus E of the order of 102–104 Pa (Forgacs et al., 1998; Engler et al., 2004; Kong et al., 2005). For time scales exceeding the largest relaxation time τ, however, viscoelastic media behave as viscous liquids with viscosity η=Eτ. Measurements of the mechanical response of various cell aggregates suggest a value of the relaxation time in the range of tens of seconds to several minutes (Forgacs et al., 1998; Schötz et al., 2008), corresponding to a viscosity in the range of 103–105 Pa s. The fastest division rates of mammalian cells are typically of the order of one division per day (Weinberg, 2007). Hence, tissue-growth dynamics takes place on time scales that are long compared to the characteristic times of cellular processes, including adhesion and detachment of the proteins that insure the integrity of the tissue under consideration. Under such conditions, it is a general result that the effective rheology on large scales appears to be that of a fluid (Frisch et al., 1986). We therefore argue that, in the context of tissue-growth dynamics, a purely viscous rheology is appropriate, which leads to the standard constitutive equation:
| (3) |
Under fixed biochemical and biophysical conditions, division and apoptosis rates—as well as pressure—are functions of cell density only. In the absence of a detailed knowledge of the pressure and rate dependences as functions of ρ, and for the sake of simplicity, we rely on an expansion to first order in ρ−ρh around the homeostatic density ρh:
| (4) |
The parameter χ is equivalent to the standard compressibility of a material and describes the variation of cell density with pressure. Similarly, the coefficient κ quantifies how the difference between division and apoptosis rates depends on density. Both χ and κ are experimentally accessible parameters that must both be positive to insure stability. In Eqs. 4, the expansion of the pressure p in terms of the cell density ρ is complementary to the expansion of kd−ka known as logistic growth, which is a common way to model growth dynamics (Sachs et al., 2001). As we have stated, the most general dependence of the pressure p as well as of the division and apoptosis function kd−ka on the biochemical environment of the tissue is encoded in the expansion coefficients χ and κ here, which are therefore constants only under fixed biochemical conditions. As we focus on tumors of small sizes under steady environmental conditions, such a dependence will not be discussed here. But our framework in principle allows us to study more complex situations where spatio-temporal inhomogeneities would play a role, simply by allowing χ and κ to vary. Cell division and apoptosis could also be coupled directly to pressure, leading to tissue competition as proposed in a similar model by Shraiman (2005). Also, studies have shown that a defective density sensing can lead to a growth advantage of cancerous cells (Chaplain et al., 2006). In this paper, we argue that competition for volume is a generic property of tissues in mechanical contact, since the pressure and effective growth rate functions take the form of Eqs. 4 close to the steady state density of the tissue.
Tumor growth dynamics
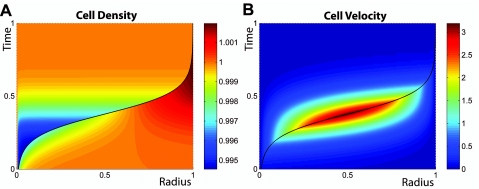
We are now in a position to show that homeostatic regulation intrinsically contains a growth mechanism for neoplastic tissues. Consider the growth of a spherical tissue T located at the center of a spherical compartment of finite volume, filled with another tissue H of lower homeostatic pressure. The spherical tissue can be either a primary tumor developing within the tissue it stems from, or a metastasis that has migrated from its original location and invaded a foreign organ. The two tissues are in mechanical contact, so that the total stress is continuous at the interface. Equations 1, 2, 3, 4 must be solved for both compartments, taking the location of the interface of the two tissues into account. A numerical solution of the associated generic growth dynamics is presented in Fig. 2. The solution shows that the tissue with higher homeostatic pressure grows at the expense of the other one and takes over the entire compartment. In vivo, however, the condition of a fixed finite volume does not hold in general. In real tissues, there is often first a displacement of the nontumor tissue before anatomical constraints limit the total volume available to the system. However, the devastating effect of malignant tumors stems from the fact that they invade and replace the functional tissues. The architecture of most tissues leads to a competition for volume in the case of neoplastic proliferation. Note also that, considering the time evolution of the boundary between the two tissues only, we get a curve that is reminiscent of the well-known, experimentally observed Gompertzian growth curves (Molski and Konarski, 2003). A quantitative illustration of this behavior obtained within our framework is illustrated in the Supplementary Material, making use of realistic parameters.
Figure 2. Numerical solution for the cell density (A) and cell velocity (B) as functions of space and time during the growth of one tissue located in the bulk of another tissue of lower homeostatic pressure.
Color coding for local cell density and velocity is given on the right hand side of panels (A) and (B), respectively. Spherical symmetry is assumed. Total integration time, compartment size, and homeostatic densities of tissues T and H are scaled to one. In both plots, the boundary between the two tissues is indicated by a black line. Parameters are chosen in order to illustrate the interplay between viscous dynamics and compartment growth (see Supplementary Material).
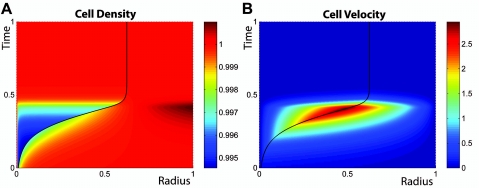
We now examine several effects that can significantly alter the tumor growth dynamics as presented above. A first example, which is motivated by the structure of benign tumors, corresponds to tissue T engulfed in a membrane, typically a thin shell of extracellular matrix, where the surface tension γ rises as T expands. If this tension increases faster than the radius of T, the additional pressure increases and the expansion of T eventually stops: a stable steady state exists at r=rs such that pT,h−pH,h=2γ(rs)∕rs, where pT,h and pH,h are the respective homeostatic pressures of T and H. A numerical solution illustrating this case is presented in Fig. 3. This dormant state is stable until genetic alterations inducing the production of proteases by the tumor cells lead to the degradation of the membrane.
Figure 3. Numerical solution for the cell density (A) and cell velocity (B) as functions of space and time during the growth of a tissue engulfed in an elastic membrane and located in the bulk of another tissue of lower homeostatic pressure.
As in Fig. 2, spherical symmetry is assumed; total integration time, compartment size, and homeostatic densities are scaled to one, and in both plots the boundary between the two tissues is indicated by a black line. Color coding is similar to the one used in Fig. 2. In this solution, the membrane is treated as purely elastic and is put under tension above a given radius x0=0.5. The explicit surface tension dependence on the boundary location x is given in the Supplementary Material. Additional parameters are given in the Supplementary Material. (A) The expansion of the inner compartment is similar to that of Fig. 2 until, at x=0.5, surface tension begins to play a role. The membrane expands until its tension balances the pressure difference between the two compartments and a stable steady state is reached. (B) Corresponding cell-velocity plot.
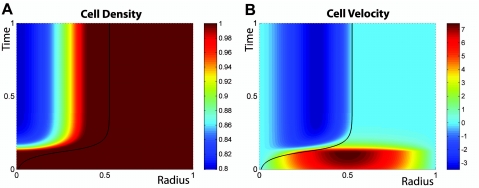
As a second example, we consider the case of a tumor that is limited in its growth, for example by nutrient or oxygen supply. It is indeed a well-known fact that tumors are poorly vascularized before they acquire the capability to trigger the growth of new blood vessels via angiogenesis (Folkman and D’Amore, 1996; Weidner et al., 1991;Hanahan and Weinberg, 2000). This limitation has profound consequences for their growth dynamics (Preziosi, 2003), often leading to the existence of a maximum size of about 1–2 mm, where they remain in a “dormant state” until the induction of angiogenesis (Folkman and D’Amore, 1996; Weinberg, 2007). In Fig. 4, we present a numerical integration of the growth dynamics of a nutrient-limited tumor in the bulk of a healthy, well vascularized tissue. While we assume a homogeneous and high enough concentration of nutrients for the healthy tissue, the neoplastic tissue is only supplied with nutrients via diffusion through its surface. Since nutrient diffusion is fast compared to growth dynamics, we calculate the nutrient concentration profile by solving a steady-state diffusion equation, taking into account nutrient consumption due to cell metabolism and division (see Supplementary Material). We choose functional dependences of the division and apoptosis rates on the nutrient concentration and cell density that correspond to a biological behavior: under very low concentrations of nutrients or oxygen, cells tend to die, but a limited supply of nutrients can also decrease cell division by triggering cell differentiation, inducing a quiescent cell state or favoring adaptation of the metabolism of the cells to the new environment. In agreement with what is known about the internal structure of dormant tumors, cells divide at the boundary where they get enough nutrients, and die at the center. This creates a steady state flow of cells from the surface toward the center of the tumor and thereby a constant cell turnover that is favorable to mutations.
Figure 4. Numerical solution of the growth dynamics with the geometrical arrangement of Fig. 2 when growth rates are nutrient limited.
Nutrients diffuse into the inner compartment through the tissue interface (see Supplementary Material). (A) The inner compartment starts growing as in the case of Fig. 2 but asymptotically reaches a maximum size. (B) Cells proliferate at the surface of the inner compartment, which is rich in nutrients, and die at the center where nutrients are scarce, resulting in an inward flow of cells. Color coding is similar to the one used in Figs. 23, but it now allows for negative values required by the inward flow.
Note that the typical length-scale at which the diffusion of nutrients becomes a limiting factor is of the order of millimeters, the size of a dormant tumor (Folkman and D’Amore, 1996; Weinberg, 2007). This scale is very large compared to the size we estimate for the critical radius introduced above. In the following, when considering the nucleation process of micro-tumors, we therefore assume a homogeneous, high enough concentration of nutrients.
Critical size and stochastic growth dynamics
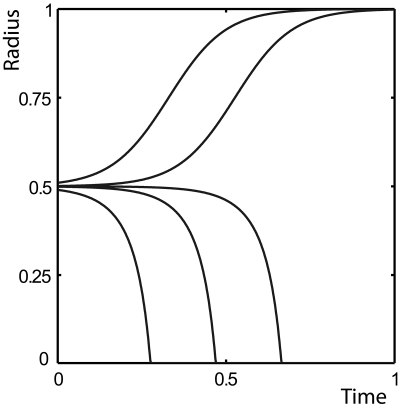
A third effect that can modify tumor growth dynamics is the presence of a constant tissue interfacial tension as introduced above. When H and T are at their homeostatic densities, there exists a particular radius rc at which mechanical equilibrium is reached, but this equilibrium, given by pT,h−pH,h=2γ∕rc, is unstable. Numerical solutions illustrating the growth dynamics around this critical radius are presented in Fig. 5.
Figure 5. Tissue boundary as a function of time during the growth of a tissue located in the bulk of another tissue of lower homeostatic pressure, with interfacial tension and in spherical geometry.
Parameters are given in the Supplementary Material, together with rc=0.5 and γ=1. The different curves show the dynamics for the following initial values r0 of r: 0.49, 0.499, 0.4999, 0.501, and 0.51. Both tissues start out at their homeostatic densities.
Given the existence of such an unstable critical radius, the question arises as to how a metastasis—or a primary tumor—can grow within a healthy tissue since in general it originates from a single cell (Talmadge and Fidler, 1982; Talmadge and Zbar, 1987; Chambers and Wilson, 1988). The answer stems from stochasticity, an aspect of the dynamics that has been ignored in the description so far. The importance of stochasticity in growth processes has already been recognized in various situations (Nowak et al., 2003). Under the assumption that stochastic tumor growth is a Poisson process, the evolution of the probability P(n,t) for a spherical tumor T inside a healthy tissue H to contain n cells at time t can be described by a master equation:
| (5) |
where and are the rates at which a tumor grows or shrinks from n to (n+1) or (n−1) cells, respectively. The rates kd and ka depend on n, and we model their dependence in the following way: For tumors small compared to the size of the healthy compartment, the healthy tissue is only slightly perturbed away from its homeostatic state. Thus, the pressure inside the tumor is given by Laplace’s law: pT=pH,h+2γ∕r. Therefore, the division and apoptosis rates of a spherical tumor of radius r are given by
| (6) |
Here, κd, κa, and k0 are three phenomenological coefficients that enter the linear expansions of kd and ka, similarly to κ in Eq. 4. To ensure the proper behavior as a function of the cell density ρ, κd needs to be positive and κa negative. Both equations for kd and ka share the same constant k0 such that Eq. 4 is satisfied with κ=κd−κa. In the master equation 5, the rates and are then given to leading order by
| (7) |
and k0>0 fixes the amount of cell turnover—and thereby the amount of stochasticity—in the system.
For an analytic treatment, we map this growth process onto a random walk with sinks at n=0 and n=nmax, which results in a linear birth-death process where all tumors either disappear or reach macroscopic sizes when time goes to infinity. The so-called “splitting probability” Πg—namely the probability for a single cell to reach the size n=nmax and not disappear in the lower sink n=0—is given by (Van Kampen, 2007)
| (8) |
In Fig. 6, we present this analytic result together with the results of a Monte Carlo simulation of Eqs. 5, 6, 7 based on a Gillespie algorithm (Gillespie, 1977).
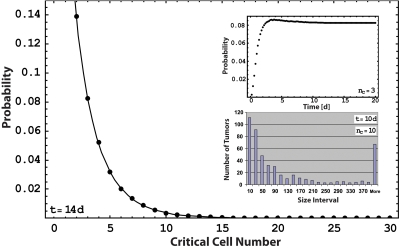
Figure 6. Splitting probability as a function of the critical cell number.
The black dots are the fraction of tumors with a size larger than the critical radius after 14 days of evolution, starting from a single cell in a Monte Carlo simulation [see Eqs. 5, 7]. The black line represents the analytic result for the splitting probability as given by Eq. 8. Parameters are chosen such that far above the critical radius, the division rate reaches a value of one division per day and the apoptosis rate is negligible. k0 in Eq. 7 is chosen to be 0.9 division per day. Note that the probability for growth on the compartment surface can be read out from the plot using half of the critical cell number of the process in the bulk. Upper inset: Fraction of tumors that are above the critical size. This fraction relaxes to a constant value in a Monte Carlo simulation. Lower inset: Distribution of tumor sizes in a Monte Carlo simulation after 10 days of evolution of 105 individual tumor cells.
Finally, it is interesting to consider the growth of neoplastic semi-spheroids on tissue boundaries. The internal pressure of a semi-spheroid is again given by Laplace’s law, but for the same radius, the number of cells within the tumor is half that of a complete spheroid. Therefore, the critical number of cells of a tumor at an interface is only half that of a tumor in the bulk. We can directly read the resulting effect on the growth probability out of Fig. 6. For example, between the critical sizes of five and ten cells, we obtain a growth probability ratio of 8.6. This characterizes a significant preference for tumors to grow on surfaces, an effect that has been observed experimentally (Cameron et al., 2000) and clinically (Weiss, 1985). Note that other mechanisms—such as the adhesion of cancerous cells to the extracellular matrix, which leads to a different initial distribution of metastatic cells—might also play a role.
DISCUSSION
In this work, we have shown that homeostatic regulation of cell density and pressure leads to a competition for space between tissues in mechanical contact. We have proposed that an increased homeostatic pressure is a characteristic trait of tumors. Gedanken experiments for measuring the homeostatic pressure and growth rates have been discussed and could be realized using current experimental techniques. For example, Helminger et al. have shown that tumors growing in agarose gels proliferate until the pressure exerted by the gel reaches 45–120 mmHg (Helminger et al., 1997). This gives an estimate of the homeostatic pressure defined in this paper. Such numbers are compatible with the pressure typically generated by actin polymerization (Footer et al., 2007; Marcy et al., 2004). An experiment of particular interest that has not been performed so far is the competition between a healthy and a tumorlike tissue separated by a piston, which could prove that mechanical effects are important for tumor growth. If the growth mechanism discussed in this paper is relevant to tumor growth, it would be interesting to measure the homeostatic pressures characterizing healthy and neoplastic tissues, together with their dependences on their biochemical environments. Of particular interest would be their dependence on oxygen, nutrients, growth factors, and drugs.
The second concept introduced in this paper is the existence of a critical size for tumor growth due to biochemical, immunological, or mechanical surface effects that can outbalance the bulk growth advantage of the neoplastic tissue for small tumor sizes. We show that this interaction can be responsible for the inefficiency of the metastatic cascade after extravasation. The growth of very few metastases to macroscopic sizes cannot be explained by size-independent growth rates, which yield a probability distribution of metastatic cell clusters that decays exponentially with cluster size. Instead, the existence of a critical size yields realistic values for metastatic inefficiency and a distribution of tumor sizes compatible with experimental observations (Luzzi et al., 1998; Cameron et al., 2000; Zijlstra et al., 2002) (see Fig. 6). Figure 6 also shows the dependence of metastatic inefficiency on the critical size: a small change in tissue-tumor interaction such as an increased interfacial tension can dramatically lower the probability for macroscopic growth. As an illustration of this effect, consider a metastatic tissue with a critical cell number of 5 in a given environment. Let us compare this situation with that of the same tissue placed in another environment where its interfacial tension is now twice as large, a situation that is well within natural variations (Foty, 1996). While for the first environment, about 3 in 100 metastatic cells form a macroscopic tumor, in the second environment, with a critical cell number of 40, less than 2 in 10 million manage to do so. This corresponds to a difference in metastatic efficiency of five orders of magnitude. We propose that this effect could account for the strong tissue specificity of metastatic growth that underlies the “seed and soil hypothesis” (Weinberg, 2007).
The concept of homeostatic pressure presented here is not an alternative to the cellular and genetic mechanisms involved in tumor growth, but rather a different level of description. Indeed, we propose that some of the fundamental biological deregulations that are characteristic of neoplastic cells lead to an increased homeostatic pressure. The framework presented here can be used to explicitly take into account such well-known properties. It can also be generalized to incorporate more general features of biological tissue behavior. For example, on long time scales, genetic instability as well as senescence render tissue properties time dependent. This could be incorporated into our framework using techniques similar to those of Hallatschek et al. (Hallatschek et al., 2007; Hallatschek and Nelson, 2008), as well as those of multiscale models of tumor growth (Ribba et al., 2006; Macklin and Lowengrub, 2007; Wise et al., 2008).
SUPPORTING INFORMATION
Supplementary Material
Supplementary, Figure S1
Supplementary, Figure S2
Supplementary, Figure S3
Supplementary, Figure S4
Supplementary, Figure S5
ACKNOWLEDGMENTS
We thank M. Bornens, M. Piel, and P. Silberzan for many helpful discussions, and P. Janmey for useful comments.
References
- Cameron, M D, Schmidt, E E, Kerkvliet, N, Nadkarni, K V, Morris, V L, Groom, A C, Chambers, A F, and MacDonald, I C (2000). “Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency: 1.” Cancer Res. 60, 2541–2546. [PubMed] [Google Scholar]
- Chambers, A F, Groom, A C, and MacDonald, I C (2002). “Dissemination and growth of cancer cells in metastatic sites.” Nat. Rev. Cancer 10.1038/nrc865 2, 563–572. [DOI] [PubMed] [Google Scholar]
- Chambers, A F, and Wilson, S (1988). “Use of Neo R B16F1 murine melanoma cells to assess clonality of experimental metastases in the immune-deficient chick embryo.” Clin. Exp. Metastasis 6, 171–182. [DOI] [PubMed] [Google Scholar]
- Chaplain, M A. J, Graziano, L, and Preziosi, L (2006). “Mathematical modelling of the loss of tissue compression responsiveness and its role in solid tumour development.” Math. Med. Biol. 23, 197–229. [DOI] [PubMed] [Google Scholar]
- Couzin, J (2003). “Tracing the steps of metastasis, cancer’s menacing ballet.” Science 299, 1002–1006. [DOI] [PubMed] [Google Scholar]
- Duguay, D, Foty, R A, and Steinberg, M S (2003). “Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants.” Dev. Biol. 10.1016/S0012-1606(02)00016-7 253, 309–323. [DOI] [PubMed] [Google Scholar]
- Engler, A J, Richert, L, Wong, J Y, Picart, C, and Discher, D E (2004) “Surface probe measurements of the elasticity of sectioned tissue, thin gels and polyelectrolyte multilayer films: Correlations between substrate stiffness and cell adhesion.” Surf. Sci. 10.1016/j.susc.2004.06.179 570, 142–154. [DOI] [Google Scholar]
- See EPAPS Document No. E-HJFOA5-3-001905 for supplemental material. This document can be reached through a direct link in the online’s HTML reference section or via the EPAPS homepage (http://www.aip.org/pubservs/epaps.html).
- Fidler, I J (2003). “The pathogenesis of cancer metastasis: the seed and soil hypothesis revisited.” Nat. Rev. Cancer 3, 453–458. [DOI] [PubMed] [Google Scholar]
- Folkman, J, and D’Amore, P A (1996). “Blood vessel formation: what is its molecular basis.” Blood 87, 1153–1155. [DOI] [PubMed] [Google Scholar]
- Footer, M J, Kerssemakers, J WJ, Theriot, J A, and Dogterom, M (2007) “Direct measurement of force generation by actin filament polymerization using an optical trap.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0607052104 104, 2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgacs, G, Foty, R A, Shafrir, Y, and Steinberg, M S (1998). “Viscoelastic properties of living embryonic tissues: a quantitative study.” Biophys. J. 74, 2227–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foty, R A (1996). “Surface tensions of embryonic tissues predict their mutual envelopment behavior.” Development 122, 1611–1620. [DOI] [PubMed] [Google Scholar]
- Foty, R A, Forgacs, G, Pfleger, C M, and Steinberg, M S (1994). “Liquid properties of embryonic tissues: Measurement of interfacial tensions.” Phys. Rev. Lett. 10.1103/PhysRevLett.72.2298 72, 2298–2301. [DOI] [PubMed] [Google Scholar]
- Frisch, U, Hasslacher, B, and Pomeau, Y (1986). “Lattice-gas automata for the Navier-Stokes equation.” Phys. Rev. Lett. 10.1103/PhysRevLett.56.1505 56, 1505–1508. [DOI] [PubMed] [Google Scholar]
- Gillespie, D (1977). “Exact stochastic simulation of coupled chemical reactions.” J. Phys. Chem. 10.1021/j100540a008 81, 2340–2361. [DOI] [Google Scholar]
- Hallatschek, O, Hersen, P, Ramanathan, S, and Nelson, D R (2007). “Genetic drift at expanding frontiers promotes gene segregation.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0710150104 104, 19926–19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallatschek, O, and Nelson, D R (2008). “Gene surfing in expanding populations.” Theor. Popul. Biol. 73, 158–170. [DOI] [PubMed] [Google Scholar]
- Hanahan, D, and Weinberg, R A (2000). “The hallmarks of cancer.” Cell 10.1016/S0092-8674(00)81683-9 100, 57–70. [DOI] [PubMed] [Google Scholar]
- Helmlinger, G, Netti, P A, Lichtenbeld, H C, Melder, R J, and Jain, R K (1997). “Solid stress inhibits the growth of multicellular tumor spheroids.” Nat. Biotechnol. 10.1038/nbt0897-778 15, 778–783. [DOI] [PubMed] [Google Scholar]
- Kong, H J, Polte, T R, Alsberg, E, and Mooney, D J (2005). “FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness.” Proc. Natl. Acad. Sci. U.S.A. 102, 4300–4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit, T, and Lenne, P F (2007). “Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis.” Nat. Rev. Mol. Cell Biol. 8, 633–644. [DOI] [PubMed] [Google Scholar]
- Luzzi, K J, MacDonald, I C, Schmidt, E E, Kerkvliet, N, Morris, V L, Chambers, A F, and Groom, A C (1998). “Multistep nature of metastatic inefficiency.” Am. J. Pathol. 153, 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklin, P, and Lowengrub, J (2007). “Nonlinear simulation of the effect of the microenvironment on tumor growth.” J. Theor. Biol. 245(4), 677–704. [DOI] [PubMed] [Google Scholar]
- Marcy, Y, Prost, J, Carlier, M F, and Sykes, C (2004). “Forces generated during actin-based propulsion: a direct measurement by micromanipulation.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0307704101 101, 5992–5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molski, M, and Konarski, J (2003). “Coherent states of Gompertzian growth.” Phys. Rev. E 10.1103/PhysRevE.68.021916 68, 021916. [DOI] [PubMed] [Google Scholar]
- Nowak, M, Michor, F, and Iwasa, Y (2003). “The linear process of somatic evolution.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.2535419100 100, 14966–14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preziosi, L (2003). Cancer modelling and simulation, Chapman & Hall/CRC, Boca Raton, FL. [Google Scholar]
- Ribba, B, Saut, O, Colin, T, Bresch, D, Grenier, E, and Boissel, J P (2006). “A multiscale mathematical model of avascular tumor growth to investigate the therapeutic benefit of anti-invasive agents.” J. Theor. Biol. 243, 532–541. [DOI] [PubMed] [Google Scholar]
- Sachs, R, Hlatky, L, and Hahnfeldt, P (2001). “Simple ODE models of tumor growth and anti-angiogenic or radiation treatment.” Math. Comput. Modell. 10.1016/S0895-7177(00)00316-2 33, 1297–1305. [DOI] [Google Scholar]
- Sahai, E (2007). “Illuminating the metastatic process.” Nat. Rev. Cancer 1910, 737–749. [DOI] [PubMed] [Google Scholar]
- Schötz, E M, Burdine, R D, Jülicher, F, Steinberg, M S, Heisenberg, C P, and Foty, R A (2008). “Quantitative differences in tissue surface tension influence zebrafish germ layer positioning.” HFSP J. 10.2976/1.2834817 2, 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shraiman, B I (2005). “Mechanical feedback as a possible regulator of tissue growth.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0404782102 102, 3318–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge, J E, and Fidler, I J (1982). “Evidence for the clonal origin of spontaneous metastases.” Science 217, 361–363. [DOI] [PubMed] [Google Scholar]
- Talmadge, J E, and Zbar, B (1987). “Clonality of pulmonaly metastases from the bladder 6 subline of the B 16 melanoma studied by southern hybridization.” J. Natl. Cancer Inst. 78, 315–320. [PubMed] [Google Scholar]
- Van Kampen, N G (2007). Stochastic Processes in Physics and Chemistry, North-Holland, Amsterdam. [Google Scholar]
- Weidner, N, Semple, J P, Welch, W R, and Folkman, J (1991). “Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma.” N. Engl. J. Med. , 324, 1–8. [DOI] [PubMed] [Google Scholar]
- Weinberg, R A (2007). The biology of cancer, Garland Science, New York. [Google Scholar]
- Weiss, L (1985). Principle of metastasis, Academic Press, Orlando, FL. [Google Scholar]
- Wise, S M, Lowengrub, J S, Frieboes, H B, and Cristini, V (2008). “Three-dimensional multispecies nonlinear tumor growth-I. Model and numerical method.” J. Theor. Biol. 253, 524–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra, A, Mellor, R, Panzarella, G, Aimes, R T, Hooper, J D, Marchenko, N D, and Quigley, J P (2002). “A quantitative analysis of rate-limiting steps in the metastatic cascade using human-specific real-time polymerase chain reaction: 1.” Cancer Res. 62, 7083–7092. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary, Figure S1
Supplementary, Figure S2
Supplementary, Figure S3
Supplementary, Figure S4
Supplementary, Figure S5