Abstract
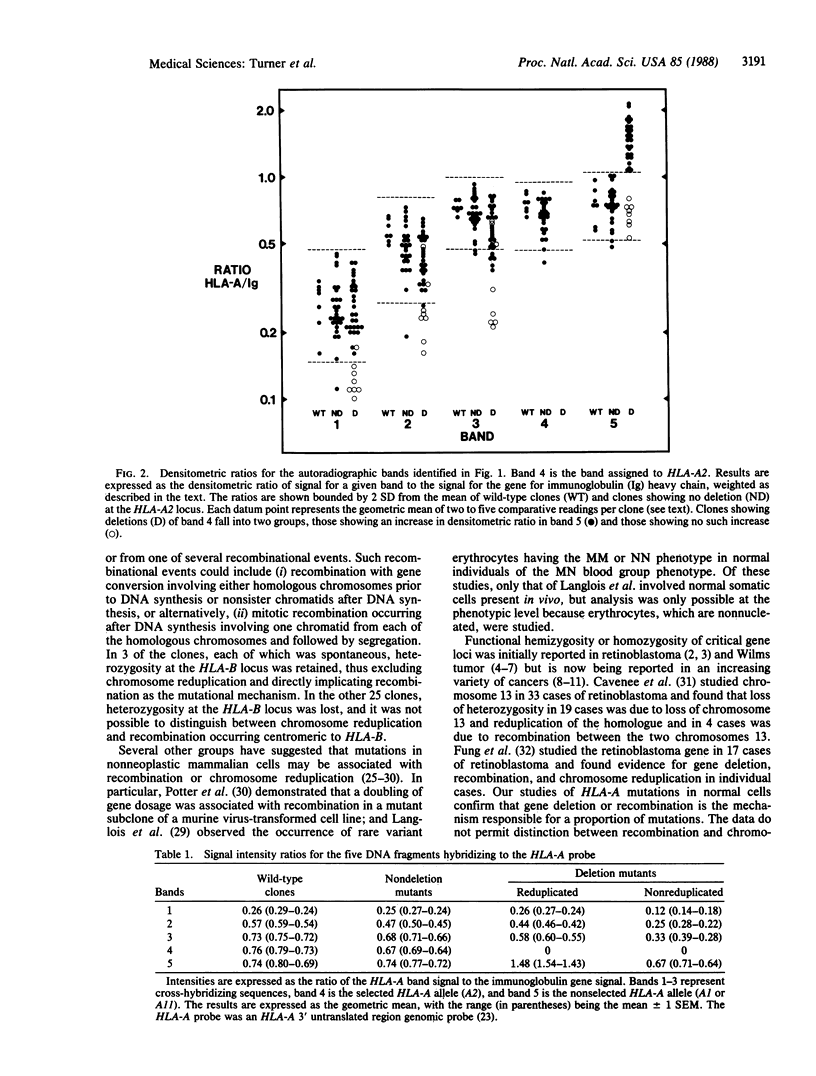
Mutations in human lymphocytes are commonly due to gene deletion. To investigate the mechanism of deletion for autosomal genes, we immunoselected lymphocytes mutated at the HLA-A locus and cloned them for molecular analysis. Of 36 mutant clones that showed deletion of the selected HLA-A allele, 8 had resulted from a simple gene deletion, whereas 28 had resulted from a more complex mutational event involving reduplication of the nonselected HLA-A allele as indicated by hybridization intensity on Southern blots. In 3 of the 28 clones, retention of heterozygosity at the HLA-B locus indicated that the reduplication was due to recombination between the two chromosomes 6; but in the remaining 25 clones, distinction could not be made between recombination and chromosome reduplication. The results indicate that mutations in normal somatic cells frequently result in hemizygosity or homozygosity at gene loci and, thereby, resemble the mutations thought to be important in the etiology of various forms of cancer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R. J., Castle K. L., Borcherding W. R. T-cell cloning to detect the mutant 6-thioguanine-resistant lymphocytes present in human peripheral blood. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6617–6621. doi: 10.1073/pnas.79.21.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini R. J., O'Neill J. P., Nicklas J. A., Heintz N. H., Kelleher P. C. Alterations of the hprt gene in human in vivo-derived 6-thioguanine-resistant T lymphocytes. Nature. 1985 Jul 25;316(6026):369–371. doi: 10.1038/316369a0. [DOI] [PubMed] [Google Scholar]
- Benedict W. F., Murphree A. L., Banerjee A., Spina C. A., Sparkes M. C., Sparkes R. S. Patient with 13 chromosome deletion: evidence that the retinoblastoma gene is a recessive cancer gene. Science. 1983 Feb 25;219(4587):973–975. doi: 10.1126/science.6336308. [DOI] [PubMed] [Google Scholar]
- Campbell C. E., Worton R. G. Segregation of recessive phenotypes in somatic cell hybrids: role of mitotic recombination, gene inactivation, and chromosome nondisjunction. Mol Cell Biol. 1981 Apr;1(4):336–346. doi: 10.1128/mcb.1.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Koufos A., Hansen M. F. Recessive mutant genes predisposing to human cancer. Mutat Res. 1986 Jul;168(1):3–14. doi: 10.1016/0165-1110(86)90019-9. [DOI] [PubMed] [Google Scholar]
- Eves E. M., Farber R. A. Expression of recessive Aprt- mutations in mouse CAK cells resulting from chromosome loss and duplication. Somatic Cell Genet. 1983 Nov;9(6):771–778. doi: 10.1007/BF01539479. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B., Feinberg A. P. Somatic deletion and duplication of genes on chromosome 11 in Wilms' tumours. Nature. 1984 May 10;309(5964):176–178. doi: 10.1038/309176a0. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Hansen M. F., Koufos A., Gallie B. L., Phillips R. A., Fodstad O., Brøgger A., Gedde-Dahl T., Cavenee W. K. Osteosarcoma and retinoblastoma: a shared chromosomal mechanism revealing recessive predisposition. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6216–6220. doi: 10.1073/pnas.82.18.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janatipour M., Trainor K. J., Kutlaca R., Bennett G., Hay J., Turner D. R., Morley A. A. Mutations in human lymphocytes studied by an HLA selection system. Mutat Res. 1988 Mar;198(1):221–226. doi: 10.1016/0027-5107(88)90058-9. [DOI] [PubMed] [Google Scholar]
- Kavathas P., Bach F. H., DeMars R. Gamma ray-induced loss of expression of HLA and glyoxalase I alleles in lymphoblastoid cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4251–4255. doi: 10.1073/pnas.77.7.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B. H., Sidwell B., DeMars R., Orr H. T. Isolation of HLA locus-specific DNA probes from the 3'-untranslated region. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5175–5178. doi: 10.1073/pnas.81.16.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Jensen R. H. Measurements of the frequency of human erythrocytes with gene expression loss phenotypes at the glycophorin A locus. Hum Genet. 1986 Dec;74(4):353–362. doi: 10.1007/BF00280485. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Stachelek J. L. Evidence for intrachromosomal gene conversion in cultured mouse cells. Cell. 1983 Nov;35(1):157–165. doi: 10.1016/0092-8674(83)90218-0. [DOI] [PubMed] [Google Scholar]
- Lundberg C., Skoog L., Cavenee W. K., Nordenskjöld M. Loss of heterozygosity in human ductal breast tumors indicates a recessive mutation on chromosome 13. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2372–2376. doi: 10.1073/pnas.84.8.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley A. A., Trainor K. J., Dempsey J. L., Seshadri R. S. Methods for study of mutations and mutagenesis in human lymphocytes. Mutat Res. 1985 Dec;147(6):363–367. doi: 10.1016/0165-1161(85)90005-6. [DOI] [PubMed] [Google Scholar]
- Morley A. A., Trainor K. J., Seshadri R. S. Cloning of human lymphocytes using limiting dilution. Exp Hematol. 1983 May;11(5):418–424. [PubMed] [Google Scholar]
- Morley A. A., Trainor K. J., Seshadri R., Ryall R. G. Measurement of in vivo mutations in human lymphocytes. Nature. 1983 Mar 10;302(5904):155–156. doi: 10.1038/302155a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Pious D., Hawley P., Forrest G. Isolation and characterization of HL-A variants in cultured human lymphoid cells. Proc Natl Acad Sci U S A. 1973 May;70(5):1397–1400. doi: 10.1073/pnas.70.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter T. A., Zeff R. A., Frankel W., Rajan T. V. Mitotic recombination between homologous chromosomes generates H-2 somatic cell variants in vitro. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1634–1637. doi: 10.1073/pnas.84.6.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Reeve A. E., Housiaux P. J., Gardner R. J., Chewings W. E., Grindley R. M., Millow L. J. Loss of a Harvey ras allele in sporadic Wilms' tumour. Nature. 1984 May 10;309(5964):174–176. doi: 10.1038/309174a0. [DOI] [PubMed] [Google Scholar]
- Sanderson B. J., Morley A. A. Exposure of human lymphocytes to ionizing radiation reduces mutagenesis by subsequent ionizing radiation. Mutat Res. 1986 Dec;164(6):347–351. doi: 10.1016/0165-1161(86)90027-0. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Martuza R. L., Gusella J. F. Loss of genes on chromosome 22 in tumorigenesis of human acoustic neuroma. Nature. 1986 Aug 14;322(6080):644–647. doi: 10.1038/322644a0. [DOI] [PubMed] [Google Scholar]
- Solomon E., Voss R., Hall V., Bodmer W. F., Jass J. R., Jeffreys A. J., Lucibello F. C., Patel I., Rider S. H. Chromosome 5 allele loss in human colorectal carcinomas. Nature. 1987 Aug 13;328(6131):616–619. doi: 10.1038/328616a0. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Turner D. R., Morley A. A., Haliandros M., Kutlaca R., Sanderson B. J. In vivo somatic mutations in human lymphocytes frequently result from major gene alterations. Nature. 1985 May 23;315(6017):343–345. doi: 10.1038/315343a0. [DOI] [PubMed] [Google Scholar]
- Wasmuth J. J., Vock Hall L. Genetic demonstration of mitotic recombination in cultured Chinese hamster cell hybrids. Cell. 1984 Mar;36(3):697–707. doi: 10.1016/0092-8674(84)90350-7. [DOI] [PubMed] [Google Scholar]