Abstract
OBJECTIVES: To compare the case-finding ability of current national guidelines for screening diabetes mellitus and characterize factors that affect testing practices in an ambulatory population.
PATIENTS AND METHODS: In this retrospective analysis, we reviewed a database of 46,991 nondiabetic patients aged 20 years and older who were seen at a large Midwestern academic physician practice from January 1, 2005, through December 31, 2007. Patients were included in the sample if they were currently being treated by the physician group according to Wisconsin Collaborative for Healthcare Quality criteria. Pregnant patients, diabetic patients, and patients who died during the study years were excluded. The prevalence of patients who met the American Diabetes Association (ADA) and/or US Preventive Services Task Force (USPSTF) criteria for diabetes screening, percentage of these patients screened, and number of new diabetes diagnoses per guideline were evaluated. Screening rates were assessed by number of high-risk factors, primary care specialty, and insurance status.
RESULTS: A total of 33,823 (72.0%) of 46,991 patients met either the ADA or the USPSTF screening criteria, and 28,842 (85.3%) of the eligible patients were tested. More patients met the ADA criteria than the 2008 USPSTF criteria (30,790 [65.5%] vs 12,054 [25.6%]), and the 2008 USPSTF guidelines resulted in 460 fewer diagnoses of diabetes (33.1%). By single high-risk factor, prediabetes (15.8%) and polycystic ovarian syndrome (12.6%) produced the highest rates of diagnosis. The number of ADA high-risk factors predicted diabetes, with 6 (23%) of 26 patients with 6 risk factors diagnosed as having diabetes. Uninsured patients were tested significantly less often than insured patients (54.9% vs 85.4%).
CONCLUSION: Compared with the ADA recommendations, the new USPSTF guidelines result in a lower number of patients eligible for screening and decrease case finding significantly. The number and type of risk factors predict diabetes, and lack of health insurance decreases testing.
Compared with the American Diabetes Association recommendations, the new US Preventive Services Task Force guidelines result in a lower number of patients eligible for screening and decrease case finding significantly. The number and type of risk factors predict diabetes, and lack of health insurance decreases testing.
ADA = American Diabetes Association; CI = confidence interval; FPG = fasting plasma glucose; HbA1c = hemoglobin A1c; ICD-9 = International Classification of Diseases, Ninth Revision; PCOS = polycystic ovarian syndrome; RG = random glucose; USPSTF = US Preventive Services Task Force; WCHQ = Wisconsin Collaborative for Healthcare Quality
Diabetes mellitus has reached epidemic proportions in the United States. National Health and Nutrition Examination Survey data from 2005-2006 determined that the prevalence of diabetes in an ambulatory sample aged 20 years and older was 12.9%.1 An additional 29.5% had either impaired fasting plasma glucose (FPG) levels, impaired glucose tolerance, or both; therefore, 42.4% of the US population aged 20 years or older has some degree of dysglycemia.1 These numbers represent an increase since 1999-2002, when the National Health and Nutrition Examination Survey reported that the diabetic prevalence was 9.3%.2 This trend is expected to continue, with 48.3 million Americans expected to have diabetes by 2050, a 198% increase compared with 2005.3
The prevalence of undiagnosed diabetes mellitus is equally alarming, with approximately 40% of the US diabetic population not knowing about their disease.1 In total, 5.1% of the US population aged 20 years and older has diabetes but is unaware of the diagnosis. These patients are of particular concern because patients without knowledge of their disease obviously cannot be treated and may sustain progressive end-organ compromise. Harris et al4 discovered retinopathy in almost 21% of patients with newly diagnosed diabetes, indicating that the disease may have been active for 4 to 7 years before the actual diagnosis. In addition, recent UK Prospective Diabetes Study data confirmed the “legacy effect” in patients with type 2 diabetes, a finding that was originally demonstrated in patients with type 1 diabetes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study.5 This phenomenon refers to the finding that a period of untreated hyperglycemia, such as what might be expected in a patient with undiagnosed diabetes, has lasting effects on cardiovascular morbidity and mortality even if blood glucose levels are later appropriately controlled.6,7 Thus, a more timely diagnosis may reduce these complications by creating an opportunity for early intervention and optimization of glycemic control.
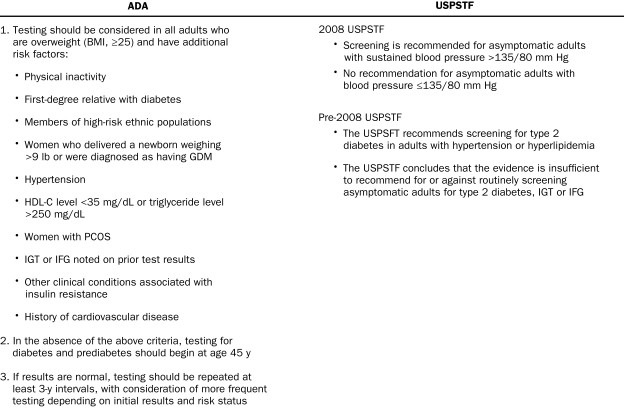
Why the prevalence of undiagnosed diabetes remains high is unclear because screening guidelines have been in place for more than a decade. Since 1997, the American Diabetes Association (ADA) has recommended diabetes screening for patients 45 years and older, as well as in younger patients with high-risk factors (Figure 1).8,9 The US Preventive Services Task Force (USPSTF) has consistently recommended diabetes screening for patients with hypertension and hyperlipidemia since the Guide to Clinical Preventive Services, Third Edition,10 was published in series beginning in 2000. However, with the USPSTF 2008 update, hyperlipidemia was deleted as a criterion and diabetes screening was advised only for patients with blood pressure greater than 135/80 mm Hg (Figure 1).10,11 These guidelines are based on evidence-based review of the literature (ADA and USPSTF) and expert opinion (ADA).
FIGURE 1.
Criteria to screen for diabetes mellitus: American Diabetes Association (ADA) and/or US Preventive Services Task Force (USPSTF). BMI = body mass index; GDM = gestational diabetes mellitus; HDL-C = high-density lipoprotein cholesterol; IFG = impaired fasting glucose; IGT = impaired glucose tolerance; PCOS = polycystic ovarian syndrome. Adapted from the ADA9 and the USPSTF,10,11 with permission.
The failure of available guidelines to effectively reduce the number of patients with undiagnosed diabetes may be due to factors such as patients not presenting for care or physicians failing to screen. However, whether the guidelines themselves are targeting the correct at-risk population to maximize diabetes case finding is unknown. Despite extensive publication and commentary of the 2 primary guidelines (ADA and USPSTF),12 it remains unclear how diabetes screening guidelines affect case finding in ambulatory practice. To our knowledge, there have been no systematic comparisons of the testing practices and case-finding ability of the ADA and USPSTF recommendations.
The primary objective of this study was to determine the diabetes case-finding ability of the ADA and USPSTF criteria when applied to clinical practice. In addition, we investigated whether patients with more ADA-designated high-risk factors were more likely to be tested, what risk factor was most predictive of a diabetes diagnosis, and whether a screening difference existed on the basis of primary care specialty or presence of health care insurance.
PATIENTS AND METHODS
We retrospectively analyzed diabetes screening practices in a large Midwestern academic physician group for the 3-year period from January 1, 2005, through December 31, 2007. The University of Wisconsin, Madison Institutional Review Board approved the study and granted a waiver of consent for the Health Insurance Portability and Accountability Act. Patients' clinical, laboratory, encounter, and demographic data were obtained from the electronic health record of a large Midwestern academic physician group practice. These data include patient health care records, billing and payer information, and physician- and clinic-specific data generated from services rendered by physicians. Patients were included if they had had most of their visits at a clinic owned and operated by the physician group. These clinics serve patients in both referral and primary care practice. Since implementation in 2003, these clinics have contributed approximately 2 million patient, 48 million encounter, 47 million laboratory, 7 million pharmacy, and 434 million transaction records to the electronic health record.
Study Population
Patients were included in the sample during a specific year if they were “currently managed” by the physician group. Specifically, patients were required to have had at least 2 primary care office encounters in an outpatient, nonurgent setting, regardless of diagnosis code, with a primary care physician (internal medicine, gynecology, family practice, or pediatrics) in a primary care location in the prior 36 months, with at least 1 of those visits in the prior 24 months. This approach uses the previously established Wisconsin Collaborative for Healthcare Quality (WCHQ) definition of a “currently managed” population. This definition is the property of WCHQ and is used herein with their permission. The WCHQ is a diverse, voluntary, state-wide partnership of health care institutions and organizations whose goal is to improve the quality of health care in Wisconsin.13 These guidelines ensure that a patient who may have a single visit to a clinic but then seeks care permanently elsewhere is not included in screening measures based on the initial and only clinic visit.
Patients included were aged 20 years or older on January 1, 2005, and met the WCHQ criteria for being currently managed for each of the years 2005, 2006, and 2007. A 3-year window was chosen on the basis of the ADA recommendation for screening every 3 years. Data from the years 2003 and 2004 were used to determine prior diagnosis of diabetes, prediabetes, preexisting comorbidities, and pregnancy. Patients with any visit for pregnancy in the years 2003 to 2007 were excluded (eAppendix 1 online linked to this article) as were patients who died during the 3-year study period. Patients with 2 or more outpatient encounters with a diagnosis of diabetes mellitus in the years 2003-2004 were excluded (eAppendix 1).14
Demographic, clinical, and laboratory data were extracted for eligible patients. For all patients, age, sex, ethnicity, insurance status, and body mass index (calculated as weight in kilograms divided by height in meters squared) were extracted. Ethnicity was included because African American, Latino, Native American, Asian American, or Pacific Islander heritage is considered a risk factor for diabetes and is incorporated into the ADA guidelines for diabetes screening.9 All evaluation and management outpatient encounter data, including date of service, physician specialty, and International Classification of Diseases, Ninth Revision (ICD-9)15 codes were extracted. Diabetes screening tests were defined as FPG measurement, random glucose (RG) measurement, 2-hour glucose tolerance test, and hemoglobin A1c (HbA1c) measurement.. The percentage of patients undergoing at least 1 test in the 3-year study period was recorded, with 74.3% having 1 or more RG measurements, 9.1% having 1 or more HbA1c measurements, 0.8% having 1 or more FPG measurements, and 0.8% having 1 or more glucose tolerance tests, with some patients having more than 1 type of test performed. Although HbA1c measurement was not an accepted diabetes screening test during the study years, it was included in our screening profile because it is known that physicians used HbA1c measurement in this manner before its recent incorporation into guidelines.16,17 Additional laboratory data extracted included levels of total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides.
Variable Definitions
Eight high-risk variables were defined on the basis of the ADA-designated risk factors for screening (Figure 1). These factors include age 45 years or older, high-risk ethnicity, hypertension, hypercholesterolemia, polycystic ovarian syndrome (PCOS), vascular disease (cardiovascular, peripheral vascular, or ischemic stroke), overweight (body mass index, ≥25), and history of prediabetes. Definitions for each high-risk variable were determined on the basis of a combination of 1 or more factors, including ICD-9 code, laboratory data, and clinical information, with detailed criteria for each high-risk factor listed in eAppendix 2 (online linked to this article). When possible, validated definitions from Grundy et al,18 Elixhauser et al,19 Chronic Condition Warehouse,20 Segars and Lea,21 Hebert et al,14 Goldstein,22 and Tirschwell and Longstreth23 were used to determine ICD-9 codes. Two diagnosis codes on 2 separate occasions within 2 years were required to determine the presence of a high-risk factor. New cases of diabetes were determined by the presence of 2 validated ICD-9 codes on 2 separate occasions within the 3-year study.14
The population that met the ADA and USPSTF screening criteria was determined on the basis of the defined high-risk factors. For ADA criteria, this included any patient 45 years or older or any overweight patient younger than 45 years with at least 1 additional high-risk factor. Eligibility for screening by USPSTF criteria was determined in 2 ways. First, screening practices were determined on the basis of the third edition of the USPSTF recommendations (pre-2008 USPSTF) to screen all hyperlipidemic and hypertensive patients because these guidelines were in place between the study years 2005 and 2007.10 Second, the results of using hypertension alone as a criterion to screen were determined to estimate how the updated 2008 USPSTF guideline may be expected to perform if applied to this population.11 The number of patients who met each screening criterion, the percentage of eligible patients screened, and the case-finding ability of diagnostic testing (number of new cases divided by number of eligible patients screened) were determined. The diabetes case-finding ability by individual risk factor and the number of high-risk factors were determined. Primary care specialty was defined as the specialty in which most or all of the patient's primary care visits occurred during the 3-year period. Screening adherence was evaluated on the basis of primary care specialty and presence or absence of insurance.
Statistical Analyses
Categorical variables were summarized using percentages. Continuous variables were summarized using means and SDs. Descriptive variables were presented overall and separately for each diabetes screening guideline. We conducted χ2 tests for 2-way contingency tables comparing diabetes screening and diagnosis by insurance status and physician specialty, with the results presented as P values. P≤.05 was considered statistically significant. To compare the proportion of patients screened, the proportion of conditions diagnosed, or the proportion of patients with diagnoses missed by different screening guidelines, 95% confidence intervals (CIs) were calculated using bootstrap techniques to replicate observations 1000 times.
RESULTS
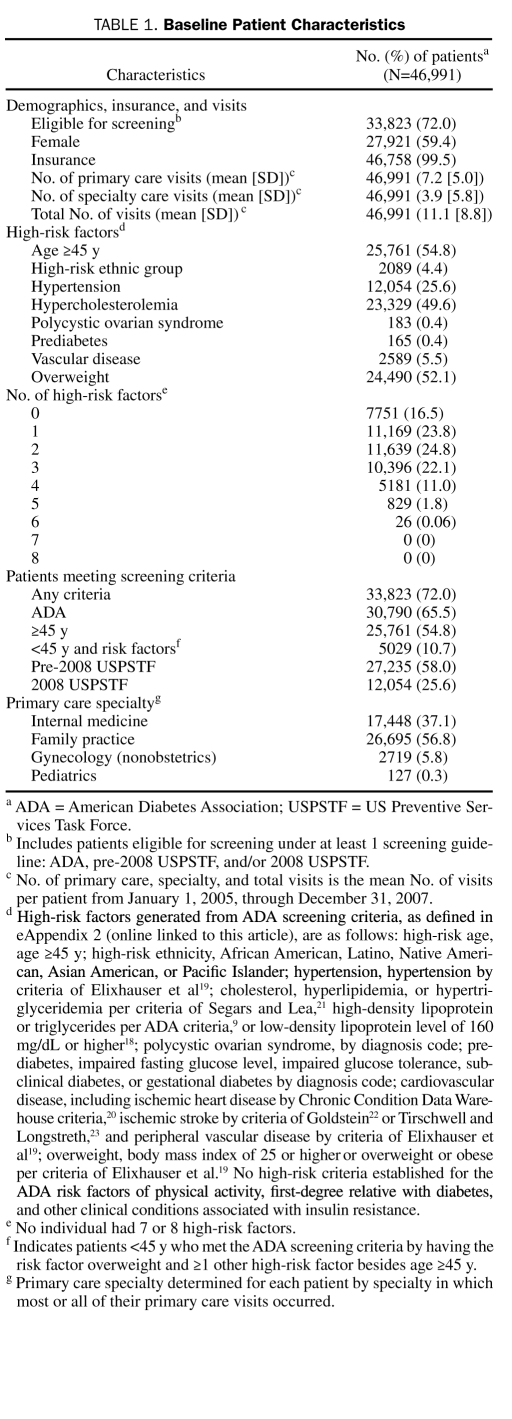
A total of 51,034 patients met the inclusion criteria. Of these, 183 (0.4%) were excluded because of pregnancy, 982 (1.9%) because of death, and 3083 (6.0%) because of prior diagnosis of diabetes (note that some individuals met more than 1 of these criteria). Of the remaining 46,991 patients, 27,921 (59.4%) were female and 46,758 (99.5%) were insured. Of those 233 patients without insurance, 146 (62.7%) were younger than 45 years, and 124 (53.2%) were male. The mean ± SD number of visits to a primary care physician was 7.2±5.0 per person during the 3-year period of 2005-2007 (Table 1).
TABLE 1.
Baseline Patient Characteristics
A total of 33,823 patients (72.0%) met at least 1 of the 3 screening criteria: ADA, pre-2008 USPSTF, or 2008 USPSTF. More patients met the ADA criteria (30,790 [65.5%]) than either the pre-2008 USPSTF (27,235 [58.0%]) or 2008 USPSTF (12,054 [25.6%]) criteria. Only 7751 patients (16.5%) had no high-risk factors. The most common high-risk factor was age 45 years or older (25,761 [54.8%]) followed by overweight (24,490 [52.1%]). Family practice (26,695 [56.8%]) was the most common primary care specialty (Table 1).
ADA and USPSTF Screening Criteria
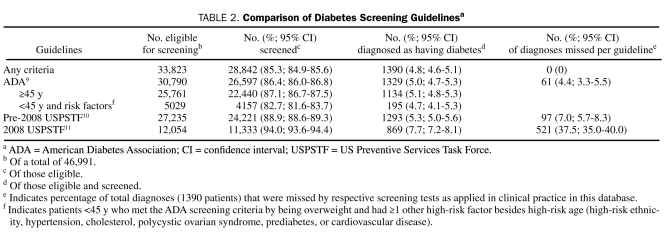
In total, 30,790 patients met the ADA screening criteria, and 26,597 (86.4%; 95% CI, 86.0%-86.6%) of those eligible had 1 or more glucose tests performed (Table 2). Of those eligible and tested, 1329 patients (5.0%; 95% CI, 4.7%-5.3%) were diagnosed as having diabetes. Of patients who met the ADA guidelines, those with criteria to screen by age alone (≥45 years) were more likely to be tested than those younger than 45 years; those younger than 45 years qualified for screening on the basis of being overweight and having at least 1 additional high-risk factor (22,440 [87.1%] of 25,761 vs 4157 [82.7%] of 5029; 95% CI, 86.7%-87.5% and 81.6%-83.7%, respectively; P<.001).
TABLE 2.
Comparison of Diabetes Screening Guidelinesa
A total of 27,235 patients met pre-2008 USPSTF criteria because of hypertension and/or hypercholesterolemia, and 24,221 (88.9%; 95% CI, 88.6%-89.3%) were screened. Of those who met the criteria and were screened, 1293 (5.3%; 95% CI, 5.0%-5.6%) were assigned a new diagnosis of diabetes. When patients with hypercholesterolemia were deleted to determine 2008 USPSTF eligibility (which is based on hypertension alone), only 12,054 patients met the criteria for screening, a reduction in 15,181 patients. Of the 2008 USPSTF patients, 11,333 (94.0%; 95% CI, 93.6%-94.4%) of 12,054 eligible patients were screened, with 869 (7.7%; 95% CI, 7.2%-8.1%) of those being diagnosed as having diabetes.
Of those 33,823 patients eligible for screening by any criteria, 28,842 (85.3%; 95% CI, 84.9%-85.6%) had at least 1 glucose test performed (Table 2). By individual screening criteria, a higher percentage of patients eligible and tested by 2008 USPSTF criteria were diagnosed as having diabetes compared with pre-2008 USPSTF and ADA criteria, respectively (7.7% vs 5.3% vs 5.0%; 95% CIs, 7.2%-8.1%, 5.0%-5.6%, and 4.7%-5.3%, respectively). However, the corresponding total number of patients diagnosed as having diabetes was lowest with the 2008 USPSTF criteria compared with the pre-2008 USPSTF and ADA criteria, respectively (869 vs 1293 vs 1329) because of the lower absolute numbers of patients eligible for screening under the 2008 USPSTF guideline.
In total, 1390 (4.8%; 95% CI, 4.6%-5.1%) of 28,842 patients eligible and tested by any criteria were diagnosed as having diabetes (Table 2). By individual guideline, when applied to clinical practice, the ADA screening criteria missed the fewest of these 1390 diagnoses, missing 61 patients (4.4%; 95% CI, 3.3%-5.5%), followed by 97 (7.0%; 95% CI, 5.7%-8.3%) missed with pre-2008 USPSTF. The 2008 USPSTF was significantly worse than either, missing 521 (37.5%; 95% CI, 35.0%-40.0%) of 1390 when applied to the study population. Of the 2 current screening guidelines, the 2008 USPSTF guidelines resulted in 460 fewer diagnoses of diabetes (33.1%) when compared with the case-finding ability of ADA criteria.
Number of Risk Factors, Primary Care Specialty, and Insurance Status
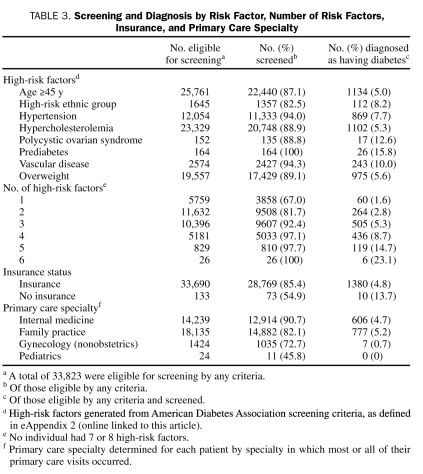
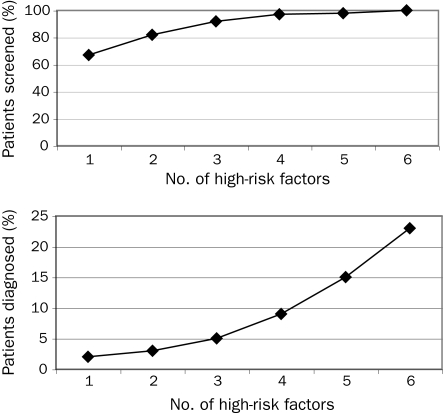
Patients most likely to be tested had prediabetes (100% of 164 patients), vascular disease (2427 [94.3%] of 2574 patients), and hypertension (11,333 [94.0%] of 12,054 patients) (Table 3). The high-risk factors associated with the highest rate of new diabetes diagnoses during the 3-year study period were prediabetes (26 [15.8%] of 164 patients), PCOS (17 [12.6%] of 135 patients), and vascular disease (243 [10.0%] of 2427 patients). Age 45 years or older (1134 [5.0%] of 22,440 patients) and hypercholesterolemia (1102 [5.3%] of 20,748 patients) produced the lowest rate of new diagnosis. The number of high-risk factors was strongly correlated with both screening and case-finding ability, with all 26 patients with 6 high-risk factors screened, and 6 (23%) of 26 diagnosed as having diabetes (Table 3 and Figure 2).
TABLE 3.
Screening and Diagnosis by Risk Factor, Number of Risk Factors, Insurance, and Primary Care Specialty
FIGURE 2.
Percentage of patients screened for and diagnosed as having diabetes mellitus by number of high-risk factors. Patients were eligible for screening by either American Diabetes Association and/or US Preventive Services Task Force criteria.
Patients without insurance were less likely to be tested with any glucose screening measurement compared with insured patients. In total, 73 (54.9%) of 133 eligible uninsured patients vs 28,769 (85.4%) of 33,690 insured patients were screened (P<.001). However, 10 (14%) of 73 uninsured patients vs 1380 (4.8%) of 28,769 insured patients were diagnosed as having diabetes, demonstrating a higher percentage of new cases of diabetes in the un insured (P=.002). Eligible patients seen by internal medicine physicians were tested most often (12,914 [90.6%] of 14,239 patients) followed by family practice physicians (14,882 [82.1%] of 18,135 patients) and gynecologists (1035 [72.7%] of 1424 patients) (P<.001) (Table 3).
DISCUSSION
This study represents a comprehensive evaluation of diabetes screening guidelines and practices in a large, ambulatory cohort. The ADA criteria identified more patients eligible for screening than either USPSTF standard, with the new 2008 USPSTF criteria recommending screening for a significantly smaller number of patients than either the pre-2008 USPSTF or ADA criteria. Most importantly, when the 2 current guidelines were applied to clinical practice, the decrease in the number of patients eligible for screening based on the new 2008 USPSTF criteria resulted in a significant reduction in diabetes case finding compared with the ADA criteria. On the basis of US Census data from 2005-2007, the prevalence of undiagnosed diabetes, and the performance of 2008 USPSTF guideline in the current study, nationwide use of the new USPSTF guideline alone would result in 3,650,390 fewer diabetes diagnoses in adults aged 20 years and older during the 3-year study period compared with the ADA guidelines.1,24 This finding is of concern because many primary care physicians view USPSTF recommendations as standard of care and therefore may miss many cases of diabetes in their practice. Indeed, the USPSTF identifies itself as the “gold standard for clinical preventive services.”25
Despite significantly better performance in clinical practice for diabetes case-finding ability, when compared with the 2008 USPSTF recommendations, the ADA criteria also failed to recommend screening for a subset of approximately 3000 patients who met at least 1 of the 2 USPSTF criteria. The specific patients missed by the ADA guideline were nonobese patients younger than 45 years with hypertension (pre-2008 and 2008 USPSTF) or hyperlipidemia (pre-2008 only). Patients younger than 45 years who met the ADA criteria for screening were significantly less likely to be tested than those 45 years and older. These high-risk, younger patients on average will have longer glycemic exposure during their lifetime because of their younger age and therefore should be an intense focus of future screening efforts.
A potential argument in favor of the new 2008 USPSTF guidelines could be the higher number of cases per number screened because 7.7% of patients screened under 2008 USPSTF standards were given a new diagnosis of diabetes, whereas only 5.0% of those screened under ADA criteria were diagnosed as having diabetes. However, the 7.7% figure is based on a much smaller number of eligible patients to begin with (12,054 vs 30,790), resulting in a significantly lower number of cases found compared with the ADA criteria. In addition, 5.0% is a high rate when compared with other well-accepted (and more costly) screening tests, such as mammography, which may produce fewer than 1 new diagnosis per 100 patients screened.26
When individual risk factors were evaluated, certain ADA high-risk factors were found to have particularly high case-finding ability; in particular, 15.8% of those with prediabetes and 12.6% of patients with PCOS were diagnosed as having diabetes during the study period. Patients with these less common but high-risk factors should be targeted for screening in clinical practice. Patients with multiple high-risk factors also present a screening priority because the amount of screening increases in a nonlinear manner, particularly with 4 or more high-risk factors.
Most of our patients eligible for screening by any criteria had been tested with at least 1 glucose screening measurement, although 15% of patients who met any screening guideline were not tested. The current study also revealed that screening practices were unequal across primary care subspecialties or by insurance status. Of patients who met the screening criteria, those seen most frequently by a gynecologist were less likely to have a diabetes screening test performed. This is worth noting because nonpregnant women of all ages seek primary care with gynecologists and should have access to the same preventive services as those in other primary care practices. In addition, this database was largely an insured population (99.5%) because of strict adherence to the WCHQ criteria mandating multiple physician visits, which almost certainly increased the frequency of screening found in this study compared with what could be expected with a larger uninsured population. However, even with the small number of uninsured patients studied, a worrisome trend in screening frequency was observed. Uninsured patients who met any screening criteria were tested significantly less, even among those patients who had clinic visits. Uninsured patients who do not come to the clinic were not captured in this study but almost certainly fare worse and remain a vulnerable population that should be targeted for public health outreach in the area of diabetes screening.27
The strengths of the current study are the large population size and the use and availability of standardized criteria to establish an accurate, comprehensive, and reproducible population. Acknowledging that every retrospective study has inherent limitations, we chose at every opportunity the most strict definition for inclusion criteria or risk factor definition. For example, we used strict WCHQ criteria for our sample definition even though it is likely that many patients seen only once (or not at all) in our clinics were still “clinic patients” and would be far less likely to be screened because of their infrequent visits. However, we could not distinguish an infrequent visitor from a patient who came to an eligible clinic once and then went elsewhere for care and was potentially screened, so we chose not to include these patients. Likewise, we used conservative criteria and determined that a patient had a risk factor only when the risk factor appeared 2 or more times in their medical record or laboratory test results by predefined, standardized criteria when possible (eAppendix 2). Therefore, we were fairly confident that the risk factor was present and that the physician should be aware of that particular comorbidity. However, our database did not allow us to create ADA high-risk factors for family history, physical inactivity, and other conditions associated with insulin resistance; thus, we were not able to include every ADA risk factor, which could have resulted in more patients being eligible for ADA screening.
We included all glucose values in our database as screening data points, although some glucose values were measured for reasons other than screening, such as those measured incidentally as part of a basic chemistry panel. In addition, our database does not have mandatory entry for fasting status, so any unlabeled FPG test by default was classified as an RG test. These factors certainly resulted in underreporting of true FPG values and together help explain the apparent high incidence of RG values. However, our goal was to ensure that all possible attempts to screen were captured. Although the screening statistics in this report are a best-case scenario by design (despite the 15% rate of unscreened patients), we present a clear starting point for analyzing screening practices. However, because we could not always determine FPG status with absolute certainty and could not determine symptoms of hyperglycemia associated with an elevated RG level as required to diagnose diabetes,9 the primary end point of our study, diagnosis of diabetes, was determined exclusively by validated diagnosis code criteria14 and not by glucose laboratory data.
This study is a comprehensive review of diabetes screening guidelines and practices in a US subpopulation, including evaluation of case-finding ability and performance characteristics of the 2 current national screening guidelines. The most important finding of this analysis is that the new 2008 USPSTF criteria fail to include a large number of patients who would be eligible for screening by current ADA criteria, resulting in a concomitant reduction in discovery of new diabetes cases. Yet almost equally concerning is the discovery that our 2 current national screening guidelines (ADA and 2008 USPSTF) recommend screening for disparate populations. We believe that these findings together argue strongly for greater standardization of screening recommendations that also maximize diabetes case finding. With the epidemic of undiagnosed diabetes in the United States, we need to improve screening efforts, especially in light of the inexpensive, low-risk, and easily performed screening tests available.
Clearly, guidelines should be evidence based. Indeed, there is increasing concern that the integrity of guidelines in general may be in question, largely because any group or organization, regardless of bias, may issue a guideline and present it as standard of care.28 However, the goal of a practice guideline is to help physicians make medical decisions on a daily basis. In most cases, the ideal evidence is unavailable or, at the very least, open to debate.29 The USPSTF guidelines have historically been exclusively based on existing evidence, resulting in an “I Statement” of insufficient evidence in many of their clinical guidelines.25 In the case of diabetes mellitus, the USPSTF acknowledged that the ideal clinical trial, randomizing screening-detected diabetes to treatment vs no treatment, would be unethical and therefore is unlikely to be performed.30 Thus, if criteria for USPSTF guidelines remain bound to their current definition of evidence, the USPSTF may never be able to recommend comprehensive screening for a disease that is a national epidemic.
Fortunately, the USPSTF recently issued a statement addressing physician frustration with their guidelines, specifically the “I Statement,” which plagues so many of their guidelines, including diabetes mellitus.31 In this statement, they describe a newly adopted model for issuing recommendations when evidence is lacking. This model should certainly improve their guidelines, although it is unclear whether these changes will go far enough in providing comprehensive national recommendations to physicians who rely on them. Until revised USPSTF diabetes screening recommendations are available, we advocate following the evidence-based and expert opinion—driven ADA criteria because they will find more cases of diabetes when applied to clinical practice, as our study demonstrated. We also need to be vigilant about screening patients with multiple high-risk factors and individual risk factors that have high diagnostic predictive value, such as PCOS. Furthermore, we need to ensure that screening practices are robust across all ages and primary care specialties and for uninsured patients.
CONCLUSION
Multiple factors likely contribute to the high prevalence of undiagnosed diabetes. Although this study was not designed to investigate all these aspects, it showed that physicians who fail to screen eligible patients may play a role because not all our patients who should have been screened had a glucose test. In addition, lack of health insurance decreases testing, even in this uninsured patient population presenting for routine clinic visits.
This study also demonstrated that number and type of risk factors predict diabetes, and these findings should be considered in screening efforts. More importantly, suboptimal guidelines may ultimately contribute to maintaining the large undiagnosed diabetic population. Specifically compared with ADA recommendations, the new USPSTF guidelines result in a lower number of patients eligible for screening and decrease case finding significantly. Health care professionals need to be aware of the blind spots in daily screening practices to effectively and promptly reduce the prevalence of undiagnosed diabetes in the the United States.
Supplementary Material
Acknowledgments
We thank Gregory Sheehy, MD, for critical review of the submitted manuscript, Michael Barbouche, MS, and Jeff Pier, BS, for database support and technical assistance, and Melissa Meredith, MD, and Teresa Darcy, MD, for assistance with laboratory test interpretation.
Footnotes
This study was supported by the University of Wisconsin Department of Medicine, and the Health Innovation Program and the Community Academic Partnerships core of the University of Wisconsin Institute for Clinical and Translational Research (UW ICTR), grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
REFERENCES
- 1.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and prediabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care 2009February;32(2):287-294 Epub 2008 Nov 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999-2002. Diabetes Care 2006;29(6):1263-1268 [DOI] [PubMed] [Google Scholar]
- 3.Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005-2050. Diabetes Care 2006;29(9):2114-2116 [DOI] [PubMed] [Google Scholar]
- 4.Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4-7 yr before clinical diagnosis. Diabetes Care 1992;15(7):815-819 [DOI] [PubMed] [Google Scholar]
- 5.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008October9;359(15):1577-1589 Epub 2008 Sep 10 [DOI] [PubMed] [Google Scholar]
- 7.Chalmers J, Cooper M. UKPDS and the legacy effect [editorial]. N Engl J Med. 2008;359(15):1618-1620 [DOI] [PubMed] [Google Scholar]
- 8.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20(7):1183-1197 [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association Standards of medical care in diabetes-2009. Diabetes Care 2009;32(suppl 1):S13-S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Preventive Services Task Force Guide to Clinical Preventive Services. 3rd ed. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=hscps3edrec&part=A26340. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=hscps3edrec&part=A26340 Accessed December 1, 2009.
- 11.US Preventive Services Task Force Screening for type 2 diabetes mellitus in adults: U.S. Preventive Services Task Force recommendation statement [published correction appears in Ann Intern Med. 2008;149(2):147] Ann Intern Med. 2008;148(11):846-854 [DOI] [PubMed] [Google Scholar]
- 12.Sheehy AM, Coursin DB, Gabbay RA. Back to Wilson and Jungner: 10 good reasons to screen for type 2 diabetes mellitus [published correction appears in Mayo Clin Proc. 2009;84(2):208] Mayo Clin Proc. 2009;84(1):38-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatahet MA, Bowhan J, Clough EA. Wisconsin Collaborative for Healthcare Quality (WCHQ): lessons learned. WMJ 2004;103(3):45-48 [PubMed] [Google Scholar]
- 14.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14(6):270-277 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization International Classification of Diseases, Ninth Revision (ICD-9) Geneva, Switzerland: World Health Organization; 1977. [Google Scholar]
- 16.Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab. 2008July;93(7):2447-2453 Epub 2008 May 6 [DOI] [PubMed] [Google Scholar]
- 17.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009July;32(7):1327-1334 Epub 2009 Jun 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Bairney Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines [published correction appears in Circulation. 2004;110(6):763] Circulation 2004;110:227-239 [DOI] [PubMed] [Google Scholar]
- 19.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27 [DOI] [PubMed] [Google Scholar]
- 20.Chronic Condition Data Warehouse User Manual, Version 2.0 West Des Moines, IA: Iowa Foundation for Medical Care; January2007. [Google Scholar]
- 21.Segars LW, Lea AR. Assessing prescriptions for statins in ambulatory diabetic patients in the United States: a national cross-sectional study. Clin Ther. 2008;30(11):2159-2166 [DOI] [PubMed] [Google Scholar]
- 22.Goldstein LB. Accuracy of ICD9-CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke 1998;29(8):1602-1604 [DOI] [PubMed] [Google Scholar]
- 23.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke 2002;33(10):2465-2470 [DOI] [PubMed] [Google Scholar]
- 24.US Census Bureau United States 2005-2007 table S0101.Age and sex: American community survey 3-year estimates. http://factfinder.census.gov/servlet/STTable?_bm=y&-geo_id=01000US&-qr_name=ACS_2007_3YR_G00_S0101&-ds_name=ACS_2007_3YR_G00_. http://factfinder.census.gov/servlet/STTable?_bm=y&-geo_id=01000US&-qr_name=ACS_2007_3YR_G00_S0101&-ds_name=ACS_2007_3YR_G00_ Accessed August 4, 2009.
- 25.US Department of Health and Human Services. United States Preventive Services Task Force. Agency for Healthcare Research and Quality About USPSTF. http://www.ahrq.gov/clinic/uspstfab.htm. http://www.ahrq.gov/clinic/uspstfab.htm Accessed August 4, 2009.
- 26.Berg WA, Blume JD, Cormack JB, et al. ACRIN 6666 Investigators Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008;299(18):2151-2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Geiss LS, Cheng YJ, Beckles GL, Gregg EW, Kahn HS. The missed patient with diabetes: how access to health care affects the detection of diabetes. Diabetes Care 2008;31(9):1748-1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaneyfelt TM, Centor RM. Reassessment of clinical practice guidelines: go gently into that good night [editorial]. JAMA 2009;301(8):868-869 [DOI] [PubMed] [Google Scholar]
- 29.Sniderman AD, Furberg CD. Why guideline-making requires reform. JAMA 2009;301(4):429-431 [DOI] [PubMed] [Google Scholar]
- 30.Norris SL, Kansagara D, Bougatsos C, Fu R. Screening adults for type 2 diabetes: a review of evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;148(11):855-868 [DOI] [PubMed] [Google Scholar]
- 31.Petitti DB, Teutsch SM, Barton MB, Sawaya GF, Ockene JK, DeWitt T, US Preventive Services Task Force Update on the methods of the U.S. Preventive Services Task Force: insufficient evidence. Ann Intern Med. 2009;150(3):199-205 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.