Abstract
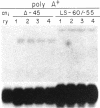
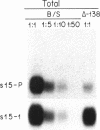
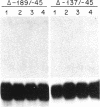
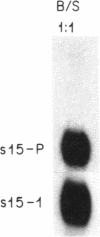
We have used germ-line transformation to dissect the cis regulatory elements responsible for the transcriptional control of an internally marked Drosophila chorion gene (s15-P) during development. A 73-base-pair segment of the proximal 5'-flanking DNA contains sequences essential for the tissue-specific expression and the precise "late" temporal regulation of that gene. A substitute s36-1 segment of similar location can provide the tissue-specific function and imparts an early temporal regulation characteristic of gene s36-1. Within the regulatory DNA of s15-P, at least three adjacent elements are recognizable: an essential operationally positive element (TCACGT) that is shared by s36-1 and other chorion genes, irrespective of temporal specificity; a second positive element that is required for the normal late expression of s15-P; and, farthest upstream, a negative element that represses precocious expression during the early choriogenic stages.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Breeden L., Abraham J., Sternglanz R., Nasmyth K. Characterization of a "silencer" in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell. 1985 May;41(1):41–48. doi: 10.1016/0092-8674(85)90059-5. [DOI] [PubMed] [Google Scholar]
- Carter P., Bedouelle H., Winter G. Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res. 1985 Jun 25;13(12):4431–4443. doi: 10.1093/nar/13.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Griffin-Shea R., Thireos G., Kafatos F. C. Organization of a cluster of four chorion genes in Drosophila and its relationship to developmental expression and amplification. Dev Biol. 1982 Jun;91(2):325–336. doi: 10.1016/0012-1606(82)90039-2. [DOI] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Chambon P. Repression of the immunoglobulin heavy chain enhancer by the adenovirus-2 E1A products. Science. 1985 Dec 20;230(4732):1391–1394. doi: 10.1126/science.2999984. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Kafatos F. C. Accepted mutations in a gene family: evolutionary diversification of duplicated DNA. J Mol Evol. 1982;19(1):87–103. doi: 10.1007/BF02100227. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Mitsialis S. A., Spoerel N., Mariani B., Lingappa J. R., Delidakis C. Studies on the developmentally regulated expression and amplification of insect chorion genes. Cold Spring Harb Symp Quant Biol. 1985;50:537–547. doi: 10.1101/sqb.1985.050.01.066. [DOI] [PubMed] [Google Scholar]
- Kalfayan L., Levine J., Orr-Weaver T., Parks S., Wakimoto B., de Cicco D., Spradling A. Localization of sequences regulating Drosophila chorion gene amplification and expression. Cold Spring Harb Symp Quant Biol. 1985;50:527–535. doi: 10.1101/sqb.1985.050.01.065. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Rubin G. M. Analysis of P transposable element functions in Drosophila. Cell. 1984 Aug;38(1):135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Kuhl D., de la Fuente J., Chaturvedi M., Parimoo S., Ryals J., Meyer F., Weissmann C. Reversible silencing of enhancers by sequences derived from the human IFN-alpha promoter. Cell. 1987 Sep 25;50(7):1057–1069. doi: 10.1016/0092-8674(87)90172-3. [DOI] [PubMed] [Google Scholar]
- Laimins L., Holmgren-König M., Khoury G. Transcriptional "silencer" element in rat repetitive sequences associated with the rat insulin 1 gene locus. Proc Natl Acad Sci U S A. 1986 May;83(10):3151–3155. doi: 10.1073/pnas.83.10.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsialis S. A., Spoerel N., Leviten M., Kafatos F. C. A short 5'-flanking DNA region is sufficient for developmentally correct expression of moth chorion genes in Drosophila. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7987–7991. doi: 10.1073/pnas.84.22.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri W. H., Wyman A. R., Kafatos F. C. Specific protein synthesis in cellular differentiation. III. The eggshell proteins of Drosophila melanogaster and their program of synthesis. Dev Biol. 1976 Mar;49(1):185–199. doi: 10.1016/0012-1606(76)90266-9. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983 Sep 24;11(18):6341–6351. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoerel N., Nguyen H. T., Kafatos F. C. Gene regulation and evolution in the chorion locus of Bombyx mori. Structural and developmental characterization of four eggshell genes and their flanking DNA regions. J Mol Biol. 1986 Jul 5;190(1):23–35. doi: 10.1016/0022-2836(86)90072-0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Spradling A. C. The organization and amplification of two chromosomal domains containing Drosophila chorion genes. Cell. 1981 Nov;27(1 Pt 2):193–201. doi: 10.1016/0092-8674(81)90373-1. [DOI] [PubMed] [Google Scholar]
- Wakimoto B. T., Kalfayan L. J., Spradling A. C. Developmentally regulated expression of Drosophila chorion genes introduced at diverse chromosomal positions. J Mol Biol. 1986 Jan 5;187(1):33–45. doi: 10.1016/0022-2836(86)90404-3. [DOI] [PubMed] [Google Scholar]
- Waring G. L., Mahowald A. P. Identification and time of synthesis of chorion proteins in Drosophila melanogaster. Cell. 1979 Mar;16(3):599–607. doi: 10.1016/0092-8674(79)90033-3. [DOI] [PubMed] [Google Scholar]
- Wong Y. C., Pustell J., Spoerel N., Kafatos F. C. Coding and potential regulatory sequences of a cluster of chorion genes in Drosophila melanogaster. Chromosoma. 1985;92(2):124–135. doi: 10.1007/BF00328464. [DOI] [PubMed] [Google Scholar]