SUMMARY
Apicomplexan parasites of the genus Eimeria are the major causative agent of avian coccidiosis, leading to high economic losses in the poultry industry. Recent results show that Eimeria tenella harbours an apicoplast organelle, and that a key biosynthetic enzyme, enoyl reductase, is located in this organelle. In related parasites, enoyl reductase is one component of a type II fatty acid synthase (FAS) and has proven to be an attractive target for antimicrobial compounds. We cloned and expressed the mature form of E. tenella enoyl reductase (EtENR) for biochemical and structural studies. Recombinant EtENR exhibits NADH-dependent enoyl reductase activity and is inhibited by triclosan with an IC50 value of 60 nM. The crystal structure of EtENR reveals overall similarity with other ENR enzymes; however, the active site of EtENR is unoccupied, a state rarely observed in other ENR structures. Furthermore, the position of the central beta-sheet appears to block NADH binding and would require significant movement to allow NADH binding, a feature not previously seen in the ENR family. We analysed the E. tenella genomic database for orthologues of well-characterized bacterial and apicomplexan FAS enzymes and identified 6 additional genes, suggesting that E. tenella contains a type II FAS capable of synthesizing saturated, but not unsaturated, fatty acids. Interestingly, we also identified sequences that appear to encode multifunctional type I FAS enzymes, a feature also observed in Toxoplasma gondii, highlighting the similarity between these apicomplexan parasites.
Keywords: Apicomplexa, apicoplast, Eimeria tenella, type I fatty acid synthase, type II fatty acid synthase, enoyl (ACP) reductase
INTRODUCTION
Intracellular parasites of the genus Eimeria belong to the phylum Apicomplexa, which includes many species of parasitic protozoa of significant medical and veterinary importance. The genus Eimeria contains a large number of species that cause coccidiosis in a variety of wild and domesticated animals, including chickens. Within the poultry industry alone, the annual economic losses associated with avian coccidiosis were estimated to be about $800 million in 1998 (Williams, 1998) and are now thought to be as high as $2400 million (Shirley et al. 2005). Although chemoprophylactic drugs such as amprolium and sulfonamides help to limit infection, there is no effective drug treatment once infection is established. Moreover, the emergence of widespread drug-resistance to commonly used chemicals further emphasizes the need for new anti-coccidial drugs. In this report, we provide evidence that Eimeria tenella harbours a fatty acid biosynthesis pathway similar to those actively pursued as drug targets in other apicomplexan parasites.
Apicomplexan parasites often contain an apicoplast, an organelle thought to have arisen through secondary endosymbiosis of an algal cell which had previously incorporated a cyanobacterium (Williamson et al. 1994; Fast et al. 2001). In T. gondii and Plasmodium falciparum, the apicoplast, which is essential for growth and survival, is thought to harbour several metabolic pathways, including a complete type II fatty acid synthase (reviewed by He et al. 2001; Wilson, 2002; Roberts et al. 2003; Ralph et al. 2004). Proteins involved in these pathways are nuclear-encoded and use an amino-terminal bipartite leader sequence to direct trafficking to the apicoplast organelle (Waller et al. 1998, 2000). Ultrastructural studies show the presence of a multi-membrane apicoplast in Eimeria (Ferguson et al. 1976), but the pathways housed in this organelle have not been studied in detail. A recent study has located enoyl-ACP reductase (ENR) to the apicoplast, indicating that this organelle is the likely site for type II fatty acid synthesis in this parasite (Ferguson et al. 2007).
Fatty acids can be synthesized by 2 related, but distinct, fatty acid synthase (FAS) pathways. Type I FAS are large multifunctional enzymes capable of catalysing all of the reactions of fatty acid biosynthesis (Smith, 1994). In contrast, for the type II FAS, each reaction is catalysed by a discrete enzyme (Magnuson et al. 1993). Type I FAS are typically found in the cytosol of fungi and higher eukaryotes, while type II pathways are found in prokaryotes and the plastids of plants and algae. The significant differences between type I and type II FAS allow antibiotics to selectively inhibit enzymes of type II, while having minimal effect on type I enzymes (Campbell and Cronan, 2001; White et al. 2005; Wang et al. 2006). The apicoplast organelles of the apicomplexan parasites P. falciparum and T. gondii have been shown to harbour type II FAS pathways (Waller et al. 1998, 2000; Zuther et al. 1999; Jelenska et al. 2001; Ferguson et al. 2005). In P. falciparum, type II FAS appears to be the only fatty acid biosynthesis pathway, whereas T. gondii possesses both FAS pathways: type II takes place in the apicoplast while type I is suggested to occur in the mitochondrion (Coppens, 2006). Conversely, the absence of an apicoplast in Cryptosporidium parvum leaves its type I FAS as the sole pathway for synthesizing fatty acids in this apicomplexan (Zhu et al. 2000a, b).
ENR is a key component of type II FAS pathways, converting a trans-2,3 enoyl moiety into a saturated acyl chain using NADH as a cofactor. ENR, together with other enzymes in the type II FAS pathway, presents potential targets for antibacterial and anti-parasitic drug discovery efforts (Campbell and Cronan, 2001; McLeod et al. 2001; Lu et al. 2005). Moreover, inhibitors of bacterial ENRs have been shown to inhibit the activity of P. falciparum (Surolia and Surolia, 2001) and T. gondii (Samuel et al. 2003) ENR, as well as inhibiting the in vitro growth of these parasites (McLeod et al. 2001; Surolia and Surolia, 2001). Eimeria tenella ENR (EtENR) could be susceptible to these inhibitors and furthermore, since the avian hosts of E. tenella use type I FAS for fatty acid synthesis (Chang and Hammes, 1989), EtENR may present a specific target for anti-coccidial drug discovery.
Herein we report the characterization of EtENR enzymatic activity and its inhibition by triclosan. The structure of EtENR was determined to 2.6Å and represents the first crystal structure of an enzyme from the genus Eimeria. Furthermore, this structure reveals significant differences from the ENR from other apicomplexan parasites. Although the E. tenella genome project is still underway, we analysed in silico the predicted protein products of a set of candidate genes which appear to encode all of the enzymes required for a type II FAS similar to that found in P. falciparum. Separately, we have shown that EtENR, although encoded in the nucleus, is trafficked to the apicoplast organelle (Ferguson et al. 2007). Together, these results suggest that Eimeria parasites contain a complete type II FAS located in the apicoplast organelle. Interestingly, the E. tenella genome also appears to encode multiple type I FAS enzymes.
MATERIALS AND METHODS
Cloning, expression and purification of recombinant EtENR
The amplification of the coding sequence of EtENR from cDNA is described elsewhere (Ferguson et al. 2007). For expression, primers (For) 5′-GGTGGTGAATTCGGCCCGCTGCCAGTGGAC-3′ and (Rev) 5′-GGTGGTGTCGACTCAAGGGGTGAGGGACTTGGAG-3′, were used to amplify nucleotides encoding amino acids 92–410 and to introduce a proximal EcoRI and distal SalI site (underlined) in the PCR product. Nucleotides encoding the amino-terminal 91 residues of EtENR were excluded because this region is principally composed of a signal peptide and an organellar transit peptide. The resulting amplicon was digested with EcoRI and SalI and ligated into the pMALcHT vector (Muench et al. 2003). The resulting plasmid (pMA001) was sequence verified and transformed into BL21-Star(DE3) cells (Invitrogen). These cells were co-transformed with the pRIL plasmid isolated from BL21-codonPlus(DE3) cells (Stratagene) and a plasmid encoding the TEV (Tobacco Etch Virus) protease (Kapust and Waugh, 2000). Cells were grown in LB medium at 37 °C to an optical density at 600 nm of 0.8 and then induced with the addition of IPTG to a final concentration of 0.4 mm. The culture was maintained in shaker flasks at 15 °C for 16 h and then harvested by centrifugation at 6000 g for 20 min.
Cell lysis buffer (20 mm Tris-HCl, pH 8.0, 100 mm NaCl, 1 mg/ml lysozyme (Sigma), 2.5 µg/ml DNAseI (Sigma), 1 mm PMSF (Sigma)) was added to the cell pellets (20 ml per litre of E. coli culture) and gently vortexed. Resuspended cells were incubated on ice for 10 min followed by 1 min of sonication. Cell lysate was cleared by centrifugation (27 000 g, 4 °C, 20 min) and applied to a 5 ml HiTrap Metal Chelating HP column (GE Healthcare) equilibrated in 20 mm Tris-HCl, pH 8.0, 50 mm NaCl, and eluted with a linear gradient to 500 mm imidazole. Fractions containing EtENR were desalted (HiPrep 26/10 desalting column, GE Healthcare) then loaded on a 5 ml HiTrap SP FastFlow column (GE Healthcare) pre-equilibrated in 18 mm Na/K phosphate pH 5.65, and eluted stepwise with 1 m NaCl. Fractions containing EtENR were pooled and adjusted to pH 8.2 with 1 m Tris base. The resulting solution was then applied to a 5 ml HiTrap Metal Chelating HP column and washed stepwise with 50 mm, 100 mm and 150 mm imidazole. EtENR was then eluted with a linear gradient to 650 mm imidazole. Finally, the pooled EtENR was buffer exchanged into 20 mm Tris-HCl, pH 8.0, 100 mm NaCl and loaded on a 50 ml amylose column (New England Biolabs) pre-equlibrated with the same buffer. This subtractive step removes residual MBP-EtENR fusion protein that may have survived in vivo cleavage with TEV protease. The amylose flow-through containing pure EtENR was concentrated in a 15 ml concentrator (Vivascience, 30 kDa molecular weight cutoff) to a final concentration of 33 mg/ml and flash frozen for further analysis.
Enzymatic assay and inhibition of ENR
We adapted a spectrophotometric assay to determine ENR activity by monitoring the consumption of NADH at 340 nm (ΔA340) (Bergler et al. 1996). Reactions contained 132 ng of EtENR (38 nm EtENR monomer), 200 µm crotonyl-CoA, 100 µm NADH, 100 mm sodium phosphate (pH 7.5) in a final volume of 100 µl. Km and Vmax values for crotonoyl-CoA were determined at a fixed concentration of NADH (100 µm) and by varying the substrate concentration (0–1 mm). Km and Vmax values for NADH were determined at variable concentrations of NADH (0–0.5 mM) and a fixed concentration of crotonoyl-CoA (200 µm). Kinetic parameters were computed by fitting the initial velocity data to the Michaelis–Menten equation. To address the effect of reducing agents dithiothreitol (DTT) and β-mercaptoethanol (βME) on EtENR activity, DTT and βME were added in the assay at final concentrations of 100 mm, 10mm, 1mm and 0.1 mm (DTT only). Toxoplasma gondii ENR (Muench et al. 2006) at a final concentration of 38 nm was used as a control in DTT and βME assays. Inhibition assays were performed in the presence of triclosan, in which a DMSO blank was included as a negative control. All reactions were carried out at room temperature in UVettes (Eppendorf) and initiated by the addition of substrate crotonyl-CoA. The ΔA340 was continuously monitored for up to 10 min, providing a quantitative measure of NADH consumption (ε340 = 6220M−1cm−1).
Crystallization, X-ray data collection and structure determination
Crystals of diffraction quality were grown by the hanging drop method with 4 mg/ml EtENR, 50 mm sodium citrate (pH 5), 5% (v/v) polyethylene glycol 400. Crystals were cryoprotected with 50% (v/v) paratone-N in mineral oil (Hampton Research and Sigma) and frozen in a stream of cold nitrogen gas at 100 °K. A MicroStar generator was used to generate X-rays, and diffraction data were collected with an X8 Proteum charge coupled device detector (Bruker Instruments). Diffraction data were processed and scaled using HKL2000 (Otwinowski and Minor, 1997). The structure of EtENR was solved by molecular replacement (CCP4, 1994), using Brassica napus ENR (1ENP.pdb) as the starting model, from which the cofactor NADH was removed prior to phasing. XtalView (McRee, 1999) was used for model building and the structure was refined with CNS (Brunger et al. 1998). Molecular graphics images were produced using the UCSF Chimera package (Pettersen et al. 2004). The atomic coordinates and structure factors of EtENR have been submitted to the Protein DataBank and are released under PDB code 2PTG (Accession code RCSB042759).
Analysis of Eimeria tenella FAS genes
We used proteins of bacterial (E. coli) and apicomplexan (P. falciparum) FAS as query sequences to search the E. tenella genome database. Putative E. tenella genes were then analysed to determine whether key residues would be conserved in the encoded proteins. In brief, we retrieved NCBI protein sequences of E. coli and P. falciparum FAS enzymes, together with those of the P. falciparum pyruvate dehydrogenase (PDH) subunits. Sequence data from the E. tenella Sequencing Group at the Sanger Institute (October 2005 contig library) were searched with tBLASTn (Blosum62 matrix) using these protein sequences as queries (Altschul et al. 1990). The N-terminal apicoplast targeting sequences of P. falciparum proteins were included for the identification of similar targeting motifs in E. tenella proteins. Resulting E. tenella sequences were analysed to determine whether key active site residues were conserved in the putative E. tenella FAS enzymes. Additionally, BLASTX was used to search the non-redundant NCBI protein database using the E. tenella genes as query sequences.
To identify type I FAS enzymes in E. tenella, we used the protein sequences of C. parvum FASi (Zhu et al. 2000b) and T. gondii FASi (GI 95007112) as query sequences to search the E. tenella genome database. Putative E. tenella genes were analysed to determine the presence of FASi modules, the individual enzyme domains, and the key catalytic residues described for C. parvum (Zhu et al. 2000b).
RESULTS AND DISCUSSION
Recombinant EtENR has enzymatic activity which is inhibited by triclosan
ENR enzymes of different origins – bacteria, plants and apicomplexa – share high sequence homology (Fig. 1A). We expressed in E. coli the mature form of EtENR (residues 92–410), without the putative N-terminal apicoplast-targeting leader peptide. Recombinant EtENR has 328 amino acid residues, including an N-terminal hexa-histidine tag and 3 extra vector-derived residues. The apparent molecular mass as determined by SDS-PAGE is 35 kDa (Fig. 1B); on a native PAGE, the recombinant protein migrates as a higher molecular mass species consistent with a tetramer (not shown).
Fig. 1.
EtENR sequence, purification and inhibition by triclosan. (A) DALI (Holm and Sander, 1995) structure-based sequence alignment of ENR enzymes from E. tenella, B. napus, T. gondii, P. falciparum, E. coli and Thermus thermophilus. Sequence numbers are based on EtENR. Identical residues are marked with a red background while certain highly conserved residues are marked with a yellow background. The green dot denotes the starting amino acid (glycine 92) of the EtENR construct used in these studies. Residues marked with blue triangles contact bound cofactor in other ENR structures. Black boxes frame the residues comprising the three disordered loops in the EtENR structure. (B) SDS-PAGE of pure recombinant EtENR protein showing the apparent molecular mass of 35 kDa. (C) The IC50 curve of EtENR inhibition by triclosan. Error bars show the standard deviations between triplicate measurements.
ENR catalyses the reduction of trans-2-enoyl-ACP to acyl-ACP using NADH as a cofactor. We detected in vitro enzymatic activity of recombinant EtENR using a spectrophotometric assay that employs crotonyl-CoA (trans-2-butenoyl coenzyme A) as a substrate analogue of crotonyl-ACP. The Km and Vmax for NADH are 60 µm and 19 µm·min−1, whereas Km and Vmax for crotonyl-CoA are 40 µm and 10 µm·min−1, respectively. These values agree well with those observed for P. falciparum ENR under similar assay conditions: Km and Vmax for crotonyl-CoA are 48 µm and 16 µm.min−1 (Perozzo et al. 2002). Interestingly, crotonyl-CoA is a poor substrate for E. coli ENR with a Km in the millimolar range, while crotonyl-ACP (Km = 22 µm) and trans-2-dodecenoyl-CoA (Km = 30 µm) are better substrates (Bergler et al. 1994; Ward et al. 1999; Sivaraman et al. 2003). Substrate Km values indicate that there are some differences between the apicomplexan ENRs and the well-studied E. coli enzyme.
Triclosan, a potent inhibitor of bacterial ENRs, inhibits the activity of EtENR with an apparent IC50 of 60 nm (Fig. 1C). This inhibitory effect of triclosan is comparable to an IC50 of 66 nm determined for P. falciparum ENR (Kapoor et al. 2004). Similarly, an octa-arginine-triclosan conjugate inhibited T. gondii ENR activity with an apparent IC50 of 40 nm when pre-incubated with TgENR for 12 h (Samuel et al. 2003). These results demonstrate a similar triclosan potency for apicomplexan ENRs, and suggest a common mode of action. Initially, IC50 values reported for E. coli ENR were approximately 2 µm (Heath et al. 1999). However, assays conducted with less enzyme (200 nm) indicate that the IC50 value for E. coli ENR is below 120 nm (Levy et al. 1999). Thus, triclosan inhibition of EtENR is similar to that observed in other bacterial and apicomplexan ENRs.
EtENR contains 3 cysteine residues in its primary sequence, however, the structure of EtENR shows that the cysteines are reduced under the conditions used for crystallization (see below). Despite the lack of a disulfide in the crystal structure, EtENR activity is affected by moderate concentrations of the reducing agents dithiothreitol (DTT) and β-mercaptoethanol (β ME). Toxoplasma gondii ENR (TgENR), which contains only 1 cysteine, is less sensitive to these reducing agents (Fig. 2). The inhibition of EtENR may be due to a decreased affinity for the NADH cofactor as 10 mm DTT does not significantly change the apparent Vmax, but increases the Km for NADH.
Fig. 2.
Effects of reducing agents (DTT and βME) on EtENR and TgENR enzyme activity. The relative enzyme activity versus control (no reducing agent) is shown for different concentrations of DTT and βME. Standard deviations between triplicate measurements are shown with error bars.
Crystal structure of EtENR
The structure of EtENR was solved by molecular replacement using as a starting model the Brassica napus ENR (BnENR, PDB accession code 1ENP) monomer, which shares 60% sequence identity with EtENR (Rafferty et al. 1995). EtENR crystals belong to space group P3221 (Table 1), in which 2 EtENR monomers occupy 1 asymmetric unit as a non-covalent dimer, and the ENR tetramer, the biological unit, is assembled from 2 dimers in adjacent asymmetric units. The quaternary structure of the EtENR tetramer is similar overall to that of other members of the ENR family (Fig. 3A).
Table 1.
Data collection and refinement statistics
| Data collection1 | |
|---|---|
| Unit Cell | α = β = 90°, γ = 120° |
| A (Å) | 105 |
| B (Å) | 105 |
| C (Å) | 93 |
| Space group | P3221 |
| Wavelength (Å) | 1·542 |
| Resolution (Å) | 50–2·6 |
| Unique reflections | 18 683 |
| Redundancy | 6·8 (5·1) |
| Completeness (%) | 99·8 (99·8) |
| Rsym2 | 0·107 (0·598) |
| <I/σ(I)> | 17·7 (2·2) |
| Refinement3 | |
| Resolution (Å) | 50–2·6 |
| No. reflections | 17 398 (869) |
| R4 (%) | 24·4 (27·9) |
| Rmsd bond lengths (Å) | 0·0076 |
| Rmsd bong angles (°) | 1·42 |
| Protein atoms | 3346 |
| Mean B-values (Å 2)5 | 52·4 (51·3) |
Numbers in parentheses are for outer shell (2·69–2·60Å)
Rsym = Σ|I−<I>|/Σ I; I, intensity.
Numbers in parentheses are for Rfree set, containing 5% of randomly chosen reflections.
R = Σ|Fobs−Fcalc|/ΣFobs.
Number in parenthesis indicates the B-factor for main chain atoms only.
Fig. 3.
(A) The quaternary structure of tetrameric EtENR (magenta) is similar overall to that of BnENR (gold), the model used for crystallographic phasing. (B) Stereo view of EtENR (magenta) monomer superimposed on apo-TgENR (yellow) and TgENR in complex with NAD+-triclosan (green). The cofactor NAD+ and inhibitor triclosan are depicted as stick models colored by atom type. The helix (residues 332–345) of TgENR which becomes ordered upon triclosan binding is highlighted in red, while the connectivity of this disordered region in the apo structure is represented as a dashed yellow line. (C) Stereo view of the cofactor binding sites of EtENR (magenta), apo-TgENR (yellow) and TgENR ternary complex (green). The cofactor NAD+, triclosan and the residues involved in cofactor binding are shown as stick models. Residue numbering refers to the EtENR sequence. (D) Close-up view of the active site of EtENR (magenta), apo-TgENR (yellow), Thermus thermophilus ENR (blue), and Aquifex aoelicus VF5 ENR (orange). The NAD+ and triclosan molecules are shown from the TgENR ternary complex to mark the location of the active site. Dotted lines corresponding to each model represent the missing residue 332–345 region and schematically show connectivity.
To date, 57 structures of ENR enzymes from 7 organisms have been reported (the Protein DataBank). Depending on whether the active site is occupied, ENR structures can be classified as apo-ENR with an empty active site, a binary complex with a bound cofactor NADH, or a ternary complex with both NAD+ and an inhibitor bound at the active site. Among the reported structures, Toxoplasma gondii ENR (TgENR) shares the highest amino acid sequence identity (68%) and homology (81%) with EtENR. This is a reflection of the close phylogenetic relationship between T. gondii and E. tenella (see below). Two TgENR structures were recently published (Muench et al. 2007): a ternary TgENR with NAD+ and triclosan bound (2O2S.pdb) and an apo-TgENR (2O50.pdb). Overall, the EtENR monomer exhibits high structural similarity with TgENR, conserving the core 6-stranded β-sheet and the flanking 6 α-helices. The positional RMSD between EtENR and TgENR is 1.7 Å(217 of 224 aligned alpha carbons), and the core secondary structural elements of EtENR can be superimposed onto their counterparts in TgENR (Fig. 3B).
A striking feature of the EtENR structure is the degree of disorder near the active site. In our final model at 2.6 Å resolution, 3 adjacent loop regions (144–175, 285–302 and 332–362) are disordered (Fig. 1A, boxed regions). The EtENR structure does not contain bound cofactor or inhibitor ligands, and this may explain some of the disorder in the active site region. One of these loops (Lys332–Lys345, EtENR numbering) is disordered in the apo-TgENR structure, but forms a helix (coloured red in Fig. 3B) in the ternary TgENR complex (Muench et al. 2007). This active site loop has been observed to pack against bound inhibitors in several bacterial and plant ENR structures (Qiu et al. 1999a; Roujeinikova et al. 1999; Levy et al. 2001; Seefeld et al. 2003). In addition to this loop, 2 other active site loops are disordered in the EtENR structure. The first loop (142–175) contains a 13-residue insert not found in the other ENRs shown in Fig. 1A, while the second loop (285–302) is highly conserved in length and amino acid sequence. Given the high level of conservation for this loop, it is surprising that this region is disordered in the EtENR structure.
To assess the effect of ligand binding on the EtENR active site loops, we tried to determine structures of EtENR with either triclosan/NAD+ or NADH alone by co-crystallization and soaking methods. In well-characterized ENR enzymes, NAD+ and triclosan have been shown to form a non-covalent complex that tightly binds to the active site (Kapoor et al. 2004). Despite several attempts, the processed electron density maps showed no indication of the cofactor (NADH or NAD+) or triclosan. This is surprising since EtENR enzymatic activity and inhibition by triclosan (discussed above) demonstrate that both compounds bind to EtENR in solution. It is possible that this crystal form selects a small subgroup within the protein mixture that is not bound to NAD+ or triclosan and that once crystallized the active site residues are ‘frozen’ into an arrangement that is not compatible with the binding of cofactor. Indeed, certain active site residues in the structure seem to occupy the cofactor binding site (discussed below).
Nearly all of the residues involved in the interactions with cofactor NAD+ in TgENR are conserved in EtENR (Fig. 1A, blue triangles). In TgENR, these residues shift very little in position after the binding of NAD+ and triclosan. In addition to the formation of the Lys332–Lys345 helix (described above), Trp134 shifts in position to form a π–π stacking interaction with the adenine ring of bound NAD+ (Fig. 3C) (Muench et al. 2007). In EtENR, the Trp134 side-chain is tilted even further away from the anticipated position of the NAD+ adenine ring. The positions of nearby residues (G107, G115, S231, and L183–V186) are similar to those found in the TgENR structure with shifts in main chain position of no more than 0.5 Å. Overall, residues that interact with the adenosine diphosphate moiety of NAD+ in TgENR are similarly positioned in the EtENR structure, forming a binding site that could accommodate this portion of the cofactor (Fig. 3C). In contrast, the architecture of the nicotinamide binding site is considerably different in the EtENR structure and appears to be incompatible with cofactor binding. Both Asn234 and Tyr283 protrude into the cofactor binding site in the EtENR structure. Similarly, the Ala328–Leu331 loop shifts towards the projected binding site for the nicotinamide moiety of NAD+ (Fig. 3C). Given the arrangement of active site residues in this structure of EtENR, it is difficult to imagine how the cofactor could bind.
Our EtENR structure does not contain bound ligands, which may explain why the electron density of some active site loops is poorly resolved. Some or all of these loops may become more ordered upon binding cofactor, inhibitor, or substrate. This phenomenon has been observed in bacterial, plant and apicomplexan ENRs (Qiu et al. 1999a; Roujeinikova et al. 1999; Levy et al. 2001; Seefeld et al. 2003; Muench et al. 2007). In our case, addition of ligands did not help with EtENR crystallization, and the ligand-free structure of EtENR provides a rare glimpse of an ENR enzyme before ligand binding. To date, 3 apo-ENR structures have been deposited to the Protein DataBank: TgENR (2O50.pdb), Thermus thermophilus ENR (TtENR, 1ULU.pdb), and Aquifex aoelicus VF5 ENR (AaENR, 2P91.pdb). Although the pairwise amino acid sequence identity among these ENRs is only around 40%, they exhibit high structural similarity. Pairwise superposition of these ENR structures yields low RMSDs (<1·5 Å) between aligned alpha carbons. More importantly, except for the 332–345 loop that shows conformational changes in TgENR upon binding of triclosan (dotted lines in Fig. 3D), all of the secondary structure elements surrounding the active site are strikingly conserved and virtually identical to that of the TgENR ternary complex. In contrast, the central β-sheet of the EtENR structure is shifted in position relative to the other 3 apo-ENR structures. The first 2 strands of this β-sheet occupy new positions that could interfere with cofactor and inhibitor binding. The first strand and its down-stream loop (residues 227–246) are significantly displaced and the loop takes on a different conformation compared with its counterparts in the other apo-ENR structures (Fig. 3D). This loop conformation appears to significantly encroach on the predicted position of triclosan. Similarly, the neighbouring strand (residues 277–283) is shifted relative to the other apo-ENR structures and may interfere with the binding of the cofactor nicotinamide group (Fig. 3D). The observation that the active site conformation of the other apo-ENR structures resembles that of the cofactor/inhibitor bound structures may suggest a pre-complex state that allows the cofactor to easily access the binding site. In the case of EtENR structure, the active site does not appear to be pre-formed for ligand binding. Thus, our EtENR structure may represent a conformation that is favourably selected during the crystallization process.
A previous EtENR model (Ferguson et al. 2007) suggested that the 2 cysteine residues, 308 and 325, could form an intramolecular disulfide linkage. This EtENR model closely resembles the crystal structure of EtENR. However, the disulfide is not observed in the crystal structure, even though the side chain Cβ atoms of cysteines 308 and 325 are 4·4 Å apart – a distance consistent with disulfide bond formation. Rotation of the cysteine 325 side-chain by 120° (from the trans to gauche+ conformer) would allow a disulfide to form without generating clashes with other EtENR residues.
Analysis of FAS gene models in Eimeria tenella
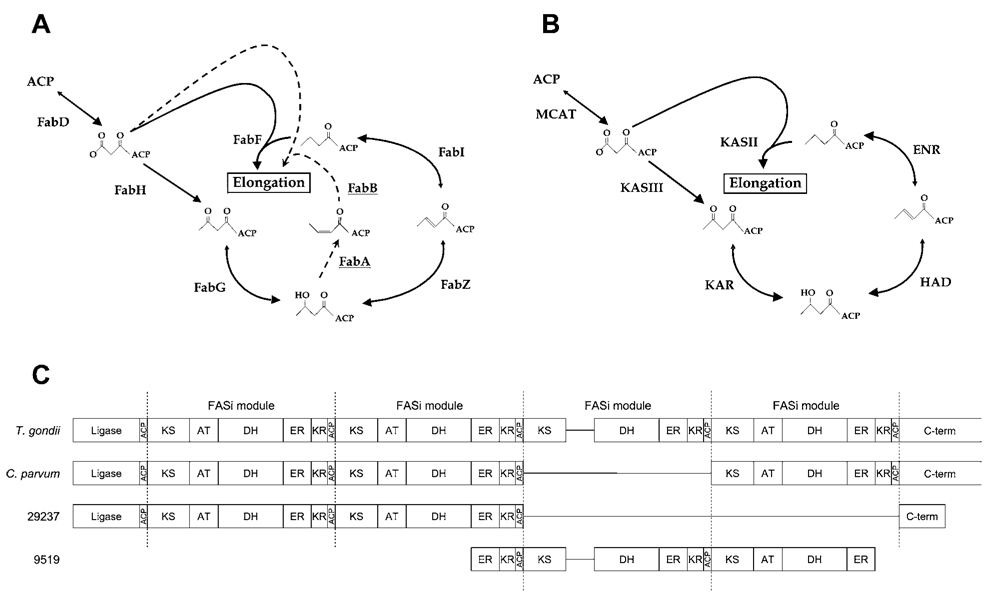
The identification of EtENR and its localization to the apicoplast of E. tenella (Ferguson et al. 2007) led us to analyse genes encoding possible type II FAS enzymes. The proteins of the E. coli type II FAS have been well characterized (reviewed by Campbell and Cronan, 2001 and White et al. 2005). Three proteins are required for the initiation steps of fatty acid biosynthesis : acyl carrier protein (ACP), malonyl-CoA:ACP transacylase (MCAT or FabD), and β-ketoacyl-ACP synthase III (KASIII or FabH). Four more enzymes are then required for fatty acid elongation: β-ketoacyl-ACP reductase (KAR or FabG), β-hydroxyacyl-ACP dehydratase (HAD or FabZ), enoyl-ACP reductase (ENR or FabI), and β-ketoacyl-ACP synthase II (KASII or FabF). These proteins produce saturated fatty acids in E. coli ; however, 2 additional enzymes are needed to synthesize unsaturated fatty acids: β-hydroxyacyl-ACP dehydratase/isomerase (FabA) and β-ketoacyl-ACP synthase I (KASI or FabB). FabA is a paralogue of FabZ, while FabB is paralogous to FabH and FabF (Fig. 4A).
Fig. 4.
FAS pathways. (A) The type II FAS pathway of E. coli. The steps of fatty acid initiation and 1 cycle of elongation are shown. Dotted arrows show the reactions catalysed by FabA and FabB to synthesize unsaturated fatty acids. See Results and Discussion section for a description of individual protein names and activities. (B) Possible type II FAS pathway of E. tenella. The putative E. tenella type II FAS proteins identified in Table 2 are shown. This pathway is identical to the type II pathway described for P. falciparum (Lu et al. 2005). (C) Schematic presentation of possible type I FAS (FASi) enzymes of E. tenella in comparison with T. gondii and C. parvum FASi. FASi modules contain 5 catalytic domains in sequential order (KS, AT, DH, ER, KR) and end with ACP (Zhu et al. 2000b); occasionally a FASi module may lack the AT domain as observed in TgFASi. Abbrevations: ACP, acyl carrier protein; KS, ketoacyl synthase; AT, acyltransferase ; DH, dehydrase; ER, enoyl reductase; KR, ketoacyl reductase.
From the latest E. tenella genomic data (Oct 2005 contig library), we identified candidate genes encoding type II FAS proteins. Table 2 shows BLAST results using type II FAS proteins from E. coli and P. falciparum as queries. Unique E. tenella sequences were identified which closely match the ACP, FabD, FabG, FabZ and FabI proteins of E. coli and P. falciparum. A cross-validation BLAST search with the putative E. tenella proteins primarily identifies orthologues in other apicomplexan para-sites, or cyanobacteria, consistent with the theory that a cyanobacterium is the primary endosymbiont in the evolution of the apicomplexan plastid (Supplemental Data [Online only], Table 2). The translated E. tenella protein sequences contain key amino acids known to be important for the function of these proteins in other systems, increasing the likelihood that functional proteins are encoded by these genes. We also identified E. tenella sequences which best match FabH and FabF. We were able to distinguish between these paralogues by observing an asparagine residue, known to be part of the catalytic triad in all FabH enzymes, which is substituted with a histidine in all FabF enzymes (Huang et al. 1998; Qiu et al. 1999b; Price et al. 2001). We did not find an orthologue of E. coli FabA, and the best match to E. coli FabB was found to be a domain fragment from a type I FAS (discussed below). Thus, the identified E. tenella genes appear to constitute a complete type II FAS pathway similar to that of P. falciparum (Fig. 4B), but lacking the FabA/B shunt found in E. coli. Since FabA and FabB are required for the synthesis of unsaturated fatty acids in E. coli, it is likely that the E. tenella type II FAS only synthesizes saturated fatty acids.
Table 2.
Eimeria tenella BLAST results using E. coli and P. falciparum type II FAS, P. falciparum PDH complex, and C. parvum and T. gondii type I FAS (FASi) as query sequences
| ProteinA | E. tenella contig | P value | Identity | |
|---|---|---|---|---|
| ACP | EcACP, 78aa | 00020221 | 1·1e-11 | 58% (41/70) |
| GI 1787336 | ||||
| PfACP, 80aa | 00020221 | 2·6e-10 | 65% (30/46) | |
| GI 124801045 | ||||
| FabD | EcFabD, 309aa | 00009244 (N-term) | 5·8e-20 | 49% (60/122) |
| GI 16129055 | ||||
| PfMCAT, 299aa | 00009244 (N-term) | 3·5e-10 | 38% (43/112) | |
| GI 124512984 | ||||
| FabH | EcFabH, 317aa | 00013835 (N-term) | 2·7e-15 | 42% (61/146) |
| GI 16129054 | 00010826 (C-term) | 1·4e-16 | 45% (75/168) | |
| PfKASIII, 311aa | 00013835 (N-term) | 3·7e-22 | 49% (63/129) | |
| GI 124801164 | 00010826 (C-term) | 4·5e-20 | 48% (84/174) | |
| FabG | EcFabG, 244aa | 00020830 | 8·1e-38 | 51% (123/241) |
| GI 16129056 | ||||
| PfKAR, 242aa | 00020830 | 8·5e-51 | 60% (146/242) | |
| GI 124507006 | ||||
| FabZ | EcFabZ, 151aa | 00031132 | 7·8e-22 | 43% (63/146) |
| GI 124529596 | ||||
| PfHAD, 143aa | 00031132 | 3·3e-37 | 55% (78/141) | |
| GI 124513228 | ||||
| FabA1 | EcFabA, 172aa | Not found | ||
| GI 16128921 | ||||
| FabI | EcFabI, 262aa | 00002007 | 2·0e-7 | 40% (56/141) |
| GI 16129249 | ||||
| PfENR, 326aa | 00002007 | 2·5e-42 | 54% (152/280) | |
| GI 86171049 | ||||
| FabF | EcFabF, 413aa | 00020791 | 8·3e-46 | 42% (170/402) |
| GI 16129058 | ||||
| PfKASI/II, 394aa | 00020791 | 1·2e-74 | 54% (218/404) | |
| GI, 86171614 | ||||
| FabB1 | EcFabB, 406aa | 000095192 | 1·1e-18 | N.D.3 |
| GI 16130258 | ||||
| FASi | CpFASi, 8242aa | 00009519 | 0 | ~37%4 |
| GI 4092069 | ||||
| TgFASi, 9940aa | 00029237 | 0 | ~56%4 | |
| GI 95007112 | ||||
| PDH5 | PfPDH E1α, 608aa | 00029262 (central) | 1·4e-51 | 53% (158/300) |
| GI 124804184 | ||||
| PfPDH E1β, 391aa | 00020940 | 3·1e-89 | 60% (161/270) | |
| GI 124809587 | ||||
| PfPDH E2, 640aa | 00009113 (C-term) | 2·1e-22 | 40% (73/182) | |
| GI 44970689 | ||||
| PfPDH E3, 666aa | 00029288 (C-term) | 2·6e-19 | 40% (126/318) | |
| GI 124512464 |
Type II FAS components of E. coli and P. falciparum are used as BLAST queries. The length of each peptide chain is listed for the corresponding protein and the GI number is also included. For P. falciparum Type II FAS, the length of the peptide chain corresponds to the mature form of the enzyme/protein without the N-terminal apicoplast-targeting leader sequence.
Orthologues of EcFabA and EcFabB are not present in the Plasmodium falciparum genome.
Contig 00009519 appears to encode a type I synthase (FASi).
N.D., not determined.
Pairwise sequence identity, as determined by the ‘bl2seq’ function at NCBI.
For components of the P. falciparum PDH complex, the length of protein precursors (full-length) is shown.
E. tenella parasites may contain unsaturated fatty acids synthesized through an alternative mechanism. An early study reported the presence of unsaturated fatty acids such as C18 : 1 and C18 : 2 in E. tenella oocysts (Weppelman et al. 1976). Fatty acid de-saturases, which can generate double bonds at specific positions in the acyl chain, may be responsible for these products. Indeed, we identified a gene model that is highly homologous to a cyanobacterial (Synechococcus elongates PCC 6301) fatty acid de-saturase (DesC, GI 56751558) (reviewed by Los and Murata, 1998). Consistent with this finding, it has recently been proposed from mining genomic data that the apicomplexan parasites, Plasmodium and Toxoplasma, may use desaturase enzymes to synthesize unsaturated fatty acids (Hee Lee et al. 2007).
The genome of E. tenella encodes at least 2 poly-peptides homologous to the apicomplexan Type I FAS enzymes of Cryptosporidium parvum (CpFASi) and T. gondii (TgFASi) (Table 2). CpFASi and TgFASi contain multiple FASi modules in which each FASi module (~2000 aa in length) contains 5 catalytic domains in a specific order and ends with ACP. CpFASi (GI 4092069) contains an N-terminal ligase domain, followed by 3 FASi modules and a C-terminal domain of unknown function (Zhu et al. 2000b). TgFASi (GI 95007112, analysed herein) contains a truncated form of the ligase domain, a C-terminal domain homologous to that of CpFASi, and 4 FASi modules, in which module-3 lacks 1 of the 5 enzyme domains (acyltransferase, AT). We identified 2 unique sequences from the E. tenella genome database that closely resemble individual FASi modules, including the order of the catalytic domains [contigs 00029237 (82 Kb) and 00009519 (12Kb)].
The long contiguous sequence (contig 00029237) is similar to CpFASi and contains 2 complete FASi modules (Supplemental Data [Online only], Table 2) flanked by an N-terminal ligase domain and a truncated version of the C-terminal domain found in CpFASi. A pairwise sequence alignment between the Et- and Cp-FASi modules revealed that the catalytic residues and important enzymatic motifs (Zhu et al. 2000b) are conserved in these sequences (not shown). Contig 00009519 encodes a FASi module flanked by 2 partial modules which are incomplete due to the boundaries of the contig. The encoded protein domains are distinct from those in contig 00029237, indicating that contig 00009519 is the central portion of a second multi-module type I FAS enzyme. Interestingly, the central FASi module of contig 00009519 lacks the acyltransferase (AT) domain, thus resembling module-3 of TgFASi (Fig. 4C). Although it is possible that these sequences may be misassembled due to the preliminary status of the E. tenella genome, our findings indicate that E. tenella contains 2 distinct type I FAS enzymes. While this manuscript was being prepared, the Eimeria genome project released the latest version of assemblies (October 2006). We retrieved the corresponding contigs from the new release using the previously identified contigs as search models (Table 2) and compared the two versions. Despite the addition of new sequences (Supplemental Data, Table 1), the predicted composition of the 2 FAS pathways remains unaltered.
In addition to the E. tenella Type II FAS pathway described above, similar pathways have been described for the apicomplexan parasites P. falciparum and T. gondii. In all 3 organisms, type II FAS enzymes have been localized to the apicoplast organelle (Waller et al. 1998, 2000; Ferguson et al. 2007). The type II FAS of T. gondii is important for the fitness and survival of the parasites. It is involved in the biogenesis of the apicoplast organelle and is required to generate lipoate for the apicoplast-localized pyruvate dehydrogenase (PDH) (Mazumdar et al. 2006; Fleige et al. 2007). Furthermore, the type II FAS found in the apicoplast of P. falciparum and T. gondii is thought to consume acetyl-CoA produced by an apicoplast-localized PDH complex (Foth et al. 2005; Fleige et al. 2007). Two independent studies on P. falciparum and T. gondii lipoate metabolism (Crawford et al. 2006; Allary et al. 2007) show that scavenged lipoate is used in the parasite mitochondrion and not in the apicoplast. These results suggest that de novo synthesis of lipoate from products of Type II FAS is required in the apicoplast organelle of these parasites. This scenario may be similar in E. tenella. We identified the four subunits of the PDH enzyme complex (E1α, E1β, E2 and E3) in the E. tenella genome (Table 2). The localization of the E. tenella PDH is not known and we could not confidently predict localization due to the poor conservation of apicoplast targeting peptides. However, it is likely that insights gleaned from studying the Type II FAS in P. falciparum and T. gondii directly apply to E. tenella.
Apicomplexan parasites employ a surprising variety of fatty acid biosynthesis strategies. P. falciparum contains only a type II FAS, while C. parvum contains only a type I FAS. T. gondii harbours an apicoplast localized type II FAS and a Type I FAS that is thought to be located in the mitochondrion (Coppens, 2006). Amongst the apicomplexan parasites discussed above, T. gondii and E. tenella share a close phylogenetic relationship (Barta et al. 2001). Indeed, based on our analysis of possible type II and type I FAS enzymes, it appears that the organization of FAS pathways in E. tenella is most similar to that found in T. gondii. In T. gondii, the presence of multiple fatty acid synthesis pathways may be tailored to the needs of different organelles. It is likely that E. tenella employs a similar compartment-specific strategy in the synthesis of fatty acids. Unlike T. gondii, there appears to be more than one Type I FAS in E. tenella and the individual roles of these pathways in fatty acid or polyketide biosynthesis remains to be determined.
Supplementary Material
Acknowledgments
We thank Dr M. Huynh for critical reading of the manuscript and members of the Prigge lab for constructive suggestions. The development of the UCSF Chimera package is supported by NIH grant (P41 RR-01081). Sequence data were produced by the E. tenella Sequencing Group at the Sanger Institute and can be obtained from ftp://ftp.sanger.ac.uk/pub/pathogens/Eimeria/tenella/. This work was supported by NIH grants AI065853 (S.T.P.); AI027530 and AI043228 (R.L.M.); a postdoctoral fellowship from the Johns Hopkins Malaria Research Institute (J.Z.L.); and the Research to Prevent Blindness Foundation (R.L.M.).
Footnotes
Note in Proof: A report on the biochemical characterization of E. tenella enoyl reductase was recently published (Cai et al. FEMS Microbiology Letters, 2007). Interestingly, the recombinanat EtENR described by Cai and coworkers was only able to oxidize NADH, but unable to transfer the electron to enoyl-CoA. Our re-combinanat EtENR catalyses the complete enoyl reductase reaction, perhaps due to differences in the constructs or the purification protocols.
REFERENCES
- Allary M, Lu JZ, Zhu L, Prigge ST. Scavenging of the cofactor lipoate is essential for the survival of the malaria parasite Plasmodium falciparum. Molecular Microbiology. 2007;63:1331–1344. doi: 10.1111/j.1365-2958.2007.05592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Barta JR, Martin DS, Carreno RA, Siddall ME, Profous-Juchelkat H, Hozza M, Powles MA, Sundermann C. Molecular phylogeny of the other tissue coccidia: Lankesterella and Caryospora. Journal of Parasitology. 2001;87:121–127. doi: 10.1645/0022-3395(2001)087[0121:MPOTOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bergler H, Fuchsbichler S, Hogenauer G, Turnowsky F. The enoyl-[acyl-carrier-protein] reductase (FabI) of Escherichia coli, which catalyzes a key regulatory step in fatty acid biosynthesis, accepts NADH and NADPH as cofactors and is inhibited by palmitoyl-CoA. European Journal of Biochemistry. 1996;242:689–694. doi: 10.1111/j.1432-1033.1996.0689r.x. [DOI] [PubMed] [Google Scholar]
- Bergler H, Wallner P, Ebeling A, Leitinger B, Fuchsbichler S, Aschauer H, Kollenz G, Hogenauer G, Turnowsky F. Protein EnvM is the NADH-dependent enoyl-ACP reductase (FabI) of Escherichia coli. Journal of Biological Chemistry. 1994;269:5493–5496. [PubMed] [Google Scholar]
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR System: A new software suite for macromolecular structure determination. Acta Crystallographica D Biological Crystallography. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Campbell JW, Cronan JE., Jr Bacterial fatty acid biosynthesis: targets for antibacterial drug discovery. Annual Review Microbiology. 2001;55:305–332. doi: 10.1146/annurev.micro.55.1.305. [DOI] [PubMed] [Google Scholar]
- CCP4. The CCP4 suite: programs for protein crystallography. Acta Crystallographica D Biological Crystallography. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Chang SI, Hammes GG. Homology analysis of the protein sequences of fatty acid synthases from chicken liver, rat mammary gland, and yeast. Proceedings of the National Academy of Sciences, USA. 1989;86:8373–8376. doi: 10.1073/pnas.86.21.8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens I. Contribution of host lipids to Toxoplasma pathogenesis. Cell Microbiology. 2006;8:1–9. doi: 10.1111/j.1462-5822.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- Crawford MJ, Thomsen-Zieger N, Ray M, Schachtner J, Roos DS, Seeber F. Toxoplasma gondii scavenges host-derived lipoic acid despite its de novo synthesis in the apicoplast. EMBO Journal. 2006;25:3214–3222. doi: 10.1038/sj.emboj.7601189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast NM, Kissinger JC, Roos DS, Keeling PJ. Nuclear-encoded, plastid-targeted genes suggest a single common origin for apicomplexan and dinoflagellate plastids. Molecular Biology and Evolution. 2001;18:418–426. doi: 10.1093/oxfordjournals.molbev.a003818. [DOI] [PubMed] [Google Scholar]
- Ferguson DJ, Birch-Andersen A, Hutchison WM, Siim JC. Ultrastructural studies on the endogenous development of Eimeria brunetti. Acta Pathologica et Microbiologica Scandinavica. Section B, Microbiology. 1976;84B:401–413. doi: 10.1111/j.1699-0463.1976.tb01959.x. [DOI] [PubMed] [Google Scholar]
- Ferguson DJ, Campbell SA, Henriquez FL, Phan L, Mui E, Richards TA, Muench SP, Allary M, Lu JZ, Prigge ST, Tomley F, Shirley MW, Rice DW, McLeod R, Roberts CW. Enzymes of type II fatty acid synthesis and apicoplast differentiation and division in Eimeria tenella. International Journal for Parasitology. 2007;37:33–51. doi: 10.1016/j.ijpara.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DJ, Henriquez FL, Kirisits MJ, Muench SP, Prigge ST, Rice DW, Roberts CW, McLeod RL. Maternal inheritance and stage-specific variation of the apicoplast in Toxoplasma gondii during development in the intermediate and definitive host. Eukaryotic Cell. 2005;4:814–826. doi: 10.1128/EC.4.4.814-826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleige T, Fischer K, Ferguson DJ, Gross U, Bohne W. Carbohydrate metabolism in the Toxoplasma gondii apicoplast: localization of three glycolytic isoenzymes, the single pyruvate dehydrogenase complex and a plastid phosphate translocator. Eukaryotic Cell. 2007 doi: 10.1128/EC.00061-07. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foth BJ, Stimmler LM, Handman E, Crabb BS, Hodder AN, McFadden GI. The malaria parasite Plasmodium falciparum has only one pyruvate dehydrogenase complex, which is located in the apicoplast. Molecular Microbiology. 2005;55:39–53. doi: 10.1111/j.1365-2958.2004.04407.x. [DOI] [PubMed] [Google Scholar]
- He CY, Striepen B, Pletcher CH, Murray JM, Roos DS. Targeting and processing of nuclear-encoded apicoplast proteins in plastid segregation mutants of Toxoplasma gondii. Journal of Biological Chemistry. 2001;276:28436–28442. doi: 10.1074/jbc.M102000200. [DOI] [PubMed] [Google Scholar]
- Heath RJ, Rubin JR, Holland DR, Zhang E, Snow ME, Rock CO. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. Journal of Biological Chemistry. 1999;274:11110–11114. doi: 10.1074/jbc.274.16.11110. [DOI] [PubMed] [Google Scholar]
- Hee Lee S, Stephens JL, Englund PT. A fatty-acid synthesis mechanism specialized for parasitism. Nature Reviews. Microbiology. 2007;5:287–297. doi: 10.1038/nrmicro1617. [DOI] [PubMed] [Google Scholar]
- Holm L, Sander C. Dali: a network tool for protein structure comparison. Trends in Biochemical Sciences. 1995;20:478–480. doi: 10.1016/s0968-0004(00)89105-7. [DOI] [PubMed] [Google Scholar]
- Huang W, Jia J, Edwards P, Dehesh K, Schneider G, Lindqvist Y. Crystal structure of beta-ketoacyl-acyl carrier protein synthase II from E. coli reveals the molecular architecture of condensing enzymes. EMBO Journal. 1998;17:1183–1191. doi: 10.1093/emboj/17.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenska J, Crawford MJ, Harb OS, Zuther E, Haselkorn R, Roos DS, Gornicki P. Subcellular localization of acetyl-CoA carboxylase in the apicomplexan parasite Toxoplasma gondii. Proceedings of the National Academy of Sciences, USA. 2001;98:2723–2728. doi: 10.1073/pnas.051629998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Reddy CC, Krishnasastry MV, Surolia N, Surolia A. Slow-tight-binding inhibition of enoyl-acyl carrier protein reductase from Plasmodium falciparum by triclosan. The Biochemical Journal. 2004;381:719–724. doi: 10.1042/BJ20031821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapust RB, Waugh DS. Controlled intracellular processing of fusion proteins by TEV protease. Protein Expression and Purification. 2000;19:312–318. doi: 10.1006/prep.2000.1251. [DOI] [PubMed] [Google Scholar]
- Levy CW, Baldock C, Wallace AJ, Sedelnikova S, Viner RC, Clough JM, Stuitje AR, Slabas AR, Rice DW, Rafferty JB. A study of the structure-activity relationship for diazaborine inhibition of Escherichia coli enoyl-ACP reductase. Journal of Molecular Biology. 2001;309:171–180. doi: 10.1006/jmbi.2001.4643. [DOI] [PubMed] [Google Scholar]
- Levy CW, Roujeinikova A, Sedelnikova S, Baker PJ, Stuitje AR, Slabas AR, Rice DW, Rafferty JB. Molecular basis of triclosan activity. Nature, London. 1999;398:383–384. doi: 10.1038/18803. [DOI] [PubMed] [Google Scholar]
- Los DA, Murata N. Structure and expression of fatty acid desaturases. Biochimica et Biophysica Acta. 1998;1394:3–15. doi: 10.1016/s0005-2760(98)00091-5. [DOI] [PubMed] [Google Scholar]
- Lu JZ, Lee PJ, Waters NC, Prigge ST. Fatty Acid synthesis as a target for antimalarial drug discovery. Combinatorial Chemistry and High Throughput Screen. 2005;8:15–26. doi: 10.2174/1386207053328192. [DOI] [PubMed] [Google Scholar]
- Magnuson K, Jackowski S, Rock CO, Cronan JE., Jr Regulation of fatty acid biosynthesis in Escherichia coli. Microbiological Reviews. 1993;57:522–542. doi: 10.1128/mr.57.3.522-542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J, Wilson EH, Masek K, Hunter CA, Striepen B. Apicoplast fatty acid synthesis is essential for organelle biogenesis and parasite survival in Toxoplasma gondii. Proceedings of the National Academy of Sciences, USA. 2006;103:13192–13197. doi: 10.1073/pnas.0603391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod R, Muench SP, Rafferty JB, Kyle DE, Mui EJ, Kirisits MJ, Mack DG, Roberts CW, Samuel BU, Lyons RE, Dorris M, Milhous WK, Rice DW. Triclosan inhibits the growth of Plasmodium falciparum and Toxoplasma gondii by inhibition of apicomplexan Fab I. International Journal for Parasitology. 2001;31:109–113. doi: 10.1016/s0020-7519(01)00111-4. [DOI] [PubMed] [Google Scholar]
- McRee DE. XtalView/Xfit-A versatile program for manipulating atomic coordinates and electron density. Journal of Structural Biology. 1999;125:156–165. doi: 10.1006/jsbi.1999.4094. [DOI] [PubMed] [Google Scholar]
- Muench SP, Prigge ST, McLeod R, Rafferty JB, Kirisits MJ, Roberts CW, Mui EJ, Rice DW. Studies of Toxoplasma gondii and Plasmodium falciparum enoyl acyl carrier protein reductase and implications for the development of antiparasitic agents. Acta Crystallographica D Biological Crystallography. 2007;63:328–338. doi: 10.1107/S0907444906053625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muench SP, Prigge ST, Zhu L, Kirisits MJ, Roberts CW, Wernimont S, McLeod R, Rice DW. Expression, purification and preliminary crystallographic analysis of the Toxoplasma gondii enoyl reductase. Acta Crystallographica. Section F, Structural Biology and Crystallization Communications. 2006;62:604–606. doi: 10.1107/S1744309106018112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muench SP, Rafferty JB, McLeod R, Rice DW, Prigge ST. Expression, purification and crystallization of the Plasmodium falciparum enoyl reductase. Acta Crystallographica D Biological Crystallography. 2003;59:1246–1248. doi: 10.1107/s0907444903008813. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods in Enzymology Macromolecular Crystallography Part A. 1997:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Perozzo R, Kuo M, Sidhu AS, Valiyaveettil JT, Bittman R, Jacobs WR, Jr, Fidock DA, Sacchettini JC. Structural elucidation of the specificity of the antibacterial agent triclosan for malarial enoyl acyl carrier protein reductase. Journal of Biological Chemistry. 2002;277:13106–13114. doi: 10.1074/jbc.M112000200. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera - a visualization system for exploratory research and analysis. Journal of Computational Chemistry. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Price AC, Choi KH, Heath RJ, Li Z, White SW, Rock CO. Inhibition of beta-ketoacyl-acyl carrier protein synthases by thiolactomycin and cerulenin. Structure and mechanism. Journal of Biological Chemistry. 2001;276:6551–6559. doi: 10.1074/jbc.M007101200. [DOI] [PubMed] [Google Scholar]
- Qiu X, Janson CA, Court RI, Smyth MG, Payne DJ, Abdel-Meguid SS. Molecular basis for triclosan activity involves a flipping loop in the active site. Protein Science. 1999a;8:2529–2532. doi: 10.1110/ps.8.11.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Janson CA, Konstantinidis AK, Nwagwu S, Silverman C, Smith WW, Khandekar S, Lonsdale J, Abdel-Meguid SS. Crystal structure of beta-ketoacyl-acyl carrier protein synthase III. Akey condensing enzyme in bacterial fatty acid biosynthesis. Journal of Biological Chemistry. 1999b;274:36465–36471. doi: 10.1074/jbc.274.51.36465. [DOI] [PubMed] [Google Scholar]
- Rafferty JB, Simon JW, Baldock C, Artymiuk PJ, Baker PJ, Stuitje AR, Slabas AR, Rice DW. Common themes in redox chemistry emerge from the X-ray structure of oilseed rape (Brassica napus) enoyl acyl carrier protein reductase. Structure. 1995;3:927–938. doi: 10.1016/S0969-2126(01)00227-1. [DOI] [PubMed] [Google Scholar]
- Ralph SA, Van Dooren GG, Waller RF, Crawford MJ, Fraunholz MJ, Foth BJ, Tonkin CJ, Roos DS, McFadden GI. Tropical infectious diseases: Metabolic maps and functions of the Plasmodium falciparum apicoplast. Nature Review. Microbiology. 2004;2:203–216. doi: 10.1038/nrmicro843. [DOI] [PubMed] [Google Scholar]
- Roberts CW, McLeod R, Rice DW, Ginger M, Chance ML, Goad LJ. Fatty acid and sterol metabolism: potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa. Molecular and Biochemical Parasitology. 2003;126:129–142. doi: 10.1016/s0166-6851(02)00280-3. [DOI] [PubMed] [Google Scholar]
- Roujeinikova A, Sedelnikova S, de Boer GJ, Stuitje AR, Slabas AR, Rafferty JB, Rice DW. Inhibitor binding studies on enoyl reductase reveal conformational changes related to substrate recognition. Journal of Biological Chemistry. 1999;274:30811–30817. doi: 10.1074/jbc.274.43.30811. [DOI] [PubMed] [Google Scholar]
- Samuel BU, Hearn B, Mack D, Wender P, Rothbard J, Kirisits MJ, Mui E, Wernimont S, Roberts CW, Muench SP, Rice DW, Prigge ST, Law AB, McLeod R. Delivery of antimicrobials into parasites. Proceedings of the National Academy of Sciences, USA. 2003;100:14281–14286. doi: 10.1073/pnas.2436169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seefeld MA, Miller WH, Newlander KA, Burgess WJ, DeWolf WE, Jr, Elkins PA, Head MS, Jakas DR, Janson CA, Keller PM, Manley PJ, Moore TD, Payne DJ, Pearson S, Polizzi BJ, Qiu X, Rittenhouse SF, Uzinskas IN, Wallis NG, Huffman WF. Indole naphthyridinones as inhibitors of bacterial enoyl-ACP reductases FabI and FabK. Journal of Medicinal Chemistry. 2003;46:1627–1635. doi: 10.1021/jm0204035. [DOI] [PubMed] [Google Scholar]
- Shirley MW, Smith AL, Tomley FM. The biology of avian Eimeria with an emphasis on their control by vaccination. Advances in Parasitology. 2005;60:285–330. doi: 10.1016/S0065-308X(05)60005-X. [DOI] [PubMed] [Google Scholar]
- Sivaraman S, Zwahlen J, Bell AF, Hedstrom L, Tonge PJ. Structure-activity studies of the inhibition of FabI, the enoyl reductase from Escherichia coli, by triclosan : kinetic analysis of mutant FabIs. Biochemistry. 2003;42:4406–4413. doi: 10.1021/bi0300229. [DOI] [PubMed] [Google Scholar]
- Smith S. The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. FASEB Journal. 1994;8:1248–1259. [PubMed] [Google Scholar]
- Surolia N, Surolia A. Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nature, Medicine. 2001;7:167–173. doi: 10.1038/84612. [DOI] [PubMed] [Google Scholar]
- Waller RF, Keeling PJ, Donald RG, Striepen B, Handman E, Lang-Unnasch N, Cowman AF, Besra GS, Roos DS, McFadden GI. Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proceedings of the National Academy of Sciences, USA. 1998;95:12352–12357. doi: 10.1073/pnas.95.21.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller RF, Reed MB, Cowman AF, McFadden GI. Protein trafficking to the plastid of Plasmodium falciparum is via the secretory pathway. EMBO Journal. 2000;19:1794–1802. doi: 10.1093/emboj/19.8.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang C, Hernandez L, Allocco J, Basilio A, Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH, Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett J, Schmatz D, Becker JW, Cully D, Singh SB. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature, London. 2006;441:358–361. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]
- Ward WH, Holdgate GA, Rowsell S, McLean EG, Pauptit RA, Clayton E, Nichols WW, Colls JG, Minshull CA, Jude DA, Mistry A, Timms D, Camble R, Hales NJ, Britton CJ, Taylor IW. Kinetic and structural characteristics of the inhibition of enoyl (acyl carrier protein) reductase by triclosan. Biochemistry. 1999;38:12514–12525. doi: 10.1021/bi9907779. [DOI] [PubMed] [Google Scholar]
- Weppelman RM, Vandenheuvel WJ, Wang CC. Mass spectrometric analysis of the fatty acids and nonsaponifiable lipids of Eimeria tenella oocysts. Lipids. 1976;11:209–215. doi: 10.1007/BF02532859. [DOI] [PubMed] [Google Scholar]
- White SW, Zheng J, Zhang YM, Rock The structural biology of type II fatty acid biosynthesis. Annual Review of Biochemistry. 2005;74:791–831. doi: 10.1146/annurev.biochem.74.082803.133524. [DOI] [PubMed] [Google Scholar]
- Williams RB. Epidemiological aspects of the use of live anticoccidial vaccines for chickens. International Journal of Parasitology. 1998;28:1089–1098. doi: 10.1016/s0020-7519(98)00066-6. [DOI] [PubMed] [Google Scholar]
- Williamson DH, Gardner MJ, Preiser P, Moore DJ, Rangachari K, Wilson RJ. The evolutionary origin of the 35 kb circular DNA of Plasmodium falciparum: new evidence supports a possible rhodophyte ancestry. Molecular & General Genetics. 1994;243:249–252. doi: 10.1007/BF00280323. [DOI] [PubMed] [Google Scholar]
- Wilson RJM. Progress with parasite plastids. Journal of Molecular Biology. 2002;319:257–274. doi: 10.1016/S0022-2836(02)00303-0. [DOI] [PubMed] [Google Scholar]
- Zhu G, Marchewka MJ, Keithly JS. Cryptosporidium parvum appears to lack a plastid genome. Microbiology. 2000a;146:315–321. doi: 10.1099/00221287-146-2-315. [DOI] [PubMed] [Google Scholar]
- Zhu G, Marchewka MJ, Woods KM, Upton SJ, Keithly JS. Molecular analysis of a Type I fatty acid synthase in Cryptosporidium parvum. Molecular and Biochemical Parasitology. 2000b;105:253–260. doi: 10.1016/s0166-6851(99)00183-8. [DOI] [PubMed] [Google Scholar]
- Zuther E, Johnson JJ, Haselkorn R, McLeod R, Gornicki P. Growth of Toxoplasma gondii is inhibited by aryloxyphenoxypropionate herbicides targeting acetyl-CoA carboxylase. Proceedings of the National Academy of Sciences, USA. 1999;96:13387–13392. doi: 10.1073/pnas.96.23.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.