Abstract
Apoptosis is controlled by a signaling equilibrium between prosurvival and proapoptotic pathways, such that unwanted apoptosis is avoided, but when required it occurs rapidly and efficiently. Many apoptosis regulators display dual roles, depending upon whether a cell has received an apoptotic stimulus or not. Here, we identify a novel and unexpected function for X-linked inhibitor of apoptosis (XIAP) that occurs when apoptosis is triggered under physiological conditions. We show that in response to loss of survival signals provided by cell adhesion, endogenous XIAP translocates from the cytosol into a mitochondrial 400-kDa complex and that this occurs very early in the apoptosis process. Membrane-associated XIAP induces mitochondrial outer membrane permeabilization leading to cytochrome c and Smac release, which is dependent on Bax and Bak. Thus, although XIAP suppresses apoptosis in healthy cells, our data indicate that XIAP may contribute to it in response to a proapoptotic signal such as loss of extracellular matrix-dependent survival signaling. We suggest that, as with Bcl-2 family proteins, more diverse functions for XIAP exist than previously identified. Moreover, switching the function of proteins from anti- to proapoptotic forms may be a common theme in the efficient execution of cell death.
Introduction
A tightly regulated balance between apoptosis and survival is critical for the development and homeostasis of metazoans. Cellular damage, or loss of survival signaling, triggers the intrinsic apoptosis pathway, resulting in activation of the caspase family of proteases (1, 2). Caspases are subject to regulation by the conserved inhibitor of apoptosis (IAP)2 family. IAPs can bind directly to caspases, leading to either their inactivation or degradation (3–5). The most potent caspase inhibitor of the IAP family is X-linked IAP (XIAP), whose potential to inhibit apoptosis downstream of the mitochondria is well documented (6, 7).
Mounting evidence suggests that many apoptotic regulatory proteins have roles in both cell survival and death. A switch in protein function drives quick and efficient suicide once an apoptotic signal is received. For example, in addition to their well established proapoptotic roles, the Bcl-2 family proteins Bid and Bad, are (respectively) involved in maintaining cellular survival following DNA damage or conditions of altered glucose metabolism (8, 9). Similarly, Bax and Bak control normal mitochondrial dynamics in the absence of death stimuli (10). It is becoming increasingly clear that the activities of IAPs are also not restricted to that of caspase regulation, and here we investigate the possibility that they may also be proapoptotic regulators (11).
Anoikis is a form of apoptosis resulting from the loss of extracellular matrix (ECM)-mediated survival signals (12–14). In mammary epithelial cells, anoikis occurs in the absence of new protein synthesis, arguing that the cells must constitutively express all of the necessary components of the apoptosis program (15). Detachment of mammary cells from ECM causes proapoptotic Bcl-2 family proteins, such as Bax and Bid, to translocate to mitochondria and form high molecular weight protein complexes, priming cells for apoptosis and ultimately inducing mitochondrial outer membrane permeabilization (MOMP) (16–19). The molecules involved in controlling commitment to MOMP are not well characterized. However, nonclassical regulators of MOMP have recently been shown to be recruited to mitochondria where they influence cytochrome c release (20).
Here, we show that following the removal of ECM-dependent survival signals, endogenous XIAP translocates to the mitochondrial membrane fraction prior to MOMP and caspase activation, where it forms a novel ∼400-kDa complex. Exogenously expressed XIAP constitutively associates with mitochondria and induces cytochrome c and Smac release in a Bax/Bak-dependent manner. This ability of XIAP to promote MOMP is separate from its better understood role in caspase activation. We suggest that the loss of ECM survival signals results in a change of XIAP function from an antiapoptotic to a proapoptotic molecule, thereby contributing to the intrinsic apoptosis pathway.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Unless otherwise stated, chemical reagents were obtained from Sigma. Monoclonal anti-XIAP (clone 2F1) and anti-Apaf-I were from Bioquote. Monoclonal mtHSP70 (MA3-028) was from Affinity Bioreagents. Anti-calnexin (C4731) was from Sigma. Monoclonal anti-cytochrome c (556432) and anti-GM130 were from BD Biosciences. Anti-histone H3, anti-cleaved caspase-3, and polyclonal anti-Myc were from Cell Signaling Technology, and polyclonal anti-Bax NT (06-499) was from Upstate Biotechnology. The LAMP-1 antibody (clone H4A3) was from the Developmental Studies Hybridoma Bank, Iowa City, IA. Horseradish peroxidase-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories.
Cells
Primary mammary epithelial cells were isolated as described (17) and cultured on collagen-coated dishes in Ham's F12 supplemented with 10% fetal calf serum, 50 μg/ml gentamycin, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.25 mg/ml Fungizone, 10 ng/ml epidermal growth factor, 5 μg/ml insulin, and 1 μg/ml hydrocortisone. FSK7 mouse mammary epithelial cells (MECs) were cultured in Dulbecco's modified Eagle's medium/F12 supplemented with 2% fetal calf serum, 10 ng/ml epidermal growth factor, 5 μg/ml insulin, 100 units/ml penicillin, and 100 μg/ml streptomycin. Wild type mouse embryonic fibroblasts (MEFs) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 100 units/ml penicillin and 100 μg/ml streptomycin. SV40-transformed Bax−/−;Bak−/− double knock-out (DKO) MEFs were kindly provided by Nika Danial (Dana Faber, Boston) and were cultured as above with the addition of 5 μm β-mercaptoethanol.
Plasmids and Transfections
XIAP IMAGE clone (IMAGE 5532247) was from MRC Geneservice. Myc-survivin was a kind gift from Stephen Taylor (University of Manchester, U.K.). Monomeric RFP (mRFP) fusion proteins were generated using pmRFPc1, a gift from Roger Tsien (University of California, San Diego). The XIAP D148A/W310A caspase-binding mutant was generously provided by Shawn Bratton (University of Texas, Austin). The XIAP C450A E3 ligase mutant was generated using QuikChange mutagenesis (Stratagene). Cells were plated at 50–80% confluence onto tissue culture dishes or coverslips and transfected as described (18). In control experiments, 0.05 μg of DNA/35-mm dish of cells caused half-maximal percent cytochrome c release, and this amount of DNA resulted in a similar level of RFP-XIAP protein as endogenous XIAP. For most experiments, we used 0.5 μg of DNA to visualize the transfected protein more easily.
Immunofluorescence
Cells were either fixed in 4% formaldehyde in phosphate-buffered saline and permeabilized with 0.2% Triton X-100/phosphate-buffered saline or subjected to a 50 μg/ml digitonin permeabilization for 5 min at 4 °C prior to fixation. Following incubation with primary antibodies, cells were incubated with Alexa Fluor-conjugated secondary antibodies and then stained with DAPI prior to mounting. A minimum of 300 cells were counted using an Axioplan2 microscope (Carl Zeiss MicroImaging). In co-localization experiments using RFP-XIAP together with mtHSP70, p11Bidv5, calnexin, GM130, and Lamp1, cytosolic RFP-XIAP was removed by permeabilizing cells with 50 mg/ml digitonin prior to fixation, to visualize the membrane associated RFP-XIAP. Note that p11Bidv5 has no apoptotic effect on cells (21). Images of XIAP membrane localization were collected on a Deltavision imaging system Olympus IX70 microscope using a ×63 PLAN-APO 1.4 NA objective. Statistical analysis was performed using Student's unpaired t test to obtain p values (***, p < 0.01; *, p < 0.05).
Cell Fractionation and Immunoblotting
Whole cell lysis was performed using radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS), supplemented with protease and phosphatase inhibitors (Calbiochem). Following primary antibody incubation, detection was performed using chemiluminescence (Pierce) with horseradish peroxidase-conjugated secondary antibodies.
For fractionation, cells were scraped or resuspended into chilled hypotonic buffer (50 mm Tris, pH 7.4, 10 mm NaCl, 1.5 mm MgCl2 plus protease inhibitors) and allowed to swell on ice for 10 min prior to homogenizing with a glass Dounce homogenizer. Homogenates were adjusted to 150 mm NaCl and spun (100,000 × g, 30 min, 4 °C) to obtain the cytosolic fraction. The remaining pellet, containing total membranes, was separated into heavy membrane (containing mitochondria and endoplasmic reticulum) and nuclear fractions by resuspending in 1% CHAPS, 10% glycerol, 0.75 m aminocaproic acid and incubation for 15-min on ice, followed by centrifugation (100,000 × g, 30 min, 4 °C). The resulting pellet, which is enriched for nuclei, was solubilized by boiling in SDS lysis buffer (2% SDS, 50 mm Tris, pH 7.4).
Blue Native PAGE
Analysis of protein complexes in cytosolic and membrane fractions was performed by BN-PAGE, as described (22) and using our protocol detailed in Ref. 23. Following fractionation (as described above), the protein concentration of the cytosol and the CHAPS-soluble membrane fractions were determined by BCA assay (Pierce), with bovine serum albumin as the standard. 40 μg of total protein, in Coomassie loading buffer (5% Coomassie Blue G-250 in 0.5 m aminocaproic acid) was resolved on a 3–15% gradient gel that was electrophoresed at 2–4 mA overnight at 4 °C. For the second dimension, a lane from BN-PAGE was cut into 15 slices, and after boiling in 2% SDS, 50 mm Tris-HCl, pH 6.8, 0.2 m dithiothreitol, and 2 m urea, the fractions were resolved by SDS-PAGE (10% gels) before immunoblotting.
RESULTS AND DISCUSSION
Endogenous XIAP Translocates to the Mitochondrial Membrane Fraction Early during Anoikis
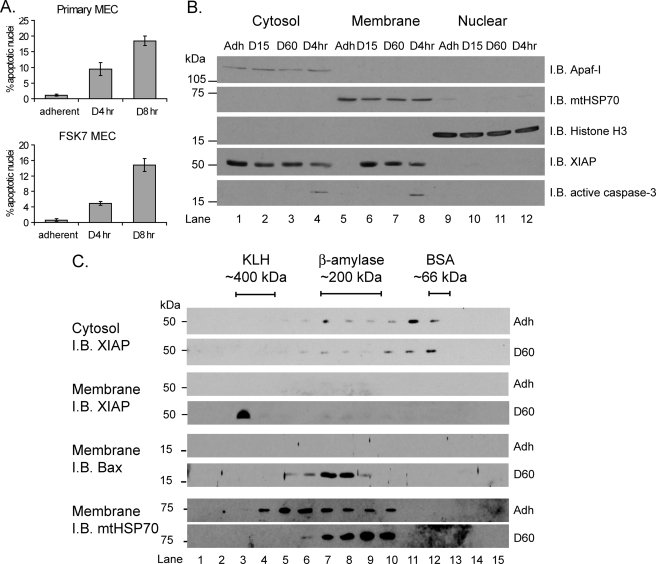
Following detachment of mammary epithelial cells from the ECM, the proapoptotic Bcl-2 family protein Bax translocates from the cytosol to mitochondria within 15 min (15, 16). Despite this rapid translocation, MOMP and cell death do not occur for a number of hours. To determine whether known antiapoptotic proteins are involved in delaying Bax-mediated MOMP during anoikis, we examined whether the localization of endogenous XIAP changed following detachment from the ECM. We used the mammary epithelial cell line FSK7 (referred to as MEC in this paper), which undergoes anoikis with kinetics similar to primary cells (Fig. 1A) (16). In adherent cells, endogenous XIAP was present exclusively in the cytosol (Fig. 1B, compare lanes 1, 5, and 9). Following detachment from the ECM, a proportion of XIAP associated with the mitochondrial membrane fraction (Fig. 1B, lanes 6–8). The enrichment of XIAP in the membrane fraction occurred within 15 min following detachment (lane 6). Caspase-3 cleavage was only apparent ∼4 h after detachment. Thus, following loss of ECM attachment, XIAP rapidly translocates from the cytosol to mitochondrial fraction, and this occurs prior to cell death.
FIGURE 1.
XIAP forms high molecular weight membrane-associated complexes in response to ECM withdrawal. A, primary mammary epithelial cells or FSK7 MECs were either left adherent or detached and plated onto poly(2-hydroxyethyl methacrylate)-coated dishes for 4 h (D4hr) or 8 h (D8hr). Cells were stained with DAPI, and apoptosis was scored by counting the percentage of nuclei with apoptotic morphology. Data represent the average of three independent experiments ± S.E. B, MECs were either left adherent (Adh) or detached for 15 min (D15), 60 min (D60), or 4 h (D4hr). Cells were then fractionated into cytosolic, membrane, and nuclear fractions and immunoblotted (I.B.) with the antibodies shown. Apaf-I, mtHSP70, and histone H3 were used as loading controls for each fraction, respectively. C, MECs were either left adherent (Adh) or detached for 60 min (D60). Cytosolic or membrane fractions were separated by BN-PAGE (first dimension), then SDS-PAGE (second dimension), and immunoblotted with the antibodies shown. Keyhole limpet hemocyanin (KLH), β-amylase, and bovine serum albumin (BSA) protein standards were used to calibrate the BN-PAGE. Membrane fractions were reprobed with anti-Bax and anti-mtHSP70.
We have demonstrated that after translocation to mitochondria, Bax associates with a high molecular weight complex prior to MOMP (23). To determine whether membrane-associated XIAP is present in protein complexes, two-dimensional BN-PAGE of cytosol and membrane fractions was performed. In adherent cells, endogenous cytosolic XIAP was in complexes of between ∼250 and ∼75 kDa (Fig. 1C, top panel, lanes 6–12). Following detachment from the ECM for 60 min (D60), the endogenous XIAP in the mitochondrial fraction was in complexes of ∼400 kDa (Fig. 1C, lane 3). In contrast, Bax complexes were approximately 200 kDa, as shown previously (23).
Together, these observations indicate that endogenous XIAP forms a novel ∼400-kDa complex on mitochondria in response to loss of adhesion signals. This complex forms rapidly and before caspase activation and cell death.
XIAP Promotes MOMP
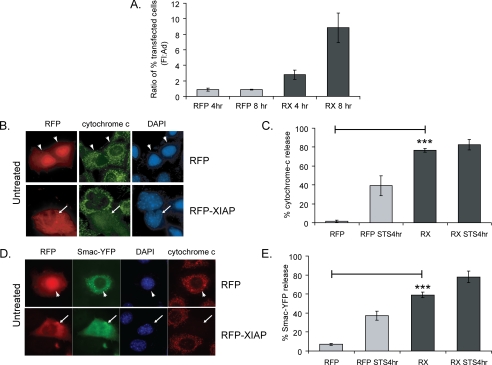
Given the antiapoptotic role of XIAP, we reasoned that its association with mitochondria might prevent Bax from initiating MOMP at early time points during anoikis. We therefore tested whether exogenous XIAP altered the kinetics of anoikis. XIAP was expressed in MECs, cells were detached from the ECM for 4 or 8 h, and nuclear morphology was quantified (supplemental Fig. S1A). There was no difference between untransfected and RFP-expressing cells, but the RFP-XIAP-expressing cells showed significantly fewer apoptotic nuclei at 4 and 8 h. Thus, as expected, XIAP suppresses the appearance of cells containing apoptotic nuclei.
Because MEC can be rescued from anoikis if ECM signals are restored before MOMP occurs, we also tested whether XIAP could alter the commitment of cells to death (24). RFP- and RFP-XIAP-expressing cells were detached from the ECM for 15 min, before allowing them to reattach to the ECM for either 4 or 8 h. We have shown previously that cells that have not committed to apoptosis before restoring ECM adhesion remain attached, whereas those that have committed to apoptosis die and detach. Thus, commitment can be determined by measuring the numbers of detached and adherent cells. The ratio of detached to adherent RFP-expressing cells was ∼1:1 at 4 and 8 h, showing that control cells that had reattached were not committed to apoptosis (Fig. 2A). In contrast, RFP-XIAP cells showed a significant time-dependent increase in the detached to adherent ratio, indicating that the majority of cells expressing XIAP had committed to apoptosis within 15-min loss of adhesion. These data indicate that although XIAP expression prevents apoptotic nuclear morphology, surprisingly, it has an adverse effect on cell viability as measured by the reattachment assay.
FIGURE 2.
XIAP-induces cytochrome c release. A, MECs expressing RFP or RFP-XIAP (RX) were detached for 15 min, then replated onto to plastic dishes for 4 or 8 h. Detached and adhered cells were collected, and the number of red cells within each population was counted. The ratio of the percent of transfected cells in the detached cells versus the adherent cells after 4 or 8 h of replating is shown. B and C, RFP- or RFP-XIAP (RX)-expressing MECs left untreated or treated for 4 h with STS were fixed and immunostained for cytochrome c. Cytochrome c release was scored by counting the percentage of transfected cells with diffuse cytochrome c staining. Note that there was no further release of cytochrome c when RFP-XIAP-expressing cells were treated with STS. In this and subsequent figures, arrowheads and arrows indicate transfected cells that have mitochondrial cytochrome c or have undergone MOMP, respectively. D and E, MECs co-transfected with Smac-YFP and RFP or RFP-XIAP were treated for 4 h with or without STS, then fixed and stained for cytochrome c. The percentage of cells showing Smac-YFP localization in the cytosol was counted.
Because the established role of XIAP is caspase inhibition, we asked whether RFP-XIAP blocked caspase activation in MECs. Cells expressing RFP or RFP-XIAP, along with a YFP-caspase-3 sensor (23), were treated with staurosporine (STS) to induce apoptosis or dimethyl sulfoxide as a control. Following cleavage by caspase-3, YFP translocates to the nucleus, seen by comparing the RFP-expressing cells treated with either dimethyl sulfoxide or STS (supplemental Fig. S1B). Quantification indicated that ∼70% of STS-treated cells had nuclear green fluorescent protein (supplemental Fig. S1C). In contrast (and as expected), RFP-XIAP completely inhibited STS-induced activation of caspase-3.
Because we observed endogenous XIAP associating with mitochondria prior to caspase activation, we asked whether RFP-XIAP affected MOMP. Cells expressing RFP or RFP-XIAP were treated with dimethyl sulfoxide or STS and immunostained for cytochrome c. RFP cells showed mitochondrial cytochrome c, which was lost following STS treatment (Fig. 2, B and C). Surprisingly, virtually all RFP-XIAP cells had released cytochrome c without receiving the apoptotic signal (Fig. 2, B and C). Similar results were obtained with Myc-tagged XIAP, indicating that the RFP-moiety did not alter XIAP function (supplemental Fig. S2). To confirm that MOMP occurred in XIAP-expressing cells, we co-expressed Smac-YFP with RFP or RFP-XIAP. Smac-YFP was mitochondrial in control RFP-expressing cells, but became cytosolic in RFP-XIAP cells (Fig. 2, D and E). Notably, cells with cytosolic Smac-YFP also had released cytochrome c, but did not have apoptotic nuclei (Fig. 2D). Finally, and in contrast to the XIAP results, Myc-survivin did not induce cytochrome c release even though it was expressed at greater levels than Myc-XIAP (supplemental Fig. S2).
Together, these data indicate a novel function for XIAP in promoting MOMP. In the overexpression studies, the RFP-XIAP cells have an apparently normal nuclear morphology, which is due to the caspase-inhibiting activity of XIAP. This was confirmed by showing that a mutant XIAP (D148A/W310A) that cannot bind caspases still induced MOMP but did not prevent the appearance of apoptotic nuclei (supplemental Fig. S3). However, because exogenous XIAP causes MOMP, it also has a detrimental effect on long term cultures. Indeed, we were readily able to obtain MEC colonies expressing RFP, but never RFP-XIAP (supplemental Fig. S4). To confirm the long term effects of RFP-XIAP on cell viability, we showed that RFP-XIAP, but not RFP, caused a loss of mitochondrial membrane potential and inhibited proliferation (supplemental Figs. S5 and S6). Thus, the caspase-binding property of XIAP prevents apoptosis, whereas a separate domain is able to trigger MOMP, which induces long term detrimental effects on cells.
XIAP-induced Cytochrome c Release Requires Bax or Bak and Is Inhibited by Bcl-XL
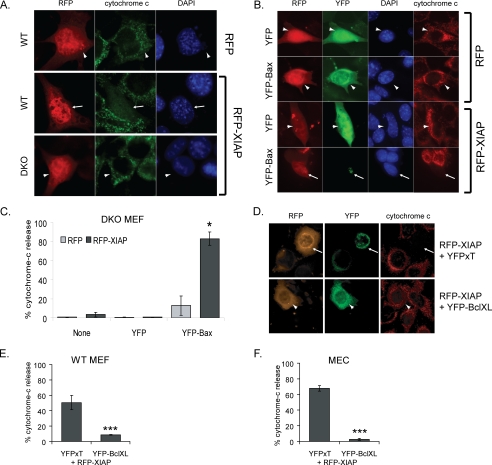
We asked whether the effect on MOMP was an intrinsic function of XIAP or whether it required the proapoptotic Bcl-2 family proteins, Bax and Bak. RFP or RFP-XIAP was expressed in either wild-type (WT) or DKO MEFs, and release of cytochrome c from mitochondria was examined (Fig. 3A). Although RFP-XIAP induced cytochrome c release in WT MEFs, it did not in DKO MEFs. To confirm this result, YFP-Bax was reexpressed in the DKO cells, together with RFP or RFP-XIAP (Fig. 3, B and C). Co-expression with YFP-Bax restored RFP-XIAP-induced cytochrome c release, whereas expression of YFP with RFP-XIAP did not. It is notable that in RFP-XIAP-expressing cells, YFP-Bax clustered into the large aggregates associated with MOMP (24, 25). Expression of YFP-Bak together with RFP-XIAP also rescued MOMP in DKO cells (data not shown).
FIGURE 3.
XIAP-induced cytochrome c release requires Bcl-2 family members. A, WT MEFs transiently expressing RFP or RFP-XIAP, or DKO MEFs expressing RFP-XIAP were fixed and scored for cytochrome c release. Note cytochrome c release in cells expressing RFP-XIAP (arrow), but mitochondrial cytochrome c in DKO cells expressing RFP-XIAP (arrowhead). B and C, DKO MEFs expressing RFP or RFP-XIAP only, or co-transfected with YFP or YFP-Bax, were scored for cytochrome c release (only transfected cells were counted). Representative images are shown in B; note that YFP-Bax is cytosolic in the RFP-expressing cell (arrowhead), whereas it has incorporated into clusters in the RFP-XIAP-expressing cell (arrow). D–F, WT MEFs and MECs expressing RFP-XIAP and either YFP-Bcl-XL or YFP-XT were scored for cytochrome c release. Representative images are shown in D.
To confirm the role of the intrinsic apoptotic pathway in RFP-XIAP-induced MOMP, we determined whether MOMP could be suppressed by the antiapoptotic Bcl-2 protein, Bcl-XL in WT cells (Fig. 3, D–F). WT MEFs or MECs were co-transfected with RFP-XIAP and either YFP-Bcl-XL- or mitochondria-targeted YFP (YFP-XT). Expression of Bcl-XL, but not YFP-XT, inhibited MOMP in response to RFP-XIAP.
Together, these results demonstrate that XIAP has a direct effect on activation of the intrinsic apoptosis pathway. Importantly, XIAP-induced MOMP occurs in both epithelial and fibroblast cell lineages, and we also noted similar effects in the human mammary line, MCF10A (supplemental Fig. S7).
XIAP Fragments That Induce Cytochrome c Release Are Membrane-associated
The results in Fig. 1 demonstrate that loss of adhesion to the ECM causes endogenous XIAP to translocate from the cytosol into a membrane-associated high molecular weight complex. We reasoned that activation of the intrinsic apoptosis pathway by exogenous XIAP in attached cells might be related to this change in endogenous XIAP localization that we observed during anoikis.
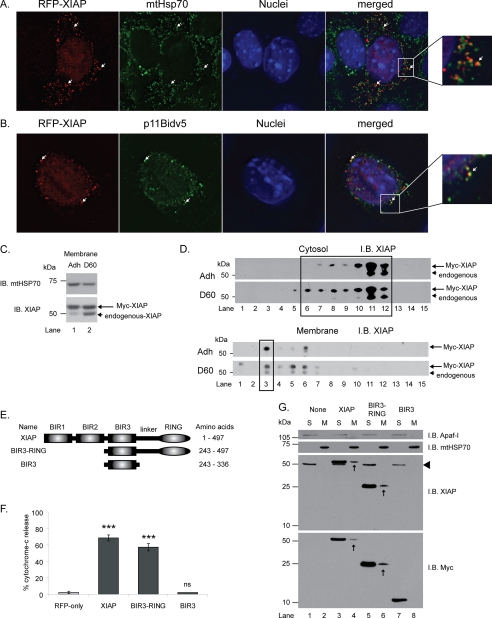
To determine whether XIAP influences MOMP through a direct association with mitochondria, we examined the subcellular localization of tagged XIAP using fractionation (Myc-XIAP) and immunofluorescence (RFP-XIAP). The transfected XIAP was present in both the cytosolic and membrane fractions (Fig. 4D), and in the latter it was located adjacent to mitochondria but was not associated with other organelle membranes (Fig. 4, A and B, and supplemental Fig. S8). Importantly, the exogenous XIAP was present in membranes even in attached cells, and detaching the cells from ECM caused no further enrichment in the membrane fraction (Fig. 4C). This membrane-associated Myc-XIAP was constitutively in the high molecular weight complex that is only formed by endogenous XIAP following ECM withdrawal (Fig. 4D, lower panel, lane 3). These results suggest that exogenous XIAP activates the intrinsic pathway by mimicking a function of endogenous XIAP, which is normally only revealed once the cell has received an apoptotic insult.
FIGURE 4.
Membrane association of XIAP corresponds with ability to induce MOMP. A, MECs transiently expressing RFP-XIAP were stained with mitochondrial HSP70 (green). Note close association of RFP-XIAP with mtHSP70 (arrows and enlarged view on right). B, to confirm mitochondrial association of RFP-XIAP, cells were co-transfected with RFP-XIAP and a V5-epitope tagged form of Bid that constitutively localizes to mitochondria (p11BidV5). C and D, MEF cells transiently expressing Myc-XIAP were either left adherent (Adh) or detached for 60 min (D60) prior to fractionation. Samples were separated by SDS-PAGE only (C) or by BN-PAGE followed by SDS-PAGE (D) and immunoblotted for XIAP. Arrow and arrowhead indicate Myc-XIAP and endogenous XIAP, respectively. Note that the cytosolic Myc-XIAP was in a complex of the same size as the endogenous protein (compare lanes 6–12 D, upper panel, and Fig. 1C, top panel). E and F, MECs transiently expressing RFP, RFP-XIAP (XIAP), RFP-BIR3-RING, or RFP-BIR3 were scored for cytochrome c release. G, cytosolic and membrane fractions of MEF cells, untransfected or transiently expressing Myc-XIAP, Myc-BIR3-RING, or Myc-BIR3 were analyzed by immunoblotting for Myc and reprobing for XIAP. Arrowhead indicates endogenous XIAP. Arrows indicate transfected, membrane-localized XIAP fragments. Note that BIR3 is only present in the cytosolic fraction and that Myc-BIR3 is only detected by the Myc antibody because it does not contain the epitope for the XIAP antibody.
To resolve whether membrane association is the mechanism for XIAP-induced MOMP, we tested whether the localization of the BIR3-RING and BIR-3 XIAP fragments (Fig. 4E) correlate with their ability to induce cytochrome c release. Transfected cells were fractionated, and full-length XIAP and the BIR3-RING were found to be present in both cytosol and membrane fractions, whereas the BIR3 domain was only present in the cytosol (Fig. 4G). In functional studies, the BIR3-RING was almost as effective as the full-length XIAP at inducing cytochrome c release, but the BIR3 domain by itself was unable to do so (Fig. 4F).
These data indicate that the novel proapoptotic function of XIAP occurs through membrane association of the protein. Further studies showed that this function of XIAP was not because of its E3 ligase domain, ability to bind caspases, or to activate c-Jun N-terminal kinase and NF-κB signaling (data not shown).
Novel Proapoptotic Role of XIAP
An emerging theme of apoptosis regulation is that proteins may have one set of functions in healthy cells but that this changes during apoptosis. For example, cytochrome c is required for ATP synthesis via the electron transport chain, but once released into the cytosol it causes apoptosome formation and caspase activation (26). Similarly, HtrA2/Omi may normally have a neuroprotective role against stress signals and regulate the physiological function of amyloid precursor protein in vivo, but in response to an acute apoptosis signal it promotes both caspase-dependent and caspase-independent death (27, 28). Although its caspase inhibitory function is well documented, recently new roles for XIAP have emerged (11, 29). In this article, we demonstrate that XIAP also has proapoptotic activity, and under certain conditions it may translocate to mitochondria and initiate Bax/Bak-dependent MOMP.
It is provocative that although XIAP is described as an apoptosis antagonist and also as a potential therapeutic target in cancer, there are very few published studies describing the generation of cell lines stably expressing exogenous XIAP despite their likely benefit for studying the role of XIAP in cancer (30–33). Furthermore, only a few XIAP transgenic mice exist, and these express XIAP in highly specialized cell types (i.e. neurons and thymocytes) (34–36). A possible explanation for the difficulty in generating cell lines and transgenic mice is the novel proapoptotic activity of XIAP shown in this article, which is revealed when exogenous XIAP is expressed. Studying the proapoptotic role of XIAP through knock-down studies may also prove challenging because of redundancy within the apoptotic cascade. For example, although XIAP has well documented prosurvival roles, XIAP−/− mice develop normally (37). It could also be possible that removing XIAP prevents both its pro- and antiapoptotic functions, resulting in no overall effect on apoptosis phenotype.
Current models dictate that MOMP is regulated by interactions between pro- and antiapoptotic Bcl-2 proteins, with the BH3-only proteins either inhibiting antiapoptotic members, such as Bcl-XL, or activating proapoptotic members such as Bax (38, 39). However, these molecules all form high molecular weight complexes on mitochondria, which suggests that other (potentially novel) molecules are also involved (23). Currently, we are screening for mitochondrial proteins involved in Bax and Bid recruitment to mitochondria and will also do so for XIAP: these proteins are likely to be different because Bax and XIAP reside in different mitochondrial complexes.
Summary
In response to an apoptosis trigger, a proportion of XIAP is rapidly removed from the cytosol to the mitochondria (∼15 min). In this location, XIAP becomes sequestered from the cytosol and may contribute to apoptosis commitment, in conjunction with other intrinsic regulators, e.g. Bax. Following the commitment event (>2 h), apoptogenic factors such as Smac are released from mitochondria, leading to the inactivation and degradation of remaining cytosolic XIAP and thereby allowing apoptosis to proceed efficiently.
Supplementary Material
Acknowledgments
We thank Dr. Shawn Bratton for providing the XIAP caspase-binding mutant.
This work was funded by the Wellcome Trust and Cancer Research United Kingdom.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S8.
- IAP
- inhibitor of apoptosis
- BIR
- baculovirus IAP repeat
- XIAP
- X-linked IAP
- BN-PAGE
- blue native-PAGE
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- DKO
- Bax−/−;Bak−/− double knock-out
- ECM
- extracellular matrix
- MEC
- mammary epithelial cell
- MEF
- mouse embryonic fibroblast
- MOMP
- mitochondrial outer membrane permeabilization
- mtHSP70
- mitochondrial heat shock protein 70
- STS
- staurosporine
- WT
- wild-type
- YFP
- yellow fluorescent protein.
REFERENCES
- 1.Cohen G. M. (1997) Biochem. J. 326, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hengartner M. O. (2000) Nature 407, 770–776 [DOI] [PubMed] [Google Scholar]
- 3.Liston P., Fong W. G., Korneluk R. G. (2003) Oncogene 22, 8568–8580 [DOI] [PubMed] [Google Scholar]
- 4.Tenev T., Zachariou A., Wilson R., Ditzel M., Meier P. (2005) Nat. Cell Biol. 7, 70–77 [DOI] [PubMed] [Google Scholar]
- 5.Ditzel M., Broemer M., Tenev T., Bolduc C., Lee T. V., Rigbolt K. T., Elliott R., Zvelebil M., Blagoev B., Bergmann A., Meier P. (2008) Mol. Cell 32, 540–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deveraux Q. L., Takahashi R., Salvesen G. S., Reed J. C. (1997) Nature 388, 300–304 [DOI] [PubMed] [Google Scholar]
- 7.Eckelman B. P., Salvesen G. S., Scott F. L. (2006) EMBO Rep. 7, 988–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danial N. N., Gramm C. F., Scorrano L., Zhang C. Y., Krauss S., Ranger A. M., Datta S. R., Greenberg M. E., Licklider L. J., Lowell B. B., Gygi S. P., Korsmeyer S. J. (2003) Nature 424, 952–956 [DOI] [PubMed] [Google Scholar]
- 9.Kamer I., Sarig R., Zaltsman Y., Niv H., Oberkovitz G., Regev L., Haimovich G., Lerenthal Y., Marcellus R. C., Gross A. (2005) Cell 122, 593–603 [DOI] [PubMed] [Google Scholar]
- 10.Karbowski M., Norris K. L., Cleland M. M., Jeong S. Y., Youle R. J. (2006) Nature 443, 658–662 [DOI] [PubMed] [Google Scholar]
- 11.Gyrd-Hansen M., Darding M., Miasari M., Santoro M. M., Zender L., Xue W., Tenev T., da Fonseca P. C., Zvelebil M., Bujnicki J. M., Lowe S., Silke J., Meier P. (2008) Nat. Cell Biol. 10, 1309–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilmore A. P. (2005) Cell Death Differ 12, Suppl. 2, 1473–1477 [DOI] [PubMed] [Google Scholar]
- 13.Streuli C. H. (2009) J. Cell Sci. 122, 171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilmore A. P., Owens T. W., Foster F. M., Lindsay J. (2009) Curr. Opin. Cell Biol. 21, 654–661 [DOI] [PubMed] [Google Scholar]
- 15.Wang P., Valentijn A. J., Gilmore A. P., Streuli C. H. (2003) J. Biol. Chem. 278, 19917–19925 [DOI] [PubMed] [Google Scholar]
- 16.Gilmore A. P., Metcalfe A. D., Romer L. H., Streuli C. H. (2000) J. Cell Biol. 149, 431–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pullan S., Wilson J., Metcalfe A., Edwards G. M., Goberdhan N., Tilly J., Hickman J. A., Dive C., Streuli C. H. (1996) J. Cell Sci. 109, 631–642 [DOI] [PubMed] [Google Scholar]
- 18.Valentijn A. J., Metcalfe A. D., Kott J., Streuli C. H., Gilmore A. P. (2003) J. Cell Biol. 162, 599–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valentijn A. J., Gilmore A. P. (2004) J. Biol. Chem. 279, 32848–32857 [DOI] [PubMed] [Google Scholar]
- 20.Owens T. W., Valentijn A. J., Upton J. P., Keeble J., Zhang L., Lindsay J., Zouq N. K., Gilmore A. P. (2009) Cell Death Differ. 16, 1551–1562 [DOI] [PubMed] [Google Scholar]
- 21.Manara A., Lindsay J., Marchioretto M., Astegno A., Gilmore A. P., Esposti M. D., Crimi M. (2009) Biochim. Biophys. Acta 1791, 997–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brookes P. S., Pinner A., Ramachandran A., Coward L., Barnes S., Kim H., Darley-Usmar V. M. (2002) Proteomics 2, 969–977 [DOI] [PubMed] [Google Scholar]
- 23.Valentijn A. J., Upton J. P., Gilmore A. P. (2008) Biochem. J. 412, 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Upton J. P., Valentijn A. J., Zhang L., Gilmore A. P. (2007) Cell Death Differ. 14, 932–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nechushtan A., Smith C. L., Lamensdorf I., Yoon S. H., Youle R. J. (2001) J. Cell Biol. 153, 1265–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li K., Li Y., Shelton J. M., Richardson J. A., Spencer E., Chen Z. J., Wang X., Williams R. S. (2000) Cell 101, 389–399 [DOI] [PubMed] [Google Scholar]
- 27.Martins L. M., Morrison A., Klupsch K., Fedele V., Moisoi N., Teismann P., Abuin A., Grau E., Geppert M., Livi G. P., Creasy C. L., Martin A., Hargreaves I., Heales S. J., Okada H., Brandner S., Schulz J. B., Mak T., Downward J. (2004) Mol. Cell. Biol. 24, 9848–9862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park H. J., Kim S. S., Seong Y. M., Kim K. H., Goo H. G., Yoon E. J., Min do S., Kang S., Rhim H. (2006) J. Biol. Chem. 281, 34277–34287 [DOI] [PubMed] [Google Scholar]
- 29.Dogan T., Harms G. S., Hekman M., Karreman C., Oberoi T. K., Alnemri E. S., Rapp U. R., Rajalingam K. (2008) Nat. Cell Biol. 10, 1447–1455 [DOI] [PubMed] [Google Scholar]
- 30.Silke J., Kratina T., Ekert P. G., Pakusch M., Vaux D. L. (2004) J. Biol. Chem. 279, 4313–4321 [DOI] [PubMed] [Google Scholar]
- 31.Verhagen A. M., Ekert P. G., Pakusch M., Silke J., Connolly L. M., Reid G. E., Moritz R. L., Simpson R. J., Vaux D. L. (2000) Cell 102, 43–53 [DOI] [PubMed] [Google Scholar]
- 32.Yang Y., Fang S., Jensen J. P., Weissman A. M., Ashwell J. D. (2000) Science 288, 874–877 [DOI] [PubMed] [Google Scholar]
- 33.Sauerwald T. M., Betenbaugh M. J., Oyler G. A. (2002) Biotechnol. Bioeng. 77, 704–716 [DOI] [PubMed] [Google Scholar]
- 34.Conte D., Liston P., Wong J. W., Wright K. E., Korneluk R. G. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 5049–5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trapp T., Korhonen L., Besselmann M., Martinez R., Mercer E. A., Lindholm D. (2003) Mol. Cell. Neurosci. 23, 302–313 [DOI] [PubMed] [Google Scholar]
- 36.Wang X., Zhu C., Wang X., Hagberg H., Korhonen L., Sandberg M., Lindholm D., Blomgren K. (2004) Neurobiol. Dis. 16, 179–189 [DOI] [PubMed] [Google Scholar]
- 37.Harlin H., Reffey S. B., Duckett C. S., Lindsten T., Thompson C. B. (2001) Mol. Cell. Biol. 21, 3604–3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chipuk J. E., Green D. R. (2008) Trends Cell Biol. 18, 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youle R. J., Strasser A. (2008) Nat. Rev. Mol. Cell Biol. 9, 47–59 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.