Abstract
Catecholamines, thought to derive from the extrinsic innervation of the ovary, participate in the regulation of ovarian development and mature gonadal function. Recently, intraovarian neurons containing tyrosine hydroxylase (TH), the rate-limiting enzyme in catecholamine biosynthesis, were described in the ovary of nonhuman primates. We now show that the primate ovary expresses both the genes encoding TH and dopamine β-hydroxylase (DBH), the key enzymes in norepinephrine (NE) biosynthesis. Ovarian neurons were identified as a site of TH and DBH gene expression, and surprisingly, oocytes were identified as an exclusive site of DBH synthesis. Oocytes contain neither TH mRNA nor protein, indicating that they are unable to synthesize dopamine (DA). They did, however, express a DA transporter gene identical to that found in human brain. The physiological relevance of this transporter system and DBH in oocytes was indicated by the ability of isolated oocytes to metabolize exogenous DA into NE. Isolated follicles containing oocytes—but not those from which the oocytes had been removed—responded to DA with an elevation in cAMP levels; this elevation was prevented by propranolol, a β-adrenoreceptor antagonist. The results suggest that oocytes and somatic cells are linked by a neuroendocrine loop consisting of NE synthesized in oocytes from actively transported DA and cAMP produced by somatic follicular cells in response to NE-induced β-adrenoreceptor activation.
Keywords: female gonad/germ cells/monamines/dopamine/dopamine transporter
All three major catecholamines used as transmitters by sympathetic neurons of the peripheral nervous system are present in the mammalian ovary (1, 2). Among them, norepinephrine (NE) is the most abundant (1, 3, 4). A variety of experimental approaches have demonstrated that NE, recognized by specific receptors of the β2-adrenergic subtype, stimulates ovarian steroidogenesis on its own and facilitates the stimulatory effect of gonadotropins on steroid production (5–7). NE also promotes follicular development (8–10). In contrast to this body of information, very little is known about the potential contribution(s) of dopamine (DA) to ovarian physiology. In one of the few studies on the subject (11), a stimulatory effect of DA on human granulosa cell steroidogenesis was blocked by propranolol, a β-adrenoreceptor antagonist, indicating that the effect of the catecholamine was indirect and most likely mediated by NE.
It is commonly assumed that catecholamines reach the ovary exclusively via its extrinsic sympathetic innervation (12). However, ovarian denervation reduces, but does not eliminate, the content of NE in the gland (13), implying the existence of an additional source of catecholamine synthesis. That this source may be intrinsic to the ovary has been suggested by the identification of neuronal cell bodies containing immunoreactive tyrosine hydroxylase (TH), the rate-limiting enzyme in catecholamine biosynthesis, in the ovary of nonhuman primates (14). Whether this is the only, or the more important, intragonadal source of catecholamines in the primate ovary is not known. Because of the potential importance of locally produced neurotransmitters in the control of ovarian function, we sought to explore this issue further. The present experiments were conducted to determine whether the primate ovary is endowed with the molecular components required for catecholamine synthesis, to identify the cellular sites where these components may be expressed, and to gain some insight into the ovarian homeostatic processes that may be subjected to the regulatory influence of this intragonadal catecholaminergic system(s).
MATERIALS AND METHODS
Tissues.
Ovaries from 11 rhesus monkeys (Macaca mulatta) ranging from 1.5 to 17 years of age were used for molecular and morphological procedures. In addition, the adrenals from two of these monkeys were collected for isolation of TH and dopamine β-hydroxylase (DBH) cDNAs. Upon collection, all tissues were frozen on dry ice or immersed in the appropriate fixative for either immunohistochemistry or in situ hybridization.
Isolation of Oocytes and Antral Follicles.
Oocytes. Ten additional monkeys were subjected to bilateral oophorectomy independent of the stage of the menstrual cycle. Germinal vesicle-intact oocytes (>100 μm vitelline diameter), surrounded by at least two layers of cumulus cells, were obtained from eight pairs of ovaries. The oocytes were mechanically isolated from antral follicles at 37°C in an air-buffered modified Tyrode’s solution containing albumin, lactate, and pyruvate (TALP) (TALP-Hepes, pH 7.4) (15). Before oocyte isolation, the follicles (of ≈1 mm in diameter) were removed individually from sagittally bisected ovaries immersed in TALP-Hepes buffer by using blunt dissection under a dissecting microscope. Thereafter, the follicular wall was punctured with a 25-gauge needle and the oocytes were extruded and collected in TALP-Hepes buffer at 37°C. The oocytes then were treated for 2–5 min with hyaluronidase (1 mg/ml) followed by gentle pipetting to remove all adherent cumulus cells. Cumulus-free oocytes were washed three-times in TALP-Hepes before either RNA extraction (two pools with six and eight oocytes per pool, respectively) or assessment of DA uptake and conversion to NE (38 oocytes, from five animals).
Antral follicles.
A total of 51 small antral follicles (average diameter 1.1 ± 0.1 mm; mean ± SEM) were harvested from the ovaries of three monkeys, as outlined above. Diameters of the follicles, carrying a minimal amount of stromal tissues, were calculated from the average of two microscopic micrometer measurements. Half of the follicles were punctured, and their oocytes (including the cumulus oophorus) were extruded whereas the other half were punctured, but the oocytes were left inside. After oocyte collection, all follicles were equilibrated in TALP-Hepes at 37°C for 30 min before initiation of the treatments.
Immunohistochemistry.
Polyclonal antibodies to DBH (1:500, EugeneTech, Ridgefield Park, NJ) were used to determine the cellular distribution of DBH in the ovary by using a procedure previously described (14, 16). Control sections were incubated with normal rabbit serum. All sections were counterstained with the nuclear dye propidium iodide, which stains double-stranded DNA (Sigma; 2 μg/ml in PBS for 2 min).
Confocal Laser Scanning Microscopy.
This procedure was performed essentially as described for the detection of TH in the ovary (14) and testis (16). Stacks of 10 optical sections at 0.5-μm intervals were collected as gray scale image, projected, rescaled to normalize contrast, and then merged via adobe photoshop 2.5 (Adobe Systems, Mountain View, CA) to produce color images.
Glyoxylic Acid Histochemistry for Catecholamines.
This method was used to visualize cells containing catecholamine-derived histofluorescence, as reported (16).
Reverse transcription–PCR (RT-PCR) Cloning.
Reverse transcription was carried out as reported (17) by using 200–500 ng of total RNA and all of the RNA obtained from two oocyte pools (six and eight oocytes, respectively).
The gene-specific oligodeoxynucleotide primers used to isolate an ovarian DBH cDNA consisted of an 18-mer sense primer complementary to nucleotides 846–863 in human DBH mRNA and a 21-mer antisense primer complementary to nucleotides 1087–1107 (18). TH cDNAs were obtained by using the same primers used to isolate testicular TH sequences (16). The primers used for isolating a fragment of the monkey DA transporter gene were 19-mers. The sense primer was complementary to nucleotides 1336–1354 in the human DA transporter mRNA; the antisense primer was complementary to nucleotides 1601–1619 (19). The PCRs were carried out as described (16, 17) with modifications in the incubation times.
The identity of the putative DBH and TH PCR products initially was assessed by Southern blot analysis of the PCR products. The DBH probe used for this procedure was a 21-mer, 32P-5′-labeled oligodeoxynucleotide complementary to nucleotides 913–933 in the human DBH sequence. The TH probe used was the same used to identify testicular TH cDNAs (16). PCR products presumed to contain DA transporter cDNAs were sequenced directly without this preliminary analysis.
Nucleic Acid Probes.
The antisense RNA probes used in these studies were transcribed (20) by using as templates monkey ovarian DBH and TH cDNAs. The cRNA transcripts were radiolabeled with 32P-UTP for RNase protection assay, and with 35S-UTP for hybridization histochemistry. Digoxygenin-labeled probes (20) also were used to better visualize DBH and TH mRNA in ovarian neurons.
RNase Protection Assay.
The RNase protection assays were carried out as reported (21) by using 32P-UTP-labeled cRNAs and 20 μg of total ovarian RNA or 2–10 μg of adrenal RNA.
Hybridization Histochemistry.
The procedure used was based on the method of Simmons et al. (22), with the modifications described for ovarian tissue (23). Three ovaries collected from adult and prepubertal monkeys (3, 4, and 12 years of age) were used. Adrenal sections were examined as well (from a 4- and a 12-year-old monkey). Nonisotopic detection of TH mRNA was carried out as described (20). Control sections were incubated with the corresponding sense RNA probes.
Dopamine Uptake by Denuded Oocytes and HPLC Determination of DA Metabolites.
Cumulus free-oocytes (n = 2–5 per group) were incubated in small vials containing 45 μl of a modified TALP-medium (without BSA, phenol red or antibiotics) for 30 min at 37°C, in the absence or presence of dopamine (10 μM). Because initial experiments suggested that newly formed NE is degraded rapidly in this in vitro system, additional experiments were performed by using iproniazid (1 μM, Research Biochemicals International, Natick, MA), a blocker of monoamine oxidase (the NE metabolizing enzyme) in the presence and absence of DA (1 μM). At the end of the incubation, 15 μl of 0.4 M perchloric acid were added to each vial, and the samples were spun for 10 min at 4°C. The supernatants were frozen until analyzed by HPLC (24). The sensitivity of this method is 2 pg NE per tube.
Effect of DA on Follicular cAMP Production.
Punctured, but otherwise intact antral follicles, and follicles lacking their oocytes (one per well, in a 96-well plate) were cultured for 9 h at 37°C in a 50-μl volume under a humidified atmosphere (95% air, 5% CO2) in TALP medium containing 10 μg/ml ascorbic acid and 0.5 mM 3-isobutyl-1-methyl-xanthine (Sigma). Experimental groups were treated with DA (10 μM), or DA plus dl-propranolol (50 μM, Sigma). At the end of the incubation, media were collected and frozen until assayed for cAMP (25). Results were analyzed by using one-way ANOVA followed by the Fisher’s posthoc test.
RESULTS
Expression of the DBH and TH Genes in the Monkey Ovary.
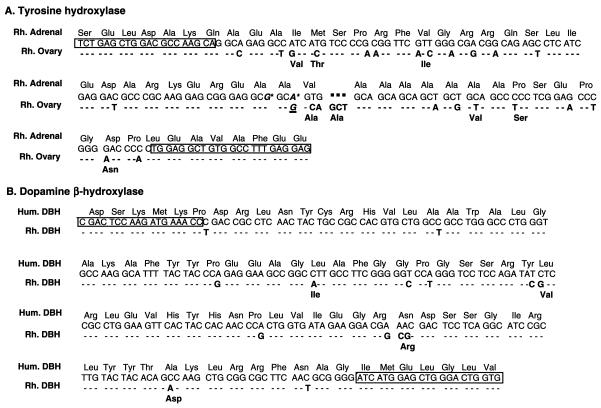
Messenger RNAs. Because the 5′ end of the mRNA encoding TH in both human and nonhuman primates is heterogeneous because of alternative splicing (26, 27), we used oligodeoxynucleotide primers complementary to conserved sequences in exons 1 and 3 of the human sequence to detect TH mRNA species that may be expressed in the monkey ovary. Four identical cDNA fragments were isolated (one from ovarian RNA and three from an ovarian cDNA library). With the exception of a silent nucleotide substitution that makes the codon identical to that in the adrenal sequence (A for G, denoted in underlined italics in Fig. 1A), ovarian TH mRNA had a sequence identical to the testicular TH mRNA form described (Fig. 1A) (16).
Figure 1.
(A) Nucleotide and amino acid sequences of a cDNA encoding the 5′ end of an ovarian form of TH mRNA type 1 isolated by RT-PCR from rhesus monkey (Rh, M. mulatta) ovaries. The ovarian sequence differs from that of testes (16), only by a silent nucleotide substitution (G instead of A, denoted by an underlined bold italics letter). The sequence is compared with that isolated from adrenal gland of M. mulatta (16). Nucleotides or amino acids in the ovarian sequence that are similar to those in the adrenal sequence are shown as dashes. Letters in bold denote nucleotide or amino acid substitutions in the ovarian sequence relative to the adrenal sequence. A three-nucleotide addition that results in an extra amino acid (Ala) is shown by a dotted line. The sequence of adrenal TH mRNA is identical to that of Macaca fuscata (26), with the exception of two conservative nucleotide substitutions (asterisks on the adrenal sequence). The latter substitution does not occur in the ovarian TH mRNA form. Boxed regions correspond to the deoxynucleotides sequences used for PCR amplification. (B) Nucleotide and amino acid sequence of a cDNA encoding the mid-portion of DBH mRNA isolated from the rhesus monkey ovary by RT-PCR. The sequence, identical to that isolated from the monkey adrenal, is compared with that of human DBH (18). For other details, see A.
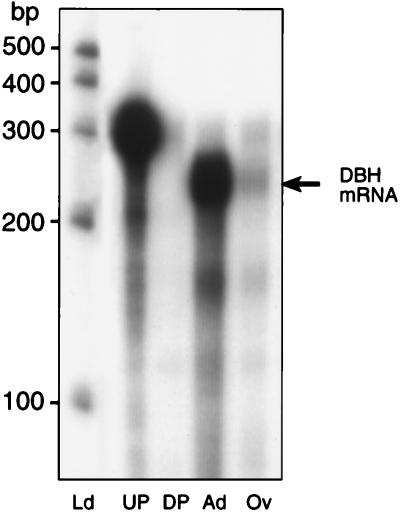
RT-PCR amplification of ovarian RNA, using primers complementary to conserved sequences of the human and rat DBH genes, yielded a cDNA encoding an mRNA species identical to monkey adrenal DBH mRNA (Fig. 1B). Three identical cDNA fragments were isolated from different PCR reactions. The degree of homology of this ovarian DBH cDNA with the human DBH sequence was 95% at both the nucleotide and amino acid level (Fig. 1B). The four amino acid substitutions found in the 87-aa sequence encoded by the PCR fragment were either conservative (Leu to Ile and Leu to Val) or semi-conservative (Asn to Arg and Ala to Asp). An antisense RNA probe transcribed from this ovarian DBH cDNA template was protected fully by both ovarian and adrenal RNA (Fig. 2), thus confirming the identity of the ovarian cDNA sequence obtained by RT-PCR to that of adrenal DBH.
Figure 2.
Detection of DBH mRNA in the monkey ovary and adrenal by RNase protection assay using an ovary-derived 32P-UTP-labeled DBH cRNA probe. Ld, ladder, RNA molecular markers; UP, undigested probe; DP, digested probe; Ad, adrenal (10 μg); Ov, ovary (20 μg).
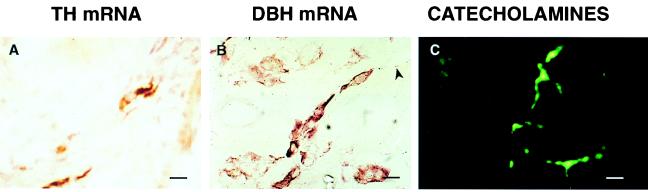
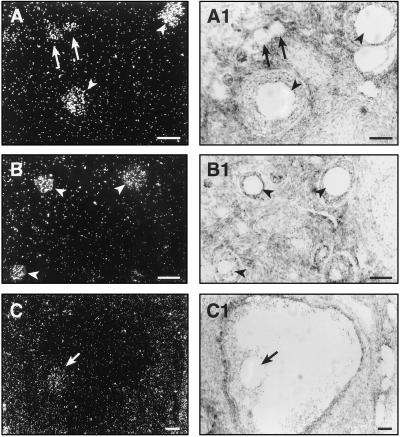
In situ hybridization experiments, using digoxygenin-UTP labeled cRNA probes, demonstrated the presence of TH mRNA in neuron-like cells (Fig. 3A). Cells with similar morphology also were found to contain DBH mRNA (Fig. 3B). That both mRNAs are active translationally was indicated by the presence of immunoreactive TH (14) and DBH (not shown) in these cells. The enzymes are active functionally as shown by the detection of catecholamine-derived glyoxylic acid histofluorescence in the neuronal cells (Fig. 3C). Additional experiments using 35S-UTP labeled cRNA probes demonstrated that, in addition to neuronal cells, DBH mRNA also was expressed in oocytes (Fig. 4). The mRNA was detected in primary (Fig. 4A), preantral (Fig. 4B), and antral follicles (Fig. 4C). No specific signal was detected when using a sense cRNA (not shown). In contrast to DBH mRNA, no TH mRNA was detected in oocytes (not shown). RT-PCR amplification of RNA from cumulus-free oocytes yielded a DBH mRNA sequence (three independent cDNA clones) identical to that isolated from the whole ovary (Fig. 1).
Figure 3.
(A) Detection of TH mRNA in ovarian neuronal cells by hybridization histochemistry by using a digoxygenin-labeled UTP-TH cRNA probe transcribed from the ovarian TH cDNA template described in Fig. 1. (B) Ovarian neuronal cells also contain DBH mRNA as determined by in situ hybridization of a digoxygenin-labeled UTP-DBH cRNA transcribed from the ovarian DBH cDNA described in Fig. 1. (C) Detection of catecholamines in ovarian neuronal cells by glyoxylic acid-derived histofluorescence. (Bars = 20 μm.)
Figure 4.
Detection of DBH mRNA in monkey oocytes by in situ hybridization using a 35S-UTP DBH cRNA transcribed from the ovarian DBH cDNA shown in Fig. 1. (A) Oocytes from primary follicles (arrows) and preantral follicles (arrowheads). (B) Oocytes from three different sizes of preantral follicles (arrowheads). (C) Oocyte (arrow) of an antral follicle. (A1–C1) Bright-field photomicrographs of the dark-field images shown in A–C. The silver grains are not apparent in the bright-field pictures because they are out of the focus plane for the underlying tissue. (Bars = 75 μm.)
DBH and TH proteins in oocytes.
Immunohistochemical localization of DBH protein by confocal scanning laser microscopy showed that oocyte DBH mRNA is translated into its protein product. DBH immunoreactivity was observed mainly in an intracellular compartment associated with the cell membrane, where it appeared to be contained in granular-like structures (Fig. 5). Some immunoreactivity also was observed in the cytoplasm. No TH protein was detected in oocytes, confirming that expression of the TH gene in the monkey ovary is limited to ovarian neurons (14).
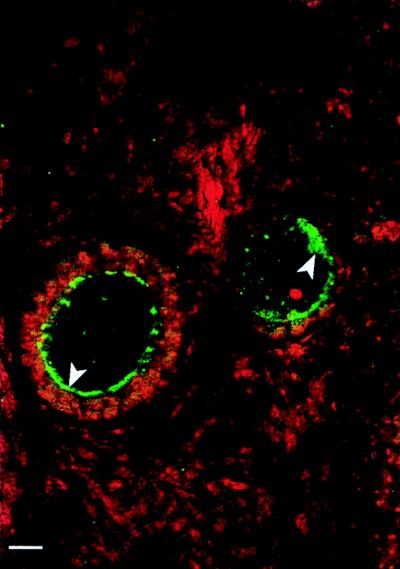
Figure 5.
Detection of DBH protein by immunohistochemistry/confocal microscopy in monkey oocytes. The DBH immunoreactivity (developed with fluorescein isothiocyanate to a green color) appears to be predominantly in subcellular granule-like structures adjacent to the cell membrane (arrowheads). Some immunoreactivity also is observed in the cytoplasm. The cell nuclei were stained (red) with propidium iodide, which binds to double stranded DNA. (Bar = 10.5 μm.)
Expression of a DA Transporter mRNA in Monkey Oocytes.
By using RT-PCR, a DA transporter cDNA was isolated from cumulus-free oocytes. The DA transporter cDNA (two independent clones) showed a high degree of homology to the human form (97% at the nucleic acid level and 100% at the amino acid level; Fig. 6) and was identical to a DA transporter cDNA isolated from the rhesus monkey brain (28).
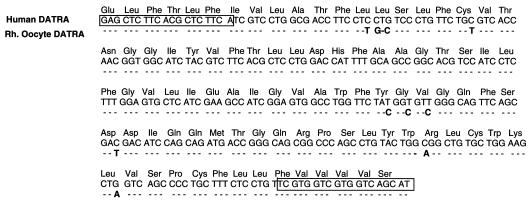
Figure 6.
Nucleotide and amino acid sequence of a cDNA encoding the rhesus monkey DA transporter (DATRA) isolated from cumulus-free monkey oocytes. The sequence is compared with that of the human gene. Nucleotides in the oocyte-derived sequence that are similar to those in human DA transporter mRNA are shown as dashes. Letters in bold denote nucleotide substitutions, and boxed regions indicate the deoxynucleotide sequences used for PCR amplification.
Biological Relevance of the Expression of DBH and a DA Transporter in Monkey Oocytes.
Isolated denuded oocytes converted exogenous DA into NE. In three of four experiments, NE was detected in the incubation medium of oocytes exposed to DA for 30 min, but not in the medium of oocytes cultured in the absence of DA (Table 1). In one experiment, only presumptive NE metabolites were detected. That newly formed NE is degraded rapidly by denuded oocytes was indicated by the finding that addition of a lower concentration of DA (1 μM instead of 10 μM), in the presence of iproniazid to reduce NE degradation, reestablished detection of NE in the culture medium (Table 1).
Table 1.
Isolated denuded oocytes metabolize DA into NE, as detected by HPLC-electrochemical detection
| Exp. | Conditions | NE released by oocytes | Oocytes per group |
|---|---|---|---|
| 1 | Control, no DA | ND | 5 |
| DA 10 μM | 2.39 ng/ml | 5 | |
| 2 | Control, no DA | ND | 5 |
| DA 10 μM | 0.60 ng/ml | 2 | |
| 3 | Control, no DA | ND | 5 |
| DA 10 μM | Metabolites | 5 | |
| 4 | Control, no DA/Ipro 1 μM | ND | 6 |
| DA 1 μM/Ipro 1 μM | 0.3 ng/ml | 5 |
Ipro, iproniazid; ND, not detectable.
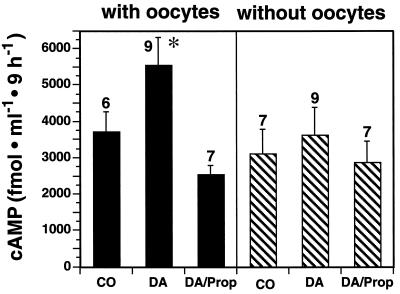
Addition of DA to single follicles containing their oocytes resulted in increased cAMP levels in the culture medium (Fig. 7). This increase was not observed in follicles without oocytes or if the β-receptor antagonist propranolol was added to the incubation medium of oocyte-intact follicles (Fig. 7). Isolated oocytes failed to respond to DA with cAMP formation (76 fmol/ml in control vs. 72 fmol/ml in DA-treated oocytes, n = 3 oocytes/group).
Figure 7.
DA induces cAMP formation in ovarian follicles only in the presence of the oocyte, and the effect is blocked by a β-adrenoreceptor antagonist. Stripped columns, antral follicles without oocytes; dark columns, follicles containing oocytes; CO, control; DA, DA at 10 μM; DA/Prop, DA at 10 μm and propranolol at 50 μM. The number of independent observations per group is indicated above each bar. ∗, P < 0.05 vs. control and DA/Prop-treated groups.
DISCUSSION
A still intriguing, but long-known, aspect of early vertebrate development is the presence of monoamines in ova, both before and after fertilization (29). In fact, the expression of neurotransmitters in ova and early embryos appears to be a highly conserved feature in evolution, as monoamines—including DA, NE, and serotonin—have been detected not only in fertilized eggs of vertebrates, such as teleosts, toads, and frogs (29), but also in invertebrates, such as molluscs, polychetes, and echinoderms (30). Studies in sea urchins demonstrated the ability of both unfertilized and fertilized eggs to take up DA and showed that the uptake is more prominent before fertilization (31). That the mammalian egg is also capable of incorporating and/or synthesizing catecholamines was suggested by the earlier experiments of Burden and Lawrence (32), who, using a fluorescence method, detected catecholamine-like activity in rat ova, zygotes and 2–4 cell embryos.
It is thus apparent that the presence of neurotransmitters in ova is a phylogenetically conserved feature of early embryogenesis. It also appears that, at least in some species, neurotransmitters may be required for egg cleavage (29, 31). We now show that there are two embryologically unrelated cell types able to produce catecholamines in the mammalian ovary: neuronal cells located in the interstitial gland and oocytes. Although neuronal cells contain TH and DBH, the two enzymes required for NE biosynthesis, oocytes appear to have developed a less conventional system of NE production. They lack TH but express both a DA transporter gene and DBH, the enzyme required for NE synthesis.
With regard to the neuronal cells, we demonstrated (14) that the primate ovary contains a network of neuron-like cells, some of which are immunopositive for TH. In the present study, we show that ovarian neurons, also containing immunoreactive DBH, express the mRNAs encoding both TH and DBH and exhibit catecholamine-derived glyoxylic acid histofluorescence. Although we did not perform studies to demonstrate colocalization of TH and DBH in the same cells, the morphological similarity of TH- and DBH-expressing cells suggest that the same neurons are able to metabolize tyrosine to NE and thus represent an intragonadal source of the catecholamine.
Sequence analysis of TH and DBH cDNAs isolated from ovarian RNA demonstrated that ovarian DBH mRNA is identical to that encoding the enzyme in the adrenal gland. In contrast, ovarian TH mRNA is similar but not identical to adrenal TH mRNA type I and indistinguishable from the TH mRNA form recently found in the nonhuman primate testis (16). As in the case of the testis, no evidence for the existence of the additional TH mRNA variants described in human and nonhuman primate nervous tissue (26, 27) was found in the primate ovary. Although the detection of catecholamine-synthesizing cells in the ovary was initially surprising (14, 16), it is now clear that other nonneuronal tissues are endowed with similar systems. Thus, an intrinsic adrenergic cell system has been identified recently in the heart (33), and nonneuronal DA- producing cells have been found in the exocrine pancreas (34), suggesting that the distribution of intrinsic catecholaminergic systems is widespread and of importance for the local regulation of tissue functions.
The present study demonstrates that, in addition to neuronal cells, oocytes are also able to produce NE and thus represent an intragonadal source of catecholamines in the primate ovary. Surprisingly, oocytes contain DBH, but not TH, suggesting that, if they were to produce NE, the necessary DA precursor would have to derive from an extrinsic source. Detection in monkey oocytes of a cDNA DA transporter strongly suggests the involvement of this transporter system in the process by which the DA required for NE synthesis becomes available to oocytes. The contribution of a DA receptor appears unlikely, as no evidence was found for the presence of DA receptors in monkey oocytes (A.M. and S.R.O., unpublished data). Confocal microscopy revealed that a substantial fraction of DBH is associated with the cell membrane and thus is immediately available to DA transported across the membrane. The large number of membrane-bound granules present in oocytes (35) may represent the structural component to which DBH is associated. As in neural cells (18, 36), some DBH immunoreactivity also was seen in the oocyte’s cytoplasm.
That oocytes are able to synthesize NE from exogenous DA was demonstrated by experiments in which NE was detected in the culture medium of isolated oocytes exposed to DA. Though seemingly low (0.3–2.4 ng/ml), the NE values attained in the presence of DA are consistent with the NE levels (1.7–2.9 ng/ml) detected by HPLC in the follicular fluid of pig ovaries (3) and are well within the concentrations (10−9 to 10−8 M) required for the activation of β-adrenoreceptors (5).
The presence of a catecholamine-producing system in oocytes raises two important questions: What are the sources of DA that may be used by oocytes to produce NE, and what is the physiological significance of such a highly specialized system? DA may derive from at least three nonexclusive sources: nerve endings associated with the ovarian follicles, the blood stream, and ovarian catecholaminergic neurons. In all cases, DA would have to reach the oocyte from its site of production, via a diffusion process that has been well demonstrated in other systems (37). That DA is indeed available to the oocyte under physiological conditions is indicated by the detection of the catecholamine in the follicular fluid of both sow (3, 4) and human ovaries (38).
With regard to the physiological significance of oocyte-derived NE, our experiments do not identify the follicular processes affected by the catecholamine, but they implicate cAMP as a potential mediator of such effects. Exposure of single small antral follicles containing their oocytes to DA resulted in an increase in cAMP levels comparable to that observed when mouse cumulus cell–oocyte complexes are exposed to a small dose of follicle-stimulating hormone (39). Only follicles containing oocytes responded to DA with cAMP formation, and this change was prevented by blockade of β-adrenoreceptors. Because oocytes themselves did not release cAMP in response to DA, and granulosa cells are endowed with β-adrenoreceptors (2, 6), the most plausible explanation is that NE released from the oocytes activates the production of cAMP from the surrounding granulosa cells by interacting with β-adrenoreceptors located on these cells. Gap-junctional communication between the oocyte and the surrounding granulosa cells and between granulosa cells themselves is extensive (40). It is thus plausible that in intact follicles the production of small amounts of NE by the oocyte would suffice to activate cAMP formation by granulosa cells and, consequently, to set in motion the neuroendocrine regulatory loop suggested by the present experiments. Oocyte-derived NE may contribute, via cAMP formation (35), to regulating oocyte maturation. This function notwithstanding, the presence of DBH in oocytes from preantral follicles, which are not competent to resume meiosis, indicates that oocyte-derived catecholamines may be involved in other developmental functions independent of meiotic competence, such as the oocyte-dependent regulation of granulosa cell function.
Recent studies have identified two key pathways underlying the reciprocal oocyte–granulosa cell communication system. One of them consists of an oocyte-derived factor(s) that inhibits progesterone secretion and stimulates estradiol formation by cumulus granulosa cells (41). The other is provided by granulosa cell-derived C29 sterols, which act on the oocyte to induce a resumption of meiosis (42). Whereas the former pathway may be important for follicular growth, because it prevents premature luteinization, the second may represent one of the somatic cell-derived signals necessary for resumption of meiosis following the preovulatory surge of gonadotropins. The contribution of oocyte-derived NE to these regulatory pathways remains to be defined.
By identifying in monkey oocytes the molecular components required for catecholamine synthesis and demonstrating the viability of this system in an in vitro context, the present study highlights the importance that locally produced catecholamines may have for the regulation of ovarian function in primate species, including human. By doing so, the results also support the emerging concept that, from rodent to primates, neuronal signals both extrinsic and intrinsic to the ovary are involved in the developmental regulation of follicular homeostasis.
Acknowledgments
Supported by grants from the National Institutes of Health (HD24870 to S.R.O., HD18185, and RR00163), the National Science Foundation (IBN 9396063 and 9631765 to M.D.), the Deutsche Forschungsgemeinschaft, Germany (Heisenberg Grant Ma 1080/4-1 and Ma 1080/10-1 to A.M.), and Volkswagen Stiftung (to A.M.).
ABBREVIATIONS
- TH
tyrosine hydroxylase
- DBH
dopamine β-hydroxylase
- NE
norepinephrine
- DA
dopamine
- RT-PCR
reverse transcription–PCR
- TALP
Tyrode’s solution containing albumin, lactate, and pyruvate
Footnotes
References
- 1.Ben-Jonathan N, Arbogast L A, Rhoades T A, Bahr J M. Endocrinology. 1984;115:1426–1431. doi: 10.1210/endo-115-4-1426. [DOI] [PubMed] [Google Scholar]
- 2.Aguado L I, Ojeda S R. Endocrinology. 1984;114:1845–1853. doi: 10.1210/endo-114-5-1845. [DOI] [PubMed] [Google Scholar]
- 3.Bahr J M, Ben-Jonathan N. Endocrinology. 1985;117:620–623. doi: 10.1210/endo-117-2-620. [DOI] [PubMed] [Google Scholar]
- 4.Fernández-Pardal J, Gimeno M F, Gimeno A L. Biol Reprod. 1986;34:439–445. doi: 10.1095/biolreprod34.3.439. [DOI] [PubMed] [Google Scholar]
- 5.Aguado L I, Petrovic S L, Ojeda S R. Endocrinology. 1982;110:1124–1132. doi: 10.1210/endo-110-4-1124. [DOI] [PubMed] [Google Scholar]
- 6.Adashi E Y, Hsueh A J W. Endocrinology. 1981;108:2170–2178. doi: 10.1210/endo-108-6-2170. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez E R, Jimenez J L, Payne D W, Adashi E Y. Endocrinology. 1988;122:1592–1602. doi: 10.1210/endo-122-4-1592. [DOI] [PubMed] [Google Scholar]
- 8.Lara H E, McDonald J K, Ojeda S R. Endocrinology. 1990;126:364–375. doi: 10.1210/endo-126-1-364. [DOI] [PubMed] [Google Scholar]
- 9.Curry T E, Jr, Lawrence I E, Jr, Burden H W. Cell Tissue Res. 1984;236:257–263. doi: 10.1007/BF00214226. [DOI] [PubMed] [Google Scholar]
- 10.Mayerhofer A, Dissen G A, Costa M E, Ojeda S R. Endocrinology. 1997;138:3320–3329. doi: 10.1210/endo.138.8.5335. [DOI] [PubMed] [Google Scholar]
- 11.Bódis J, Tinneberg H R, Török A, Cledon P, Hanf V, Papenfuss F. Acta Endocrinol. 1993;129:165–168. doi: 10.1530/acta.0.1290165. [DOI] [PubMed] [Google Scholar]
- 12.Mohsin S, Pennefather J N. Clin Exp Pharmacol Physiol. 1979;6:335–354. doi: 10.1111/j.1440-1681.1979.tb01255.x. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence I E, Jr, Burden H W. Anat Rec. 1980;196:51–59. doi: 10.1002/ar.1091960106. [DOI] [PubMed] [Google Scholar]
- 14.Dees W L, Hiney J K, Schultea T D, Mayerhofer A, Danilchik M, Dissen G A, Ojeda S R. Endocrinology. 1995;136:5760–5768. doi: 10.1210/endo.136.12.7588334. [DOI] [PubMed] [Google Scholar]
- 15.Boatman D E. In: The Mammalian Preimplantation Embryo: Regulation of Growth and Differentiation In Vitro. Bavister B D, editor. New York: Plenum; 1987. pp. 273–308. [Google Scholar]
- 16.Mayerhofer A, Danilchik M, Pau K-Y F, Lara H E, Russell L D, Ojeda S R. Biol Reprod. 1996;55:509–518. doi: 10.1095/biolreprod55.3.509. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y J, Costa M E, Ojeda S R. Neuroendocrinology. 1994;60:346–359. doi: 10.1159/000126769. [DOI] [PubMed] [Google Scholar]
- 18.Lamouroux A, Vigny A, Faucon Biguet N, Darmon M C, Franck R, Henry J-P, Mallet J. EMBO J. 1987;6:3931–3937. doi: 10.1002/j.1460-2075.1987.tb02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giros B, Mestikawy S E, Godinot N, Zheng K, Han H, Yang-Feng T, Caron M G. Mol Pharmacol. 1992;42:383–390. [PubMed] [Google Scholar]
- 20.Berg-von der Emde K, Dees W L, Hiney J K, Hill D F, Dissen G A, Costa M E, Moholt-Siebert M, Ojeda S R. J Neurosci. 1995;15:4223–4237. doi: 10.1523/JNEUROSCI.15-06-04223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Y J, Dissen G A, Rage F, Ojeda S R. Methods Companion Methods Enzymol. 1996;10:273–278. doi: 10.1006/meth.1996.0102. [DOI] [PubMed] [Google Scholar]
- 22.Simmons D M, Arriza J L, Swanson L W. J Histotechnol. 1989;12:169–181. [Google Scholar]
- 23.Dissen G A, Newman Hirshfield A, Malamed S, Ojeda S R. Endocrinology. 1995;136:4681–4692. doi: 10.1210/endo.136.10.7664689. [DOI] [PubMed] [Google Scholar]
- 24.Levine J E, Wolfe A M, Porkka-Heiskanen T, Meredith J M, Norgle J R, Turek F W. Methods Neurosci. 1994;20:129–161. [Google Scholar]
- 25.Ojeda S R, Urbanski H F, Katz K H, Costa M E. Brain Res. 1988;441:339–351. doi: 10.1016/0006-8993(88)91412-6. [DOI] [PubMed] [Google Scholar]
- 26.Ichikawa S, Ichinose H, Nagatsu T. Biochem Biophys Res Commun. 1990;173:1331–1336. doi: 10.1016/s0006-291x(05)80933-7. [DOI] [PubMed] [Google Scholar]
- 27.Grima B, Lamouroux A, Boni C, Julien J-F, Javoy-Agid F, Mallet J. Nature (London) 1987;326:707–711. doi: 10.1038/326707a0. [DOI] [PubMed] [Google Scholar]
- 28.Choi W S, Ronnekleiv O K. Dev Brain Res. 1996;96:249–260. doi: 10.1016/0165-3806(96)00123-x. [DOI] [PubMed] [Google Scholar]
- 29.Buznikov G A. Adv Exp Med Biol. 1991;296:33–48. doi: 10.1007/978-1-4684-8047-4_5. [DOI] [PubMed] [Google Scholar]
- 30.Deeb S S. J Exp Zool. 1972;181:79–86. [Google Scholar]
- 31.Carginale V, Borrelli L, Capasso A, Parisi E. Mol Reprod Dev. 1995;40:379–385. doi: 10.1002/mrd.1080400315. [DOI] [PubMed] [Google Scholar]
- 32.Burden H W, Lawrence I E., Jr Am J Anat. 1973;136:251–257. doi: 10.1002/aja.1001360210. [DOI] [PubMed] [Google Scholar]
- 33.Huang M-H, Friend D S, Sunday M E, Singh K, Haley K, Austen K F, Kelly R A. J Clin Invest. 1996;98:1298–1303. doi: 10.1172/JCI118916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mezey E, Eisenhofer G, Harta G, Hansson S, Gould L, Hunyady B, Hoffman B J. Proc Natl Acad SciUSA. 1996;93:10377–10382. doi: 10.1073/pnas.93.19.10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wassarman P M, Albertini D F. In: The Physiology of Evolution. Knobil E, Neill J D, editors. New York: Raven; 1994. pp. 79–122. [Google Scholar]
- 36.Potter L T, Axelrod J. J Pharmacol Exp Ther. 1963;142:291–298. [PubMed] [Google Scholar]
- 37.Kang U J, Fisher L J, Joh T H, O’Malley K L, Gage F H. J Neurosci. 1993;13:5203–5211. doi: 10.1523/JNEUROSCI.13-12-05203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bódis J, Hartmann G, Török A, Bognár Z, Tinneberg H-R, Cledon P, Hanf V. Exp Clin Endocrinol. 1993;101:178–182. doi: 10.1055/s-0029-1211227. [DOI] [PubMed] [Google Scholar]
- 39.Tirone E, D’Alessandris C, Hascall V C, Siracusa G, Salustri A. J Biol Chem. 1997;272:4787–4794. doi: 10.1074/jbc.272.8.4787. [DOI] [PubMed] [Google Scholar]
- 40.Eppig J J. BioEssays. 1991;13:569–574. doi: 10.1002/bies.950131105. [DOI] [PubMed] [Google Scholar]
- 41.Vanderhyden B C, Tonary A M. Biol Reprod. 1995;53:1243–1250. doi: 10.1095/biolreprod53.6.1243. [DOI] [PubMed] [Google Scholar]
- 42.Byskov A G, Andersen C Y, Nordholm L, Thogersen H, Xia G, Wassmann O, Andersen J V, Guddal E, Roed T. Nature (London) 1995;374:559–562. doi: 10.1038/374559a0. [DOI] [PubMed] [Google Scholar]