Abstract
Objective
To determine whether administration of a vasoactive peptide, human adrenomedullin (AM), in combination with its binding protein (i.e., AMBP-1), prevents or minimizes hepatic ischemia-reperfusion (I/R) injury.
Summary Background Data
Hepatic I/R injury results from tissue hypoxia and subsequent inflammatory responses. Even though numerous pharmacological modalities and substances have been studied to reduce I/R-induced mortality, none have been entirely successful. We have shown that administration of AM/AMBP-1 produces significant beneficial effects under various pathophysiological conditions. However, it remains unknown if human AM/AMBP-1 has any protective effects on hepatic I/R-induced tissue damage and mortality.
Methods
70% hepatic ischemia was induced in male adult rats by placing a microvascular clip across the hilum of the left and median lobes for 90 min. After removing the clip, human AM alone, human AMBP-1 alone, human AM in combination with human AMBP-1 or vehicle was administered intravenously over a period of 30 min. Blood and tissue samples were collected 4 h after reperfusion for various measurements. In additional groups of animals, the non-ischemic liver lobes were resected at the end of 90-min ischemia. The animals were monitored for 7 days and survival was recorded.
Results
After hepatic I/R, plasma levels of AM were significantly increased while AMBP-1 levels were markedly decreased. Likewise, gene expression of AM in the liver was increased significantly while AMBP-1 expression was markedly decreased. Administration of AM in combination with AMBP-1 immediately after the onset of reperfusion downregulated inflammatory cytokines, decreased hepatic neutrophil infiltration, inhibited liver cell apoptosis and necrosis, and reduced liver injury and mortality in a rat model of hepatic I/R. On the other hand, administration of human AM alone or human AMBP-1 alone after hepatic I/R failed to produce significant protection.
Conclusions
Human AM/AMBP-1 may be a novel treatment to attenuate tissue injury after an episode of hepatic ischemia.
Keywords: Ischemia-reperfusion, liver, vasoactive peptide, inflammatory cytokines, tissue injury, survival
INTRODUCTION
Liver injury induced by hepatic ischemia and reperfusion (I/R) is a major cause of hepatic failure after severe trauma, thermal injury, hemorrhagic and septic shock, liver resection, or liver transplantation. The current strategies for hepatic I/R treatment are either preventional (e.g., ischemic preconditioning or remote preconditioning) or pharmacological. Ischemic preconditioning is the application of short periods of ischemia and reperfusion to an organ prior to prolonged ischemia whereas in remote preconditioning cycles of brief ischemia are applied to a remote organ (e.g., the lower limb) prior to prolonged ischemia of the target organ (e.g., the liver, heart). Studies with both techniques have shown some reduction in hepatic I/R injury 1–3, however, their application is extremely limited and sometimes impractical. Pharmacological modulation may have more universal application. Unfortunately, even though numerous pharmacological modalities and substances have been studied to reduce I/R-induced mortality, none have been entirely successful.4,5 As such, the development of novel and effective strategies for preventing and treating local and remote organ injury after hepatic I/R is critical for the improvement of patient outcome under such conditions.
Adrenomedullin (AM), a 52-amino acid vasoactive peptide, was first isolated from human pheochromocytomas more than a decade ago.6 Subsequent studies have shown that AM is expressed in a large number of tissues and exhibits various properties including the cardiovascular and fluid regulation, regulation of growth and differentiation, and secretions of other hormones.7 It is implicated in a variety of disease states such as cardiovascular and renal disorders, sepsis, cancer and diabetes. AM acts as a circulating hormone which elicits various biological activities in a paracrine or autocrine manner. Recently, a novel specific AM binding protein (i.e., AMBP-1) in human plasma was identified and the purified protein was reported to be identical to human complement factor H.8,9 The presence of AMBP-1 in tissues may affect the autocrine/paracrine actions of AM. Our recent studies have shown that AM in combination with AMBP-1 produces significant beneficial effects under various pathophysiological conditions.10–13 However, it remains unknown whether AM/AMBP-1 has any protective effects on hepatic I/R-induced tissue damage and mortality. We therefore hypothesize that administration of human AM/AMBP-1 improves hepatic function, attenuates organ injury and inflammation, and reduces mortality following hepatic I/R injury. The current study was conducted to test this hypothesis.
MATERIALS AND METHODS
Experimental animals
Male Sprague-Dawley rats (275–325g, Charles River Laboratories, Wilmington, MA) were housed in a temperature-controlled room on a 12-h light/dark cycle and fed on a standard Purina rat chow diet. Prior to the experiment, rats were fasted overnight but allowed water ad libitum. The experiments were performed in accordance with the National Institutes of Health guidelines for the use of experimental animals. This project was approved by the Institutional Animal Care and Use Committee of The Feinstein Institute for Medical Research.
Animal model of hepatic ischemia and reperfusion (I/R)
A rat model of segmental hepatic ischemia was used. Briefly, the animals were anesthetized with isoflurane inhalation with an anesthesia machine, following which the ventral abdomen and groin were shaved, followed by 10% povidone-iodine wash. A 3-cm midline abdominal incision was performed. The ligamentous attachments connecting the liver, diaphragm, and abdominal wall were divided. After the liver was located, the hilus was exposed to find the hepatic artery and the portal vein. A vascular micro clip was placed across the hilum of the left and median lobes to produce a 70% ischemia for 90 min. The microvascular clip was then removed to allow reperfusion. The rats were immediately resuscitated with 1 ml normal saline over a period of 30 min (i.e., the rats received low volume crystalloid resuscitation). Sham animals underwent the same surgical procedure with the exception of the hilum clamping or the administration of treatment. Liver and blood samples were collected at 4 h after reperfusion for the measurement of AM and AMBP-1, as described below.
RNA extraction and determination of AM and AMBP-1 genes
Total RNA was extracted from the liver by Tri-Reagent (Molecular Research Center, Cincinnati, OH). 150 mg tissue was homogenized in 1.5 ml Tri-Reagent and the homogenate was separated into aqueous and organic phases by chloroform addition and centrifugation. RNA was precipitated from the aqueous phase by addition of isopropanol, and washed with ethanol. The pellet was dissolved in 0.1% DEPC-treated, deionized distilled water. RNA concentration and purity were determined by measuring the absorbance at 260 and 280 nm. 5 µg of RNA from each tissue was reverse-transcribed in a 20 µl reaction volume containing 50 mmol/L KCl, 10 mmol/L Tris-HCl, 5 mmol/L MgCl2, 1 mmol/L dNTP, 20 U RNase inhibitor, 2.5 mmol/L oligod (T) 16 primer, and 50 U reverse transcription. The reverse transcription reaction solution was incubated at 42°C for 1 hour, followed by heating at 95°C for 5 minutes; 1 µl cDNA was amplified with 0.15 µmol/L each of 3' and 5' primers, specific for the rat AM and AMBP-1. Rat glyceraldehyde 3-phosphate dehydrogenase (G3PDH) was used as the housekeeping gene. The primers are as follows: 5'-TGG GTT CGC TCG CCG TTC TCG-3' (forward) and 5'-CGT CCT TGT CTT TGT CTG TAA-3' (reverse) for AM (BC061775), 5'-CAC TTC CTT TTG CCT TGC TT-3' (forward) and 5'-TCA ATT ATC CCA CCT GCT CA-3' (reverse) for AMBP-1 (AA819055), 5'-TGA AGG TCG GTG TCA ACG GAT TTG GC-3' (forward) and 5'-CAT GTA GGC CAT GAG GTC CAC CAC-3' (reverse) for G3PDH (M17701). The PCR was conducted at 20 cycles for AMBP-1, 25 cycles for AM and 30 cycles for G3PDH. Each cycle consisted of 30 seconds at 94°C, 30 seconds at 60°C, and 45 seconds at 72°C. After the RT-PCR procedure, the PCR amplification products were electrophoresed by using a 1.6% agarose gel containing 0.22 µg/ml ethidium bromide. The gel was then developed and band intensities were normalized by G3PDH using the Bio-Rad image system (Hercules, CA).
Measurement of plasma AM levels
Plasma levels of AM were assayed according to the procedure provided by the manufacturer, using a radioimmunoassay kit specific for rat AM from Peninsula Labs (Belmont, CA). Briefly, 1.5 ml blood samples were collected into a polypropylene tube containing EDTA (1 mg/ml) and aprotinin (500 KIU/ml) at 4 h after reperfusion, and plasma was separated immediately. The collected plasma was used for AM extraction by C18 Sep-Column. Each RIA incubation mixture was composed of 100 µl standard AM or an unknown sample, and 200 µl anti-serum diluted with RIA buffer containing 0.5% normal rabbit serum. After 16 h incubation, 100 µl 125I-labeled tracers (15,000 cpm) were added. After additional 24 h incubation, 100 µl anti-rabbit IgG goat serums were added. Free and bound tracers were separated after 90 min incubation by centrifugation at 3,000 rpm for 30 min. After aspiration of the supernatant, radioactivity in the pellet was counted with a γ-counter. AM levels were assessed according to the manufacturer’s instructions.
Measurement of plasma AMBP-1 levels
One microliter of plasma was fractionated on a 4–12% Bis-Tris gel and then transferred to a 0.2-nm nitrocellulose membrane. Nitrocellulose blots were blocked by incubation in TBST (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween 20) containing 5% milk for 1 hr. Blots were incubated with rabbit antihuman complement factor H polyclonal antibodies (1:2,000, Accurate Chemical, Westbury, NY) overnight at 4°C. The blots were then washed in TBST five times for 10 min, incubated with horseradish peroxidase-linked anti-rabbit immunoglobulin G for 1 hr at room temperature, and then washed five times in TBST for 10 mins. A chemiluminescent peroxidase substrate (ECL, Amersham Biosciences, Piscataway, NJ) was applied according to the manufacturer’s instructions, and the membranes were exposed briefly to radiography film. The band densities were determined using a Bio-Rad image system (Hercules, CA). Our preliminary results indicate that the antihuman complement factor H antibodies recognize rat AMBP-1. We used these antibodies because anti-rat AMBP-1 antibodies are not currently commercially available. The levels of AMBP-1 (band densities) were determined with the use of a Bio-Rad Laboratories image system.
Administration of AM and AMBP-1
At the beginning of reperfusion after 90-min hepatic ischemia, human AM (12 µg/kg BW, Phoenix Pharmaceuticals, Belmont, CA) alone, human AMBP-1 (40 µg/kg BW, Cortex Biochem, San Leandro, CA) alone, human AM (12 µg/kg BW) in combination with human AMBP-1 (40 µg/kg BW), or vehicle (1 ml normal saline) were administered over a period of 30 min via a femoral vein catheter in additional animals. The purity of synthetic human AM is more than 99% (by HPLC). Human AMBP-1 is isolated from human serum/plasma with a purity of more than 98% (by SDS-PAGE). The human AMBP-1 preparation has been tested at the serum/plasma donor level by FDA-approved methods and found negative for HIV I/II, and HCV antibodies and hepatitis B surface antigens. Blood and liver samples were collected at 3.5 h after the completion of treatment (i.e., 4 h from the beginning of reperfusion).
Determination of liver enzymes
Blood samples were centrifuged at 2000 g for 10 min to collect serum and stored at −80°C for determination Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) were measured by using commercial assay kits according to the manufacturer’s instructions (Pointe Scientific, Lincoln Park, MI).
Myeloperoxidase (MPO) immunohistochemistry
Liver biopsies were taken from the left liver lobe after 4 h reperfusion. The tissue was fixed overnight in 10% buffered formalin phosphate. The tissue was then cut into 4 µm sections using a vibrotome. The paraffin sections were dewaxed and rehydrated, followed by microwave antigen retrieval procedure. Slides were soaked in 20% citric acid buffer, pH 6.0 (Vector Labs, Burlingame, CA) and heated in the microwave oven and maintain the temperature at 95 °C for 15 min. The slides were cooled in room temperature for 5 min and then rinsed with Tris buffer saline (TBS, PH 7.5). Endogenous peroxidase was blocked by 2% H2O2 in 60% methanol for 20 min. Normal goat serum (2%) was used to block the nonspecific binding sites. The sections were then incubated in 1:2000 rabbit anti-MPO polyclonal antibodies (Abcam, Cambridge, MA) for 1.5 h at room temperature. After washing with TBS, the sections were reacted in 1:200 biotinylated anti-rabbit IgG (Vector Labs, Burlingame, CA) for 0.5 h. Vectastain ABC reagent and DAB kit (Vector, Burlingame, CA) were used to reveal the immunohistochemical reaction. For the negative control, the primary antibody was substituted by normal rabbit IgG.
Granulocyte myeloperoxidase assessment
Neutrophil accumulation within the liver was estimated using the MPO activity assay as we described previously.14 Briefly, liver tissues (100 mg wet weight) were homogenized in 1 mL buffer (0.5% hexadecyltrimethylammonium bromide in 50 mmol/L phosphate buffer, pH 6.0) and sonicated at 30 cycles, twice, for 30 seconds on ice. Homogenates were cleared by centrifuging at 12,000 rpm at 4°C, and the supernatants were stored at −80°C. Protein content in the samples was determined (BioRad, Hecules, CA). The samples were incubated with a substrate o-dianisidine hydrochloride. This reaction was carried out in a 96-well plate by adding 290 µl 50 mmol/L phosphate buffer, 3 µl substrate solution (containing 20 mg/mL o-dianisidine hydrochloride), and 3 µl H2O2 (20 mmol/L). Sample (10 µl) was added to each well to start the reaction. Light absorbance at 460 nm was read over a period of 5 min. MPO activity (1 unit defined as change in absorbance of 1 per minute) was expressed as units per gram of tissue.
Western blotting analysis of cleaved caspase-3 protein levels in the liver
Liver cell apoptosis was determined by measuring the expression of cleaved caspase 3 protein. Briefly, liver samples (0.1 g) were lysed and homogenized in 1 ml of lysis buffer (10 mM Tris-buffered saline, 1 mM EDTA, 1 mM EGTA, 2 mM sodium orthovanadate, 0.2 mM PMSF, 2 µg/ml leupeptin, 2 µg/ml aprotinin, and 1% Triton X-100) for 30 min on ice and cleared by centrifugation at 14,000 rpm for 15 min at 4°C. Twenty-five micrograms of protein were fractionated on 4–12% Bis-Tris gel and transferred to 0.2-µm nitrocellulose membrane. Nitrocellulose blots were blocked by incubation in TBST (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% Tween 20) containing 5% milk for 1 h. Blots were incubated with cleaved caspase-3 antibodies (1:1,000) (Asp175, Cell Signaling Technology) overnight at 4°C. The blots were then washed in TBST three times for 15 min, incubated with horseradish peroxidase-linked anti-rabbit immunoglobulin G for 1 h at room temperature, and then washed five times in TBST for 10 min. A chemiluminescent peroxidase substrate (ECL, Amersham Biosciences, Piscataway, NJ) was applied according to the manufacturer’s instructions, and the membranes were exposed briefly to radiography film. The band densities were normalized by β-actin with the use of the Bio-Rad Image System.
Histologic evaluation of liver injury
Liver biopsies were taken from the left liver lobe after 4 h reperfusion. The specimens fixed in 10% formalin were embedded in paraffin. Sections of 4 µm were cut and stained with hematoxylin and eosin. Semiquantitative light microscopic evaluation was performed on all sections in a blinded fashion to assess liver parenchymal injury. The assessment was expressed as the sum of the individual score grades from 0 (no findings), 1 (mild), 2 (moderate), to 3 (severe) for each of the following 6 parameters from liver sections: cytoplasmatic color fading, vacuolization, nuclear condensation, nuclear fragmentation, nuclear fading, and erythrocyte stasis.15
Determination of serum levels of TNF-α and IL-6
The serum levels of TNF-α and IL-6 were determined by using commercially obtained enzyme-linked immunosorbent assay (ELISA) kits specific for rat-TNF-α and IL-6 (BioSource International, Camarillo, CA). The assay was carried out according to the instructions provided by the manufacturer.
Survival Study
Recent publications have shown that 70% hepatic ischemia for 90 min followed by reperfusion is not severe enough to produce ~50% mortality16,17. Therefore, in order to determine whether administration of AM plus AMBP-1 improves survival after hepatic I/R, the remaining 30% non-ischemic liver lobes were resected at the end of 90-min ischemia before the onset of reperfusion. Human AM/AMBP-1 (12/40 µg/kg BW) or vehicle (1 ml normal saline) was then administered intravenously over 30 min as described above. The animals were monitored for 7 days to record survival.
Statistical analysis
All data are expressed as mean ± SEM and compared by one-way analysis of variance (ANOVA) and the Student-Newman-Keuls test. The survival rate was estimated by Kaplan-Meier method and compared by the log-rank test. Differences in values were considered significant if P<0.05.
RESULTS
Alterations in AM and AMBP-1 expression after hepatic I/R
At 4 h after hepatic I/R, AM gene expression in the liver increased by 77% (P<0.05, Fig. 1A). Similarly, a moderate but significant increase in circulating levels of AM was found at 4 h after hepatic I/R as shown in Figure 1B. In contrast, the gene expression of AMBP-1 in the liver decreased by 37% at 4 h after reperfusion (Fig. 1C). Likewise, plasma levels of AMBP-1 also decreased by 33% at 4 h after reperfusion (Fig. 1D). The results suggest that a decrease in the production of AMBP-1 occurs after hepatic I/R.
Figure 1.
Alterations in AM (A) and AMBP-1 (C) mRNA expressions in the liver and plasma levels of AM (B) and AMBP-1 (D) at the end of 4 h reperfusion after 90 min hepatic ischemia/reperfusion (I/R) or sham operation (Sham). Representative blots are also presented. Data are presented as means ± SE (n=8/group) and compared by compared by Student t-test: * P<0.05 versus Sham group.
Effects of human AM/AMBP-1 treatment on liver injury after hepatic I/R
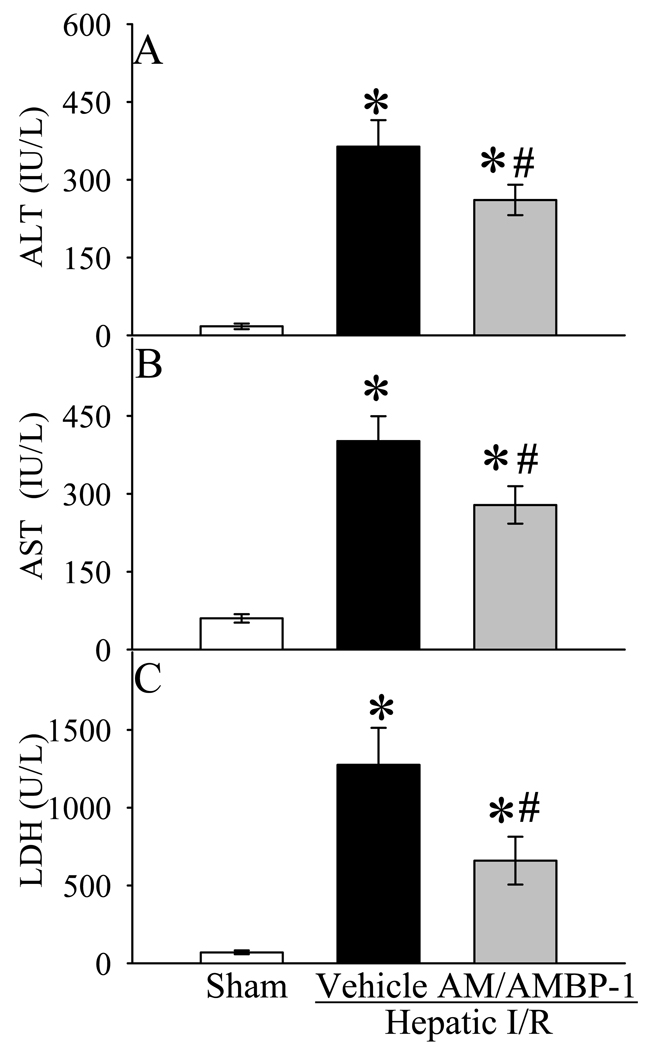
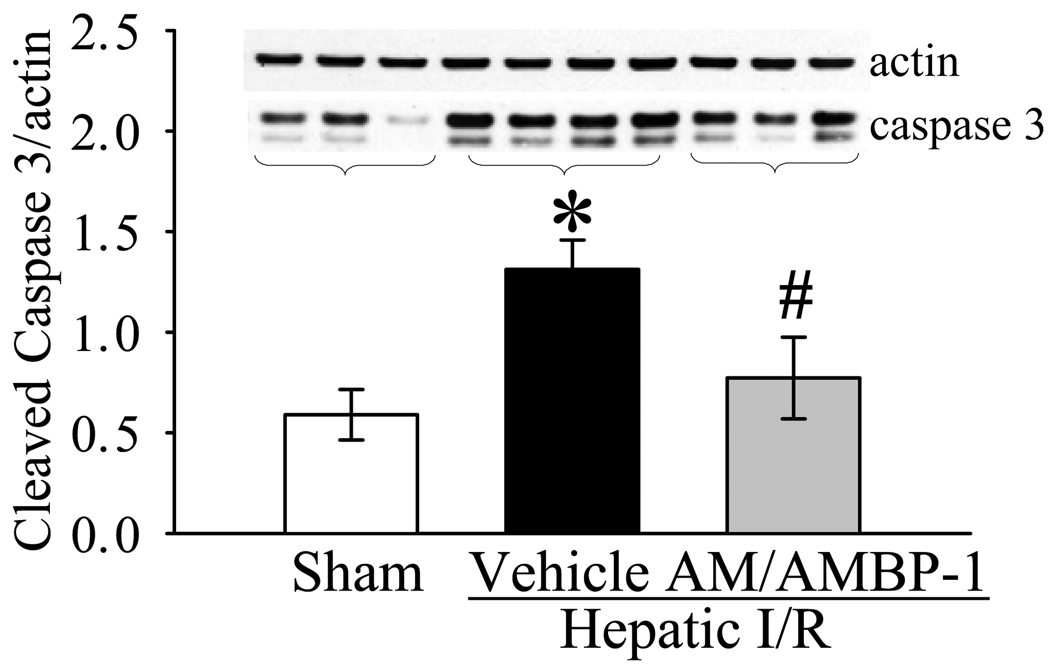
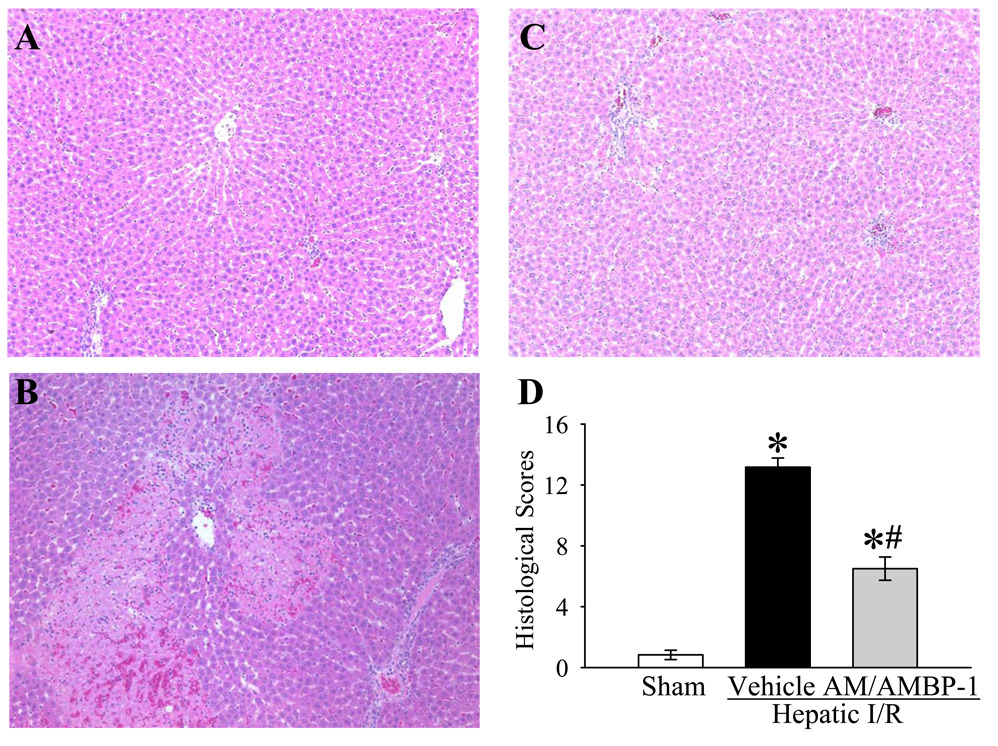
As indicators of hepatic injury, serum levels of liver enzymes (i.e., ALT, AST and LDH) increased by 20, 5.7 and 17 fold, respectively, after hepatic I/R (Fig. 2A–C). Administration of AM/AMBP-1 significantly attenuated serum levels of ALT, AST and LDH by 31%, 28% and 48%, respectively (P<0.05, Figs. 2A–C). To determine the alterations in neutrophil infiltration, immunohistochemical staining of liver sections for MPO was performed. As shown in Figure 3A, minimal immunostaining of MPO was detected in the liver of sham-operated animals. MPO immunostainings increased markedly at 4 h after reperfusion in vehicle-treated rats (Fig. 3B). administration of human AM/AMBP-1 after hepatic ischemia dramatically reduced the MPO immunostaining (Fig. 3C). As a control for specificity of detection, substitution of the primary antibody with a nonimmunized rabbit serum resulted in negative staining (data not shown). Consistent with the immunohistochemical data, MPO activity in the liver increased by 7.5 fold after hepatic I/R. Administration of AM/AMBP-1 after hepatic ischemia markedly reduced the liver MPO activity (P<0.05, Fig. 3D). As a measure of liver cell apoptosis, the expression of cleaved caspase 3 protein in the liver was more than doubled at 4 h after hepatic I/R (P<0.05, Fig. 4). AM/AMBP-1 treatment decreased liver cleaved caspase 3 levels by 41% (P<0.05, Fig. 4), which are similar to those in sham animals. Figures 5A–C show liver histological alterations. At 4 h after reperfusion, vehicle treated livers developed significant structural abnormalities, such as sinusoidal derangement and congestion. Extensive hepatic coagulation necrosis, localized mostly in the centrilobular zones, and neutrophil infiltration were also detected in the liver of hepatic I/R vehicle treated animals (Fig. 5B). In contrast, these changes were remarkably attenuated in all AM/AMBP-1 treated animals (Fig. 5C). As indicated in Figure 5D, the liver injury score was significantly increased at 4 h after hepatic I/R in vehicle treated animals as compared with that in sham operated animals (P<0.05). Administration of human AM/AMBP-1 markedly reduced the liver injury score by 51% (P<0.05). Taken together, these data suggest that administration of AM/AMBP-1 at the beginning of reperfusion confers significant protection against liver injury after hepatic I/R.
Figure 2.
Alterations in serum levels of ALT (A), AST (B) and LDH (C) in sham-operated animals (Sham) and hepatic ischemia/reperfusion (I/R) animals treated with normal saline (Vehicle) or AM/AMBP-1 at 4 h after reperfusion. Data are presented as means ± SE (n=8/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test: * P<0.05 versus Sham group, # P<0.05 versus Vehicle group.
Figure 3.
Alterations in MPO immunohistochemical stainings of the liver. A, Photomicrograph of a hepatic section from a sham-operated rat; B, Photomicrograph of a hepatic section from an I/R rat at 4 h after hepatic ischemia/reperfusion treated with vehicle; C, Photomicrograph of a hepatic section from an I/R rat at 4 h after hepatic ischemia/reperfusion treated with AM/AMBP-1. Dark brown staining represents the positive cell. Original magnification × 200. Alterations in hepatic MPO activity (D) in sham-operated animals (Sham) and hepatic I/R animals treated with normal saline (Vehicle) or AM/AMBP-1 at 4 h after reperfusion. Data are presented as means ± SE (n=8/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test: * P<0.05 versus Sham group, # P<0.05 versus Vehicle group.
Figure 4.
Alterations in hepatic cleaved caspase-3 protein levels in sham-operated animals (Sham) and hepatic ischemia/reperfusion (I/R) animals treated with normal saline (Vehicle) or AM/AMBP-1 at 4 h after reperfusion. A representative blot is also presented. Data are presented as means ± SE (n=8/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test: * P<0.05 versus Sham group, # P<0.05 versus Vehicle group.
Figure 5.
Alterations in morphology of the liver. A, Photomicrograph of a hepatic section from a sham-operated rat; B, Photomicrograph of a hepatic section from an I/R rat at 4 h after hepatic ischemia/reperfusion treated with vehicle; C, Photomicrograph of a hepatic section from an I/R rat at 4 h after hepatic ischemia/reperfusion treated with AM/AMBP-1; D, histopathologic scoring of liver injury in sham-operated animals (Sham) and hepatic ischemia/reperfusion (I/R) animals treated with normal saline (Vehicle) or AM/AMBP-1 at 4 h after reperfusion. Data are presented as means ± SE (n=6) and compared by one-way ANOVA and Student-Newman-Keuls test: * P<0.05 versus Sham group; # P<0.05 versus Vehicle group. Original magnification × 200.
Effects of human AM/AMBP-1 treatment on serum levels of TNF-α and IL-6 after hepatic I/R
At 4 h after reperfusion, serum levels of TNF-α increased by 3.5 fold in vehicle group as compared with those in sham group (P<0.05, Fig. 6A). Administration of AM/AMBP-1 significantly decreased serum TNF-α levels by 36% (Fig. 6A). The changes in serum levels of IL-6 after hepatic I/R were similar to those of TNF-α. Serum IL-6 levels were significantly increased by 2.8 fold after hepatic I/R (Fig. 6B). Administration of AM/AMBP-1 reduced circulating IL-6 by 43% (P<0.05, Fig. 6B).
Figure 6.
Alterations in serum levels of TNF-α (A) and IL-6 (B) in sham-operated animals (Sham) and hepatic ischemia/reperfusion (I/R) animals treated with normal saline (Vehicle) or AM/AMBP-1 at 4 h after reperfusion. Data are presented as means ± SE (n=8/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test: * P<0.05 versus Sham group, # P<0.05 versus Vehicle group.
Effects of human AM/AMBP-1 treatment on survival after hepatic I/R
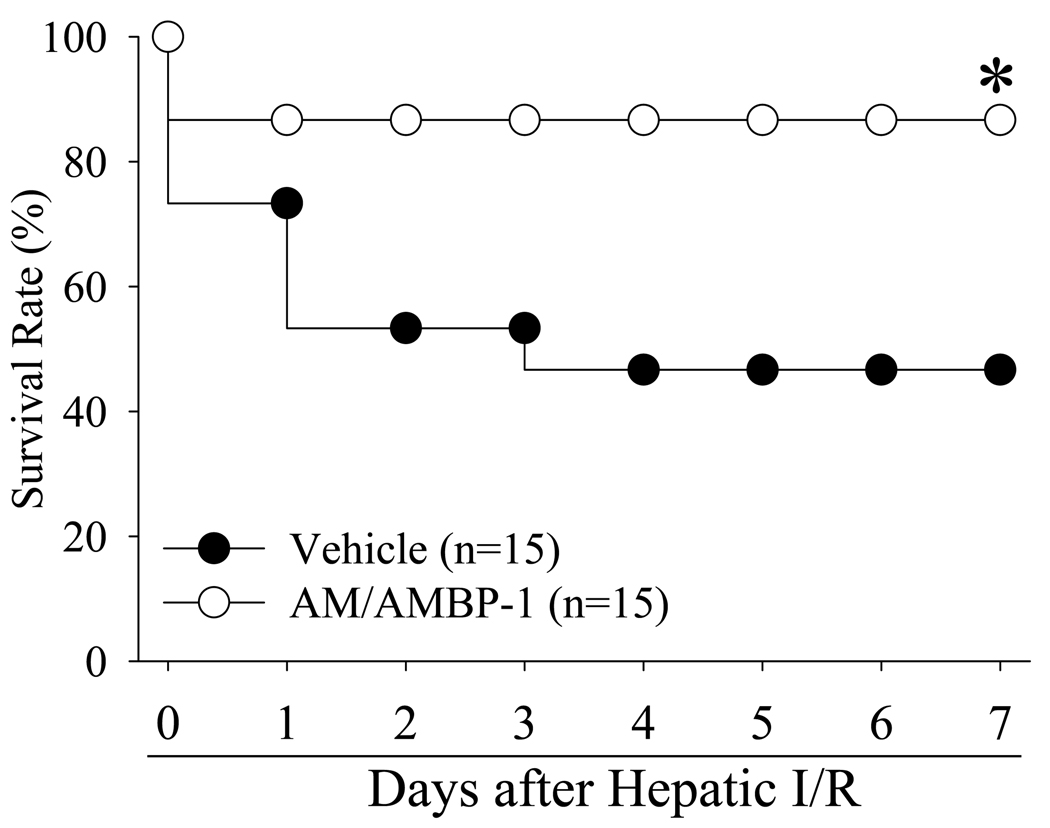
The survival rate after hepatic I/R with vehicle administration was 73% at day 1 and decreased to 47% at days 4 to 7 (Fig. 7). Treatment with AM/AMBP-1, however, improved the survival rate to 87% at days 1 to 7, which was higher than the hepatic I/R vehicle group (P<0.05, Fig. 7).
Figure 7.
Alterations in the survival rate at 7 days after hepatic ischemia/reperfusion (I/R) with normal saline treatment (Vehicle) and gut ischemia/reperfusion with AM/AMBP-1 treatment (AM/AMBP-1). There were 15 animals in each group. The survival rate was estimated by the Kaplan-Meier method and compared by using the log-rank test. * P< 0.05 vs. Vehicle group.
Effects of human AM alone or human AMBP-1 alone on serum levels of ALT, AST and LDH after hepatic I/R
As shown in Table 1, neither human AM alone nor human AMBP-1 alone prevented the significant increase in ALT, AST and LDH at 4 h after the reperfusion. Although ALT, AST and LDH were slightly reduced after human AM alone or AMBP-1 alone treatment, the decrease is not statistically significant from vehicle-treated animals. Thus, administration of human AM alone or human AMBP-1 alone after hepatic I/R does not produce significant protection.
Table 1.
Effects of AM alone or AMBP-1 alone on serum levels of ALT, AST and LDH after hepatic I/R
| Sham | Hepatic I/R-Vehicle | Hepatic I/R-AM | Hepatic I/R-AMBP-1 | |
|---|---|---|---|---|
| ALT (IU/L) | 22.7±4 | 352.4±25* | 346.8±34* | 330.2±29* |
| AST (IU/L) | 42.9±4 | 406.4±31* | 378.8±45* | 395.3±36* |
| LDH (U/L) | 75.3±13 | 1453.3±131* | 1303.1±140* | 1323.3±162* |
P <0.05 versus sham-operated animals. Values are presented as means ± SE (n = 5/group) and compared by one-way ANOVA and Student-Newman-Keuls test.
DISCUSSION
Recent advances in surgical techniques and pharmacological interventions have moderately improved the outcome of trauma surgery, liver resection, liver transplantation, and shock. However, liver failure due to ischemia-reperfusion (I/R) injury continues to be a major complication in the clinical arena. The processes initiated during hepatic I/R lead to liver dysfunction and remote organ injury, which in turn culminate in multiple organ failure and death. It is obvious that there is an urgent medical need for the development of novel treatments to prevent or at least minimize hepatic I/R injury.
Since the discovery of AM in 1993 6, it has attracted the interest of investigators in the cardiovascular field because of AM’s potent and long-lasting vasoactive property. The hypotensive effect of AM was demonstrated by ex vivo studies with isolated rat aortae and with perfused mesenteric arteries.18,19 Various studies have demonstrated that circulating levels of AM increase in patients with ischemia-reperfusion injury 20,21, hemorrhagic and cardiogenic shock 22,23, systemic inflammatory response syndrome 24,25, and following major surgery 26 or hypoxia 27–30. Consistent with these findings, our present study has also shown that both AM gene expression in the liver and its plasma levels increased significantly after hepatic I/R. Hepatic I/R involves microcirculatory disturbances, leading to underperfused areas in the liver that may further worsen the injury.31 In this regard, the elevation in AM levels may be a compensative mechanism to counteract cardiovascular disorders under such conditions.32
The recent discovery of the interaction between AM and its binding protein, AMBP-1, has opened a new avenue for further understanding the regulation of AM activity. The finding that AMBP-1 potentiates AM-induced cAMP accumulation in cultured Rat-2 fibroblast cells9 suggests that AMBP-1 may play an important role in AM-induced vascular relaxation. A study from our laboratory has shown that AMBP-1 in an organ bath at concentrations of 2 and 5 nM is able to enhance AM-induced relaxation of aortic rings taken from normal animals.33 AMBP-1 alone is associated with only minimal vascular relaxation. Thus, circulating AMBP-1 may affect the bioactivity of AM under normal and pathological conditions. AMBP-1 levels decreases by 67% in the plasma of calves undergoing an acute phase response to a parasitic infection, when compared to healthy calves.8 Similarly, a deficiency of AMBP-1 in humans is associated with higher susceptibility to recurrent infections.34 Our recent animal studies have also shown that the decreased level of AMBP-1 leads to the reduced vascular responsiveness to AM, which in turn contributes to the circulatory collapse after hemorrhagic shock or severe sepsis.10,13 The primary site of AMBP-1 biosynthesis is the liver.35–37 In this regard, we have measured AMBP-1’s levels after hepatic I/R. Our results showed that plasma levels of AMBP-1 and its gene expression in the liver decreased significantly after hepatic I/R, indicate the potential role of AMBP-1 deficiency in the development of I/R-induced organ injury.
The pathophysiology of hepatic I/R is complex.5,38,39 During the two hour period of the early phase, liver Kupffer cells become activated, leading to the production of pro-inflammatory cytokines, such as TNF-α and IL-6. These cytokines have a direct cytotoxic effect on hepatocytes and endothelial cells, but they also induce the expression of adhesion molecules and recruitment of neutrophils. These activated neutrophils release reactive oxygen species (ROS) and proteases which are responsible for the induced oxidative stress during the late phase (3–48 h post-reperfusion). The injury during the late phase is much more severe. Thus, hepatic I/R injury involves several contributory factors, making it difficult to attain effective protection by targeting only an individual mechanism.
Although AM was originally discovered as a vasodilative peptide, recent studies have revealed its pleiotropic effects. The anti-inflammatory property of AM following various pathophysiological conditions is well described by us and others.40–46 Studies indicate that AM suppresses secretion of TNF-α and IL-6 from murine macrophage-like RAW264.7 cells stimulated by endotoxin.47 Our recent studies indicate that while AM alone decreases endotoxin-induced TNF-α production from isolated Kupffer cells by only 52%, AM and AMBP-1 in combination reduce TNF-α production by 90%.45 Moreover, AM has been shown to downregulate chemokine levels both in vivo and in vitro.48,49 It can inhibit neutrophil activation and migration to inflammatory sites like the liver by suppressing upregulation of the adhesion molecule CD11.50 Thus, AM can target all the important pathways involving hepatic I/R injury (i.e., microcirculatory disturbances, Kupffer cells activation, neutrophils infiltration).
The primary biological feature of AM is a potent, long-lasting, blood pressure lowering effect with reduced peripheral resistance after intravenous bolus injection or infusion in a relatively short period of time.6,51,52 However, the beneficial effect of low dosages of AM (which do not induce significant hypotension) is extremely limited.12,45,53,54 Low-dose AM in combination with AMBP-1 (which does not have any adverse cardiovascular effects), on the other hand, produces significant beneficial effects under various pathophysiological conditions.10–13 Binding to AMBP-1 increases receptor-mediated effects of AM but suppresses its receptor-independent antimicrobial activity.55 AMBP-1 does not change the affinity of AM receptors for AM, but has sequences which may bind to cell surface adhesion molecules and could therefore bring AM near its receptors and raise the effective concentration of AM.9 AM binding with AMBP-1 may also create a locally contained reservoir of AM at high concentrations. Thus, AMBP-1 may increase the AM effectiveness without modifying the affinity of its receptor. It is also possible that AMBP-1 inhibits the degradation of the biologically active AM. In this regard, our present study has also demonstrated that administration of a low dose of AM in combination with AMBP-1 immediately after the onset of reperfusion downregulated inflammatory cytokines, decreased hepatic neutrophil infiltration, inhibited liver cell apoptosis and necrosis, and reduced liver injury and mortality in a rat model of hepatic I/R. On the other hand, administration of human AM alone or human AMBP-1 alone after hepatic I/R failed to produce significant protection. Since the survival rate after hepatic I/R was significantly improved from 47% to 87% when 12 µg/kg BW human AM and 40 µg/kg BW human AMBP-1 were administered at the beginning of reperfusion, a dose response study was not conducted. In our future studies, however, we will determine the optimal dosage(s) of delayed administration of human AM/AMBP-1 after the onset of hepatic I/R (i.e., delayed administration). It is concluded that administration of human AM in combination with human AMBP-1 appears to be a novel, promising therapeutic approach to reduce mortality after hepatic I/R injury.
ACKNOWLEDGEMENTS
This study was in part supported by National Institutes of Health grants.
REFERENCES
- 1.Banga NR, Homer-Vanniasinkam S, Graham A, et al. Ischaemic preconditioning in transplantation and major resection of the liver. Br J Surg. 2005;92:528–538. doi: 10.1002/bjs.5004. [DOI] [PubMed] [Google Scholar]
- 2.Koti RS, Seifalian AM, Davidson BR. Protection of the liver by ischemic preconditioning: a review of mechanisms and clinical applications. Dig Surg. 2003;20:383–396. doi: 10.1159/000072064. [DOI] [PubMed] [Google Scholar]
- 3.Kharbanda RK, Mortensen UM, White PA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- 4.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86–93. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 7.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21:138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- 8.Elsasser TH, Kahl S, Martinez A, et al. Adrenomedullin binding protein in the plasma of multiple species: characterization by radioligand blotting. Endocrinol. 1999;140:4908–4911. doi: 10.1210/endo.140.10.7157. [DOI] [PubMed] [Google Scholar]
- 9.Pio R, Martinez A, Unsworth EJ, et al. Complement factor H is a serum binding protein for adrenomedullin. The resulting complex modulates the bioactivities of both partners. J Biol Chem. 2001;276:12292–12300. doi: 10.1074/jbc.M007822200. [DOI] [PubMed] [Google Scholar]
- 10.Yang S, Zhou M, Chaudry IH, et al. Novel approach to prevent the transition from the hyperdynamic phase to the hypodynamic phase of sepsis: role of adrenomedullin and adrenomedullin binding protein-1. Ann Surg. 2002;236:625–633. doi: 10.1097/00000658-200211000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou M, Simms HH, Wang P. Adrenomedullin and adrenomedullin binding protein-1 attenuate vascular endothelial cell apoptosis in sepsis. Ann Surg. 2004;240:321–330. doi: 10.1097/01.sla.0000133253.45591.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrizo GJ, Wu R, Cui X, et al. Adrenomedullin and adrenomedullin-binding protein-1 downregulate inflammatory cytokines and attenuate tissue injury after gut ischemia-reperfusion. Surgery. 2007;141:245–253. doi: 10.1016/j.surg.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Wu R, Cui X, Dong W, et al. Mechanisms responsible for vascular hyporesponsiveness to adrenomedullin after hemorrhage: the central role of adrenomedullin binding protein-1. Ann Surg. 2005;242:115–123. doi: 10.1097/01.sla.0000167849.10599.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwivedi AJ, Wu R, Nguyen E, et al. Adrenomedullin and adrenomedullin binding protein-1 prevent acute lung injury after gut ischemia-reperfusion. J Am Coll Surg. 2007;205:284–293. doi: 10.1016/j.jamcollsurg.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Heijnen BH, Straatsburg IH, Gouma DJ, et al. Decrease in core liver temperature with 10 degrees C by in situ hypothermic perfusion under total hepatic vascular exclusion reduces liver ischemia and reperfusion injury during partial hepatectomy in pigs. Surgery. 2003;134:806–817. doi: 10.1016/s0039-6060(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 16.Schauer RJ, Gerbes AL, Vonier D, et al. Glutathione protects the rat liver against reperfusion injury after prolonged warm ischemia. Ann Surg. 2004;239:220–231. doi: 10.1097/01.sla.0000110321.64275.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Dou K, Song Z, et al. The Na+/H+ exchange inhibitor: a new therapeutic approach for hepatic ischemia injury in rats. Transplant Proc. 2003;35:3134–3135. doi: 10.1016/j.transproceed.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa H, Hirata Y, Kakoki M, et al. Role of nitric oxide-cGMP pathway in adrenomedullin-induced vasodilation in the rat. Hypertension. 1999;33:689–693. doi: 10.1161/01.hyp.33.2.689. [DOI] [PubMed] [Google Scholar]
- 19.Nuki C, Kawasaki H, Kitamura K, et al. Vasodilator effect of adrenomedullin and calcitonin gene-related peptide receptors in rat mesenteric vascular beds. Biochem Biophys Res Commun. 1993;196:245–251. doi: 10.1006/bbrc.1993.2241. [DOI] [PubMed] [Google Scholar]
- 20.Nagaya N, Nishikimi T, Uematsu M, et al. Plasma adrenomedullin as an indicator of prognosis after acute myocardial infarction. Heart. 1999;81:483–487. doi: 10.1136/hrt.81.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyao Y, Nishikimi T, Goto Y, et al. Increased plasma adrenomedullin levels in patients with acute myocardial infarction in proportion to the clinical severity. Heart. 1998;79:39–44. doi: 10.1136/hrt.79.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehlenz K, Koch B, Preuss P, et al. High levels of circulating adrenomedullin in severe illness: Correlation with C-reactive protein and evidence against the adrenal medulla as site of origin. Exp Clin Endocrinol Diabetes. 1997;105:156–162. doi: 10.1055/s-0029-1211745. [DOI] [PubMed] [Google Scholar]
- 23.Kikumoto K, Kubo A, Hayashi Y, et al. Increased plasma concentration of adrenomedullin in patients with subarachnoid hemorrhage. Anesth Analg. 1998;87:859–863. doi: 10.1097/00000539-199810000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Ueda S, Nishio K, Minamino N, et al. Increased plasma levels of adrenomedullin in patients with systemic inflammatory response syndrome. Am J Respir Crit Care Med. 1999;160:132–136. doi: 10.1164/ajrccm.160.1.9810006. [DOI] [PubMed] [Google Scholar]
- 25.Nishio K, Akai Y, Murao Y, et al. Increased plasma concentrations of adrenomedullin correlate with relaxation of vascular tone in patients with septic shock. Crit Care Med. 1997;25:953–957. doi: 10.1097/00003246-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Fujioka S. Increased plasma concentration of adrenomedullin during and after major surgery. Surg Today. 2001;31:575–579. doi: 10.1007/s005950170089. [DOI] [PubMed] [Google Scholar]
- 27.Hofbauer KH, Jensen BL, Kurtz A, et al. Tissue hypoxygenation activates the adrenomedullin system in vivo. Am J Physiol Regul Integr Comp Physiol. 2000;278:R513–R519. doi: 10.1152/ajpregu.2000.278.2.R513. [DOI] [PubMed] [Google Scholar]
- 28.Nagata D, Hirata Y, Suzuki E, et al. Hypoxia-induced adrenomedullin production in the kidney. Kidney Int. 1999;55:1259–1267. doi: 10.1046/j.1523-1755.1999.00361.x. [DOI] [PubMed] [Google Scholar]
- 29.Trollmann R, Schoof E, Beinder E, et al. Adrenomedullin gene expression in human placental tIssue and leukocytes: a potential marker of severe tIssue hypoxia in neonates with birth asphyxia. Eur J Endocrinol. 2002;147:711–716. doi: 10.1530/eje.0.1470711. [DOI] [PubMed] [Google Scholar]
- 30.Cejudo-Martin P, Morales-Ruiz M, Ros J, et al. Hypoxia is an inducer of vasodilator agents in peritoneal macrophages of cirrhotic patients. Hepatology. 2002;36:1172–1179. doi: 10.1053/jhep.2002.36371. [DOI] [PubMed] [Google Scholar]
- 31.Vollmar B, Glasz J, Leiderer R, et al. Hepatic microcirculatory perfusion failure is a determinant of liver dysfunction in warm ischemia-reperfusion. Am J Pathol. 1994;145:1421–1431. [PMC free article] [PubMed] [Google Scholar]
- 32.Shimosawa T, Fujita T. Adrenomedullin and its related peptide. Endocr J. 2005;52:1–10. doi: 10.1507/endocrj.52.1. [DOI] [PubMed] [Google Scholar]
- 33.Zhou M, Ba ZF, Chaudry IH, et al. Adrenomedullin binding protein-1 modulates vascular responsiveness to adrenomedullin in late sepsis. Am J Physiol Regul Integr Comp Physiol. 2002;283:R553–R560. doi: 10.1152/ajpregu.00544.2001. [DOI] [PubMed] [Google Scholar]
- 34.Naked GM, Florido MP, Ferreira dP, et al. Deficiency of human complement factor I associated with lowered factor H. Clin Immunol. 2000;96:162–167. doi: 10.1006/clim.2000.4878. [DOI] [PubMed] [Google Scholar]
- 35.Friese MA, Hellwage J, Jokiranta TS, et al. FHL-1/reconectin and factor H: two human complement regulators which are encoded by the same gene are differently expressed and regulated. Mol Immunol. 1999;36:809–818. doi: 10.1016/s0161-5890(99)00101-7. [DOI] [PubMed] [Google Scholar]
- 36.Schwaeble W, Zwirner J, Schulz TF, et al. Human complement factor H: expression of an additional truncated gene product of 43 kDa in human liver. Eur J Immunol. 1987;17:1485–1489. doi: 10.1002/eji.1830171015. [DOI] [PubMed] [Google Scholar]
- 37.Luo W, Vik DP. Regulation of complement factor H in a human liver cell line by interferon-gamma. Scand J Immunol. 1999;49:487–494. doi: 10.1046/j.1365-3083.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- 38.Glantzounis GK, Salacinski HJ, Yang W, et al. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver Transpl. 2005;11:1031–1047. doi: 10.1002/lt.20504. [DOI] [PubMed] [Google Scholar]
- 39.Lentsch AB, Kato A, Yoshidome H, et al. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 40.Nishikimi T, Saito Y, Kitamura K, et al. Increased plasma levels of adrenomedullin in patients with heart failure. J Am Coll Cardiol. 1995;26:1424–1431. doi: 10.1016/0735-1097(95)00338-X. [DOI] [PubMed] [Google Scholar]
- 41.Rademaker MT, Charles CJ, Lewis LK, et al. Beneficial hemodynamic and renal effects of adrenomedullin in an ovine model of heart failure. Circulation. 1997;96:1983–1990. doi: 10.1161/01.cir.96.6.1983. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama M, Takahashi K, Murakami O, et al. Adrenomedullin in monocytes and macrophages: possible involvement of macrophage-derived adrenomedullin in atherogenesis. Clin Sci (Colch ) 1999;97:247–251. [PubMed] [Google Scholar]
- 43.Chini EN, Chini CCS, Bolliger C, et al. Cytoprotective effects of adrenomedullin in glomerular cell injury: Central role of cAMP signaling pathway. Kidney Int. 1997;52:917–925. doi: 10.1038/ki.1997.413. [DOI] [PubMed] [Google Scholar]
- 44.Yoshibayashi M, Kamiya T, Nishikimi T, et al. Elevated plasma levels of adrenomedullin in congenital cyanotic heart disease. Clin Sci (Colch ) 1999;96:543–547. [PubMed] [Google Scholar]
- 45.Wu R, Zhou M, Wang P. Adrenomedullin and adrenomedullin binding protein-1 downregulate TNF-alpha in macrophage cell line and rat Kupffer cells. Regul Pept. 2003;112:19–26. doi: 10.1016/s0167-0115(03)00018-1. [DOI] [PubMed] [Google Scholar]
- 46.Yang S, Zhou M, Fowler DE, et al. Mechanisms of the beneficial effect of adrenomedullin and adrenomedullin-binding protein-1 in sepsis: down-regulation of proinflammatory cytokines. Crit Care Med. 2002;30:2729–2735. doi: 10.1097/00003246-200212000-00018. [DOI] [PubMed] [Google Scholar]
- 47.Kubo A, Minamino N, Isumi Y, et al. Production of adrenomedullin in macrophage cell line and peritoneal macrophage. J Biol Chem. 1998;273:16730–16738. doi: 10.1074/jbc.273.27.16730. [DOI] [PubMed] [Google Scholar]
- 48.Iwamoto M, Osajima A, Tamura M, et al. Adrenomedullin inhibits pressure-induced mesangial MCP-1 expression through activation of protein kinase A. J Nephrol. 2003;16:673–681. [PubMed] [Google Scholar]
- 49.Gonzalez-Rey E, Chorny A, Varela N, et al. Urocortin and adrenomedullin prevent lethal endotoxemia by down-regulating the inflammatory response. Am J Pathol. 2006;168:1921–1930. doi: 10.2353/ajpath.2006.051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saito Y, Nakagawa C, Uchida H, et al. Adrenomedullin suppresses fMLP-induced upregulation of CD11b of human neutrophils. Inflammation. 2001;25:197–201. doi: 10.1023/a:1011092532100. [DOI] [PubMed] [Google Scholar]
- 51.Ishiyama Y, Kitamura K, Ichiki Y, et al. Hemodynamic effects of a novel hypotensive peptide, human adrenomedullin, in rats. Eur J Pharmacol. 1993;241:271–273. doi: 10.1016/0014-2999(93)90214-3. [DOI] [PubMed] [Google Scholar]
- 52.Ishiyama Y, Kitamura K, Ichiki Y, et al. Haemodynamic responses to rat adrenomedullin in anaesthetized spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1995;22:614–618. doi: 10.1111/j.1440-1681.1995.tb02075.x. [DOI] [PubMed] [Google Scholar]
- 53.Cui X, Wu R, Zhou M, et al. Adrenomedullin and its binding protein attenuate the proinflammatory response after hemorrhage. Crit Care Med. 2005;33:391–398. doi: 10.1097/01.ccm.0000153416.41398.a9. [DOI] [PubMed] [Google Scholar]
- 54.Wu R, Dong W, Zhou M, et al. A novel approach to maintaining cardiovascular stability after hemorrhagic shock: Beneficial effects of adrenomedullin and its binding protein. Surgery. 2005;137:200–208. doi: 10.1016/j.surg.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Beltowski J, Jamroz A. Adrenomedullin - what we know 10 years since its discovery? Pol J Pharmacol. 2004;56:5–27. [PubMed] [Google Scholar]