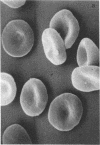
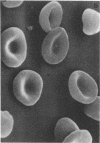
Abstract
The fluoropyrimidine deoxyribonucleotide 5-fluoro-2'-deoxyuridine 5'-monophosphate (FdUMP) was encapsulated in human erythrocytes by a procedure based on hypotonic hemolysis and isotonic resealing. Encapsulated FdUMP (up to 9 mumol/ml of packed erythrocytes) did not affect erythrocyte metabolism or morphology. Hemolysates were found to catalyze efficient dephosphorylation of FdUMP to yield nearly stoichiometric amounts of the corresponding deoxyribonucleoside 5-fluoro-2'-deoxyuridine (FdUrd), an antineoplastic drug showing selective cytotoxicity toward liver metastases from colorectal carcinomas. The dephosphorylation reaction had an apparent Km of 7.7 +/- 1.2 mM FdUMP at pH 7.4 and was remarkably slower at pH 8.2. ATP, GTP, and UTP inhibited both the disappearance of FdUMP and the formation of FdUrd in hemolysates. The enzyme responsible for the FdUMP-to-FdUrd conversion was identified with the deoxyribonucleotide-specific isozyme of erythrocyte pyrimidine 5'-nucleotidase (EC 3.1.3.5). Intracellular formation and subsequent release of FdUrd were observed in intact erythrocytes loaded with FdUMP. Inhibition of FdUrd release from these erythrocytes was obtained by raising the pH intracellularly and, alternatively, by coencapsulation of ATP. Autologous FdUMP-loaded erythrocytes might be used as endogenous bioreactors designed for time-programmed and liver-targeted delivery of FdUrd.
Full text
PDF

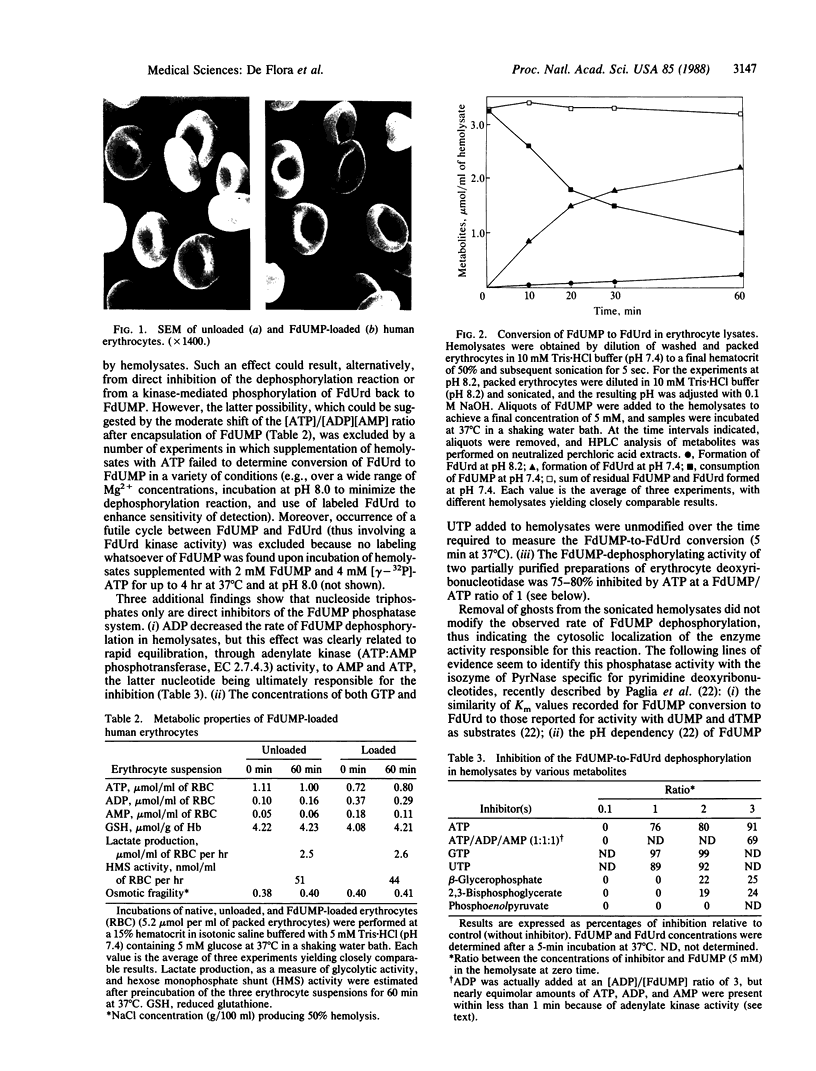
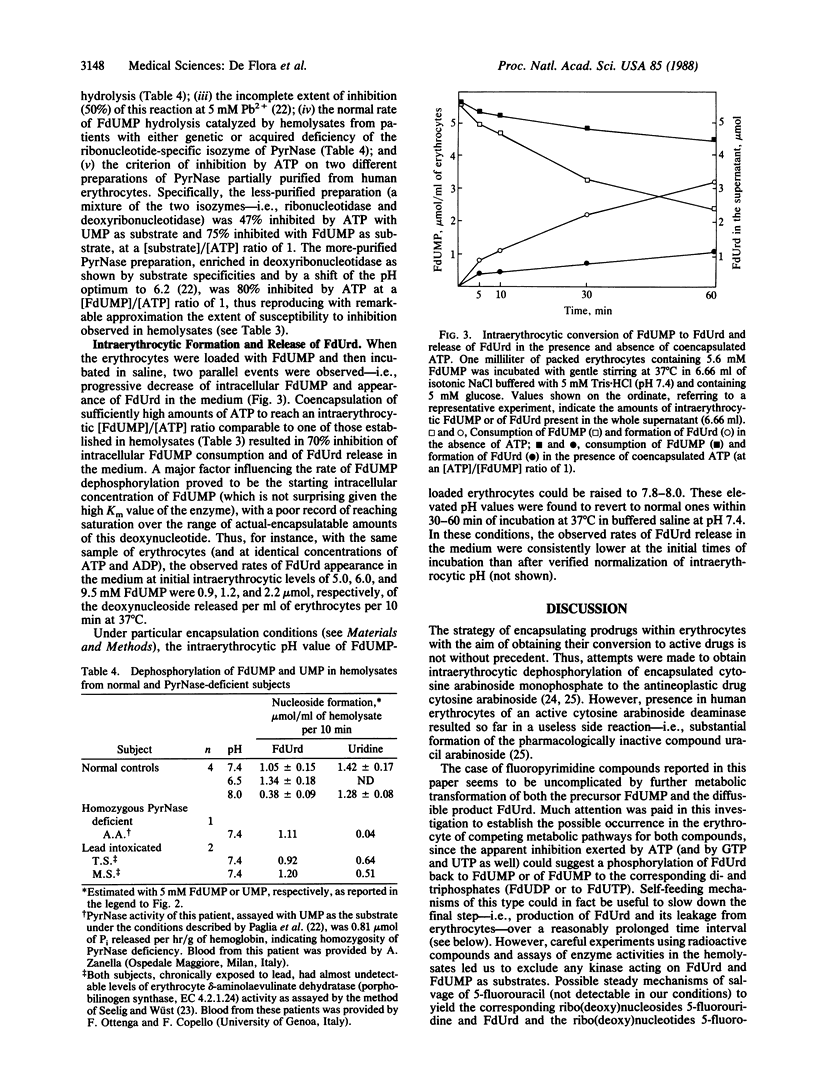

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch C. M., Urist M. M., Soong S. J., McGregor M. A prospective phase II clinical trial of continuous FUDR regional chemotherapy for colorectal metastases to the liver using a totally implantable drug infusion pump. Ann Surg. 1983 Nov;198(5):567–573. doi: 10.1097/00000658-198311000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Cummings F. J., Hoovis M. L., Calabresi P. Phase I study of 5-fluorodeoxyuridine plus cytosine arabinoside infusions in patients with solid tumors. Cancer Treat Rep. 1979 Aug;63(8):1371–1374. [PubMed] [Google Scholar]
- Dale G. L., Villacorte D. G., Beutler E. High-yield entrapment of proteins into erythrocytes. Biochem Med. 1977 Oct;18(2):220–225. doi: 10.1016/0006-2944(77)90093-x. [DOI] [PubMed] [Google Scholar]
- De Flora A., Benatti U., Guida L., Zocchi E. Encapsulation of adriamycin in human erythrocytes. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7029–7033. doi: 10.1073/pnas.83.18.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Flora A., Guida L., Zocchi E., Tonetti M., Benatti U. Construction of glucose oxidase-loaded human erythrocytes: a model of oxidative cytotoxicity. Ital J Biochem. 1986 Sep-Oct;35(5):361–367. [PubMed] [Google Scholar]
- DeLoach J. R., Barton C. Circulating carrier erythrocytes: slow-release vehicle for an antileukemic drug, cytosine arabinoside. Am J Vet Res. 1982 Dec;43(12):2210–2212. [PubMed] [Google Scholar]
- DeLoach J. R., Barton C. Glutaraldehyde-treated carrier erythrocytes for organ targeting of methotrexate in dogs. Am J Vet Res. 1981 Nov;42(11):1971–1974. [PubMed] [Google Scholar]
- DeLoach J. R. Encapsulation of exogenous agents in erythrocytes and the circulating survival of carrier erythrocytes. J Appl Biochem. 1983 Jun;5(3):149–157. [PubMed] [Google Scholar]
- DeLoach J. R., Tangner C. H., Barton C. Hepatic pharmacokinetics of glutaraldehyde-treated methotrexate-loaded carrier erythrocytes in dogs. Res Exp Med (Berl) 1983;183(3):167–175. doi: 10.1007/BF01855639. [DOI] [PubMed] [Google Scholar]
- Ensminger W. D., Rosowsky A., Raso V., Levin D. C., Glode M., Come S., Steele G., Frei E., 3rd A clinical-pharmacological evaluation of hepatic arterial infusions of 5-fluoro-2'-deoxyuridine and 5-fluorouracil. Cancer Res. 1978 Nov;38(11 Pt 1):3784–3792. [PubMed] [Google Scholar]
- Ihler G., Lantzy A., Purpura J., Glew R. H. Enzymatic degradation of uric acid by uricase-loaded human erythrocytes. J Clin Invest. 1975 Sep;56(3):595–602. doi: 10.1172/JCI108129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli A., Benatti U., Salamino F., Sparatore B., Michetti M., Melloni E., Pontremoli S., De Flora A. In vitro correction of erythrocyte glucose 6-phosphate dehydrogenase (G6PD) deficiency. Arch Biochem Biophys. 1979 Oct 15;197(2):543–550. doi: 10.1016/0003-9861(79)90278-9. [DOI] [PubMed] [Google Scholar]
- Nicolau C., Teisseire B. P., Ropars C., Vallez M. O., Herigault R. A. Incorporation of allosteric effectors of hemoglobin in red blood cells. Physiological effects. Bibl Haematol. 1985;(51):92–107. doi: 10.1159/000410232. [DOI] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N., Brockway R. A. Identification of thymidine nucleotidase and deoxyribonucleotidase activities among normal isozymes of 5'-nucleotidase in human erythrocytes. Proc Natl Acad Sci U S A. 1984 Jan;81(2):588–592. doi: 10.1073/pnas.81.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Characteristics of a pyrimidine-specific 5'-nucleotidase in human erythrocytes. J Biol Chem. 1975 Oct 25;250(20):7973–7979. [PubMed] [Google Scholar]
- Plagemann P. G., Wohlhueter R. M., Erbe J. Nucleoside transport in human erythrocytes. A simple carrier with directional symmetry and differential mobility of loaded and empty carrier. J Biol Chem. 1982 Oct 25;257(20):12069–12074. [PubMed] [Google Scholar]
- Ropars C., Avenard G., Chassaigne M. Large-scale entrapment of drugs into resealed red blood cells using a continuous-flow dialysis system. Methods Enzymol. 1987;149:242–248. doi: 10.1016/0076-6879(87)49062-9. [DOI] [PubMed] [Google Scholar]
- Ropars C., Chassaigne M., Villereal M. C., Avenard G., Hurel C., Nicolau C. Resealed red blood cells as a new blood transfusion product. Bibl Haematol. 1985;(51):82–91. doi: 10.1159/000410231. [DOI] [PubMed] [Google Scholar]
- Sansone G., Chiappara M. Morphological alterations of red blood cells in favism. A study with Nomarski optics and electron microscopy. Pathologica. 1984 Mar-Apr;76(1042):191–199. [PubMed] [Google Scholar]
- Sprandel U., Zöllner N. Osmotic fragility of drug carrier erythrocytes. Res Exp Med (Berl) 1985;185(1):77–85. doi: 10.1007/BF01851531. [DOI] [PubMed] [Google Scholar]
- Teisseire B. P., Ropars C., Vallez M. O., Herigault R. A., Nicolau C. Physiological effects of high-P50 erythrocyte transfusion on piglets. J Appl Physiol (1985) 1985 Jun;58(6):1810–1817. doi: 10.1152/jappl.1985.58.6.1810. [DOI] [PubMed] [Google Scholar]
- Zocchi E., Guida L., Benatti U., Canepa M., Borgiani L., Zanin T., De Flora A. Hepatic or splenic targeting of carrier erythrocytes: a murine model. Biotechnol Appl Biochem. 1987 Oct;9(5):423–434. [PubMed] [Google Scholar]