Abstract
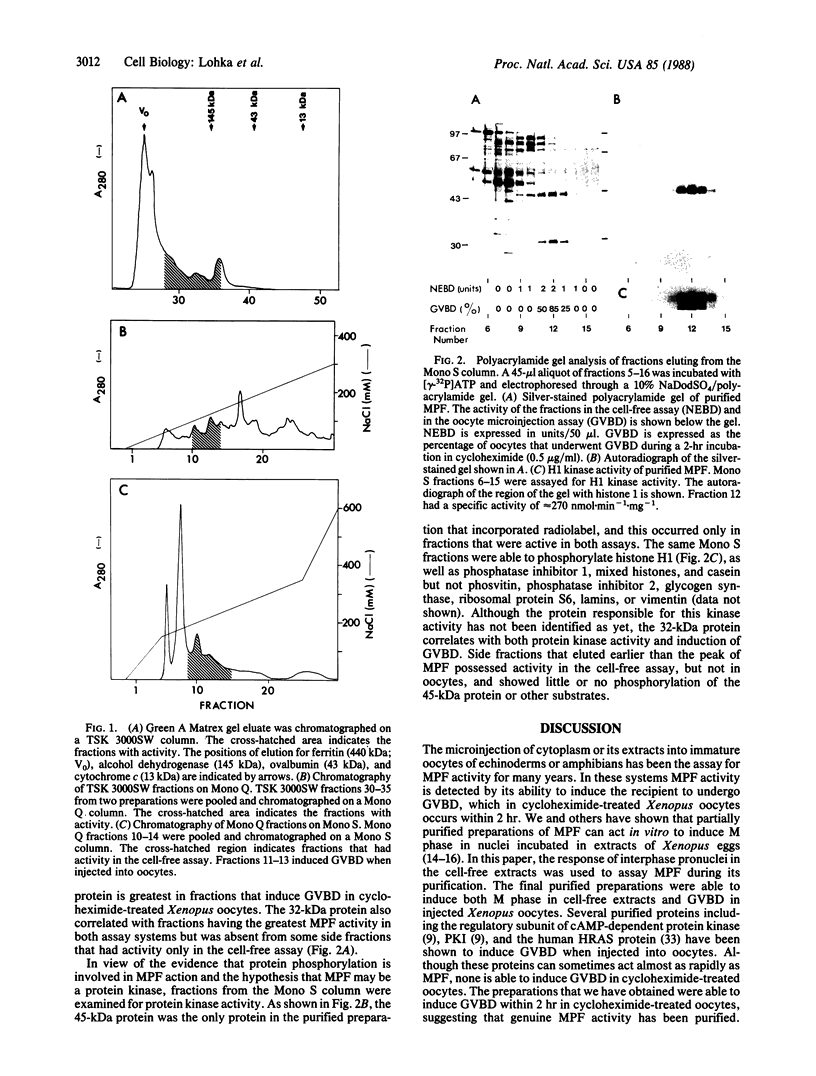
Maturation-promoting factor causes germinal vesicle breakdown when injected into Xenopus oocytes and can induce metaphase in a cell-free system. The cell-free assay was used to monitor maturation-promoting factor during its purification from unfertilized Xenopus eggs. Ammonium sulfate precipitation and six chromatographic procedures resulted in a preparation purified greater than 3000-fold that could induce germinal vesicle breakdown within 2 hr when injected into cycloheximide-treated oocytes. Proteins of 45 kDa and 32 kDa were correlated with fractions of highest activity in both assays. These fractions contained a protein kinase activity able to phosphorylate the endogenous 45-kDa protein, as well as histone H1, phosphatase inhibitor 1, and casein. The highly purified preparations described here should help to identify the mechanism of action of maturation-promoting factor and to elucidate the role of protein kinases in the induction of metaphase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlakha R. C., Wright D. A., Sahasrabuddhe C. G., Davis F. M., Prashad N., Bigo H., Rao P. N. Partial purification and characterization of mitotic factors from HeLa cells. Exp Cell Res. 1985 Oct;160(2):471–482. doi: 10.1016/0014-4827(85)90194-6. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Broek D., Wigler M. ras proteins can induce meiosis in Xenopus oocytes. Cell. 1985 Dec;43(3 Pt 2):615–621. doi: 10.1016/0092-8674(85)90233-8. [DOI] [PubMed] [Google Scholar]
- Ford C. C. Maturation promoting factor and cell cycle regulation. J Embryol Exp Morphol. 1985 Nov;89 (Suppl):271–284. [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Wu M., Cyert M., Kirschner M. M-phase promoting factors from eggs of Xenopus laevis. Cytobios. 1985;43(174S):335–347. [PubMed] [Google Scholar]
- Graziani Y., Erikson E., Erikson R. L. Characterization of the Rous sarcoma virus transforming gene product. J Biol Chem. 1983 May 25;258(10):6344–6351. [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Kyes J. L., Maller J. L. Metaphase protein phosphorylation in Xenopus laevis eggs. Mol Cell Biol. 1987 Feb;7(2):760–768. doi: 10.1128/mcb.7.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka M. J., Maller J. L. Induction of nuclear envelope breakdown, chromosome condensation, and spindle formation in cell-free extracts. J Cell Biol. 1985 Aug;101(2):518–523. doi: 10.1083/jcb.101.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Effects of Ca2+ ions on the formation of metaphase chromosomes and sperm pronuclei in cell-free preparations from unactivated Rana pipiens eggs. Dev Biol. 1984 Jun;103(2):434–442. doi: 10.1016/0012-1606(84)90331-2. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Krebs E. G. Regulation of oocyte maturation. Curr Top Cell Regul. 1980;16:271–311. doi: 10.1016/b978-0-12-152816-4.50012-1. [DOI] [PubMed] [Google Scholar]
- Maller J. L. Regulation of amphibian oocyte maturation. Cell Differ. 1985 Jun;16(4):211–221. doi: 10.1016/0045-6039(85)90570-6. [DOI] [PubMed] [Google Scholar]
- Maller J., Wu M., Gerhart J. C. Changes in protein phosphorylation accompanying maturation of Xenopus laevis oocytes. Dev Biol. 1977 Jul 15;58(2):295–312. doi: 10.1016/0012-1606(77)90093-8. [DOI] [PubMed] [Google Scholar]
- Marcus M., Fainsod A., Diamond G. The genetic analysis of mammalian cell-cycle mutants. Annu Rev Genet. 1985;19:389–421. doi: 10.1146/annurev.ge.19.120185.002133. [DOI] [PubMed] [Google Scholar]
- Masui Y., Markert C. L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971 Jun;177(2):129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Miake-Lye R., Kirschner M. W. Induction of early mitotic events in a cell-free system. Cell. 1985 May;41(1):165–175. doi: 10.1016/0092-8674(85)90071-6. [DOI] [PubMed] [Google Scholar]
- Nguyen-Gia P., Bomsel M., Labrousse J. P., Gallien C. L., Weintraub H. Partial purification of the maturation-promoting factor MPF from unfertilized eggs of Xenopus laevis. Eur J Biochem. 1986 Dec 15;161(3):771–777. doi: 10.1111/j.1432-1033.1986.tb10506.x. [DOI] [PubMed] [Google Scholar]
- Nose K., Katsuta H. Arrest of cultured rat liver cells in G2 phase by the treatment with dibutyryl cAMP. Biochem Biophys Res Commun. 1975 Jan 2;64(3):983–988. doi: 10.1016/0006-291x(75)90144-8. [DOI] [PubMed] [Google Scholar]
- Nurse P., Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981 Aug 6;292(5823):558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Osmani S. A., May G. S., Morris N. R. Regulation of the mRNA levels of nimA, a gene required for the G2-M transition in Aspergillus nidulans. J Cell Biol. 1987 Jun;104(6):1495–1504. doi: 10.1083/jcb.104.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Piggott J. R., Rai R., Carter B. L. A bifunctional gene product involved in two phases of the yeast cell cycle. Nature. 1982 Jul 22;298(5872):391–393. doi: 10.1038/298391a0. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Hadwiger J. A., Lörincz A. T. Protein kinase activity associated with the product of the yeast cell division cycle gene CDC28. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4055–4059. doi: 10.1073/pnas.82.12.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P., Nurse P. The mitotic inducer nim1+ functions in a regulatory network of protein kinase homologs controlling the initiation of mitosis. Cell. 1987 May 22;49(4):569–576. doi: 10.1016/0092-8674(87)90459-4. [DOI] [PubMed] [Google Scholar]
- Simanis V., Nurse P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell. 1986 Apr 25;45(2):261–268. doi: 10.1016/0092-8674(86)90390-9. [DOI] [PubMed] [Google Scholar]
- Smith L. D., Ecker R. E. The interaction of steroids with Rana pipiens Oocytes in the induction of maturation. Dev Biol. 1971 Jun;25(2):232–247. doi: 10.1016/0012-1606(71)90029-7. [DOI] [PubMed] [Google Scholar]
- Stambrook P. J., Velez C. Reversible arrest of Chinese hamster V79 cells in G2 by dibutytyl AMP. Exp Cell Res. 1976 Apr;99(1):57–62. doi: 10.1016/0014-4827(76)90679-0. [DOI] [PubMed] [Google Scholar]
- Swenson K. I., Farrell K. M., Ruderman J. V. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986 Dec 26;47(6):861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D., Gonzalez C., Calkins D., Fischer E. H. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1977–1985. [PubMed] [Google Scholar]
- Wu M., Gerhart J. C. Partial purification and characterization of the maturation-promoting factor from eggs of Xenopus laevis. Dev Biol. 1980 Oct;79(2):465–477. doi: 10.1016/0012-1606(80)90131-1. [DOI] [PubMed] [Google Scholar]