In C. elegans, hydrogen sulfide (H2S) exposure results in Hif-1 stabilization. hif-1 is required for survival in H2S and constitutive HIF-1 stabilization confers resistance to H2S. H2S-induced HIF-1 reporter activity appears to be independent of VHL-1, whereas VHL-1 is required for hypoxic regulation of HIF-1 reporter activity.
Abstract
Rapid alteration of gene expression in response to environmental changes is essential for normal development and behavior. The transcription factor hypoxia-inducible factor (HIF)-1 is well known to respond to alterations in oxygen availability. In nature, low oxygen environments are often found to contain high levels of hydrogen sulfide (H2S). Here, we show that Caenorhabditis elegans can have mutually exclusive responses to H2S and hypoxia, both involving HIF-1. Specifically, H2S results in HIF-1 activity throughout the hypodermis, whereas hypoxia causes HIF-1 activity in the gut as judged by a reporter for HIF-1 activity. C. elegans require hif-1 to survive in room air containing trace amounts of H2S. Exposure to H2S results in HIF-1 nuclear localization and transcription of HIF-1 targets. The effects of H2S on HIF-1 reporter activity are independent of von Hippel–Lindau tumor suppressor (VHL)-1, whereas VHL-1 is required for hypoxic regulation of HIF-1 reporter activity. Because H2S is naturally produced by animal cells, our results suggest that endogenous H2S may influence HIF-1 activity.
INTRODUCTION
In nature, oxygen and hydrogen sulfide (H2S) together create redox environments in which eukaryotes thrive (Fenchel and Finlay, 1995). In fiords, for example, ciliates and flagellates are most abundant at depths containing chemically reactive mixtures of oxygen and H2S. In the terrestrial atmosphere, where oxygen is abundant, animal cells produce H2S (Stipanuk and Beck, 1982). Although the role of endogenous H2S is unclear, exposure to exogenous H2S has profound physiological effects including improved outcome after myocardial infarction in mammals (Elrod et al., 2007; Simon et al., 2008) and increased life span in nematodes (Miller and Roth, 2007).
Mice exposed to trace amounts of H2S consume 10-fold less oxygen and exhibit a corresponding reduction in basal metabolic rate (Blackstone et al., 2005). Treatment with H2S also improves survival of mice in hypoxia (Blackstone and Roth, 2007). The response to hypoxia is coordinated by the evolutionarily conserved transcription factor hypoxia-inducible factor (HIF)-1 (Semenza, 2004). First identified as the protein responsible for the hypoxia-dependent transcription of erythropoietin (Wang and Semenza, 1993), HIF-1 activity increases as a function of decreasing oxygen levels. The activity of HIF-1 is contingent upon escaping degradation (Huang et al., 1998), nuclear localization (Wang and Semenza, 1993), and coactivator binding (Arany et al., 1996). Degradation of HIF-1 is mediated by an oxygen-dependent hydroxylation event and subsequent ubiquitin-dependent degradation (Epstein et al., 2001). In C. elegans, the enzymes regulating these events are the EGg Laying defective (EGL)-9 prolyl hydroxylase and the von Hippel–Lindau tumor suppressor (VHL)-1 ubiquitin ligase, respectively.
Here, we use C. elegans to study the influence of H2S on HIF-1. We find that hif-1 is required when nematodes are exposed to H2S. In addition, an elevated level of HIF-1 activity dramatically increases the maximum tolerable concentration of H2S. We show both H2S and hypoxia cause an increase of both HIF-1 protein concentration and nuclear localization throughout the animal. However, H2S and hypoxia treatments are distinct, with different patterns of HIF-1 transcriptional activity and H2S can activate HIF-1 in the absence of vhl-1.
MATERIALS AND METHODS
Strains
Wild-type C. elegans (N2 Bristol) and mutant strains were grown as described previously (Brenner, 1974) at room temperature on nematode growth medium (NGM) plates seeded with live Escherichia coli OP50 food. The following mutant strains were obtained from the C. elegans genetic stock center: CB5602, vhl-1(ok161); JT307, egl-9(sa307); ZG31 hif01(ia04); CB6088, egl-9(sa307), hif-1(ia04); and CB6090, vhl-1(ok161), hif-1(ia04). The strain ZG120 nhr-57::gfp(iaIs07) was a gift from Jo Anne Powell-Coffman (Iowa State University) (Shen et al., 2006). Strains bearing the transgene were generated using standard genetic procedures.
Atmospheric Chambers
Atmospheric chambers were constructed as described previously (Nystul and Roth, 2004). In brief, atmospheres were constructed by mixing defined compressed H2S stock mixtures with room air at a controlled rate to produce the desired final gas concentration. All compressed gas mixtures were obtained from Airgas (Radnor, PA) and were certified standard to within 2% of the indicated concentration. All experiments used continuous flow gas mixtures hydrated by using gas wash bottles. The 5 ppm H2S atmosphere was constructed by mixing 500 ppm H2S (balanced with nitrogen gas) with room air. All other H2S containing atmospheres were constructed by mixing a 5000 ppm H2S tank with room air. The hypoxic chamber was 5000 ppm O2 balanced with nitrogen.
Viability Test
For age-dependent viability tests, nematodes were synchronized by hypochlorite treatment and arrested as starved L1 animals in M9 solution. The larvae were released onto NGM plates seeded with OP50 food and placed into the chamber at the time indicated. Survival was scored 24 h after introduction into the atmosphere.
For viability tests of mutant animals, L4 larvae were moved from a mixed stage NGM plate onto a fresh NGM plates seeded with live OP50 E. coli. These were moved into the H2S containing chamber for 24 h, at which time worms were scored for survival to adulthood or death as L4 larvae. For adaptation experiments, worms were introduced into a chamber containing 50 ppm H2S and grown for at least one generation in that atmosphere before challenge with a higher concentration of H2S.
Antibody Production
A plasmid encoding the N terminus (amino acids 103–465) of C. elegans HIF-1 cloned into the pMal vector (New England Biolabs, Ipswich, MA) was a gift from Joanne Powell-Coffman. The plasmid was expressed in XL1 Blue cells and the recombinant protein was purified on an amylose column according to the manufacturer's instructions (New England Biolabs). The purified protein was used to immunize mice and generate the monoclonal antibody (mAb), by using established protocols (Wayner and Carter, 1987).
Immunostaining
Nematode strains were synchronized by hypochlorite treatment and grown to larval stage (L)4 on peptone-enriched NGM plates seeded with live E. coli NA22 food. Nematodes were rinsed in M9 solution until the supernatant looked clear of bacteria. The worms were placed onto unseeded NGM plates and exposed to gas. After 45-min exposure to H2S or hypoxia, as indicated, the worms were washed off the plate, minced with a razor blade, collected onto glass slides, and frozen on dry ice as described previously (Moore et al., 1999). Fixation was in N,N-dimethylformamide at −20°C for 3.5 min and then rinsed two times with phosphate-buffered saline (PBS) with 1 mM MgCl2 (PBSM). The specimens were ringed with a PAP pen (Thermo Fisher Scientific, Waltham, MA) and incubated in blocking solution (2% bovine serum albumin [BSA] in PBSM) for 30 min at room temperature. The slides were incubated with the conditioned media diluted 1:5 in blocking solution for 60 min at room temperature. The slides were then washed three times for 5 min in blocking solution and then incubated with Alexa Fluor 488-conjugated goat anti-mouse immunoglobulin (Ig)G (Invitrogen, Carlsbad, CA) secondary antibody diluted 1:2000 with 4′,6-diamidino-3-phenylindole dihydrochloride (DAPI) for 60 min. The slides were then washed with blocking solution four times for 10 min each. Coverslips were mounted onto the slides with 50% glycerol in PBSM with DAPI. The slides were immediately examined on a 510 confocal microscope (Carl Zeiss, Thornwood, NY).
Confocal image stacks were acquired with a 40×/1.3 PlanNeofluar oil immersion objective, on a LSM 510 microscope (Carl Zeiss). Green fluorescent protein (GFP) and Alexa 488 fluorescence was excited with the 488 nm line of an Argon laser and imaged through a 500–550 nm bandpass filter. DAPI was excited in two-photon mode with a pulsed femtosecond Chameleon laser tuned to 780 nm (Coherent, Santa Clara, CA), and detected through a 435–485 nm bandpass filter.
Western Blot Analysis
Nematodes were synchronized by hypochlorite treatment and arrested as starved L1 animals in M9 solution. The worms were counted and 10,000 animals were dispensed onto each of three 15-cm NGM plates seeded with OP50 bacteria (1 plate per treatment). When the worms reach L4, they were exposed to 45 min of gas treatment and then pelleted, brought up in sample buffer (72.5 mM Tris, pH 6.8, 1.8% SDS, 9% glycerol, 9 mM EDTA, 10% 2-Mercaptoethanol, and a trace amount of bromphenol blue), frozen in liquid nitrogen, and stored at −80 C. The time between opening the atmospheric chamber and freezing did not exceed 90 s. This was repeated on three different days to generate replicate samples. Before loading, the samples were heated to 95°C for 15 min, vortexed vigorously, heated for another 15 min, and then centrifuged to remove insoluble matter. Polyacrylamide gel electrophoresis (PAGE) and transfer was performed in an XCell SureLock Mini-Cell and XCell II Blot Module system (Invitrogen). Electrophoresis was performed NuPAGE gel (4–12% Bis-Tris; 1.0 mm × 15 well) with 3-(N-morpholino)propanesulfonic acid buffer, by using 12.5 μl of sample. After electrophoresis, the protein was transferred to nitrocellulose, stained with Ponceau S, rinsed, and incubated with block (PBS/Tween 20 [PBST] with 2% normal goat serum and 5% heat-inactivated nonfat dried milk) for 1 h. The blot was then incubated with conditioned media diluted 1:5 in block for 1 h. The blot was rinsed three times with PBST and then incubated with goat anti-mouse IgG-horseradish peroxidase (HRP) (Southern Biotechnology Associates, Birmingham, AL) diluted 1:20,000 for 1 h. The blot was then washed eight times with PBST, incubated with SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, IL), and immediately exposed to film (F-BX810; Phenix Research Products, Candler, NC). The membrane was then stripped by placing it in a solution of 50 mM Tris-HCl, pH 6.8, 2% SDS, and 100 mM 2-mercaptoethanol at 60°C for 30 min. The membrane was then rinsed three times and blocked for 30 min in PBST + 2% BSA. The membrane was then incubated with an anti-actin antibody (catalog no. sc-1616-R; Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:3000 in PBST + 2%BSA for 1 h with rocking. The membrane was washed three times with PBST and then incubated with a secondary antibody-HRP (catalog no. 111-035-003; Jackson ImmunoResearch Laboratories, West Grove, PA) diluted 1:5000 in PBST + 2% BSA for 30 min. This was then washed eight times with PBST and incubated with ECL Western Blotting Substrate (catalog no. 32209; Pierce Chemical, Rockford, IL) and immediately exposed to film. The film was scanned into a computer, and the bands were quantified using the program Quantity One (Bio-Rad Laboratories, Hercules, CA).
mRNA Quantification
Synchronized L4 C. elegans were exposed to air, 60 ppm H2S, or 0.5% O2 for 45 min. Then, 1 ml of TRIzol (Invitrogen) was added to 0.1 ml of packed worms and the worms were frozen in liquid nitrogen. RNA was prepared as described in the TRIzol instruction manual. cDNA was synthesized using a Protoscript First Strand Synthesis kit (New England Biolabs) according to the manufacturer's instructions. Quantitative polymerase chain reaction (PCR) was performed on an iCycler IQ system (Bio-Rad Laboratories) by using Platinum SYBR Green QPCR Supermix (Invitrogen) in 0.03-ml reactions. Standard curves were generated with cDNA, and data were analyzed using the Pfaffl analysis method (Pfaffl, 2001). cDNA levels were normalized to sir-2.1 cDNA, which has been shown previously not to change expression in H2S (Miller and Roth, 2007).
Live Animal Fluorescence Microscopy
Mixed stage NGM plates were sealed in the chamber for the time indicated, after which L4 C. elegans were picked into a drop of 1 mM levamisole in M9. After 5 min, the animals were pipetted onto a pad of 2% agarose in water. The nematodes were visualized on a 510 confocal microscope (Carl Zeiss).
RESULTS
Survival of C. elegans in H2S
We exposed C. elegans to atmospheres containing H2S in room air for 24 h. Animals either survived, seemed healthy, and were indistinguishable from untreated animals, or they died; sickly animals were not observed. Survival was dose dependent, with wild-type worms surviving 50 ppm H2S but not 150 ppm H2S.
Hif-1 Mediates Survival of Worms in Hydrogen Sulfide
C. elegans has a single conserved homologue of mammalian hypoxia-inducible factor α subunit, hif-1 (Jiang et al., 2001). Animals with the hif-1(ia04) null mutation seem normal and are viable in normal culture conditions, but they display reduced embryonic and larval viability in hypoxia compared with wild-type animals (Jiang et al., 2001; Padilla et al., 2002; Nystul et al., 2003). We observed that hif-1(ia04) worms cannot survive exposure to 15 ppm H2S (Table 1). In contrast, even at 50 ppm H2S, wild-type worms survive with high viability and no obvious changes on growth rate or morphology (Miller and Roth, 2007). These results indicate that HIF-1 is necessary for responding appropriately to H2S.
Table 1.
HIF-1 confers resistance to H2S
| Genotype | % animals surviving after 24 h ± SD (N) |
||||
|---|---|---|---|---|---|
| Room air | 15 ppm H2S | 50 ppm H2S | 150 ppm H2S | 500 ppm H2S | |
| Wild type | 100* (106) | 100* (95) | 99 ± 2 (105) | 0* (106) | 0* (59) |
| hif-1(ia04) | 100* (93) | 1 ± 1 (91) | 0* (110) | 0* (109) | 0* (63) |
| vhl-1(ok161) | 100* (89) | 100* (86) | 100* (99) | 99 ± 3 (96) | 2 ± 4 (45) |
| egl-9(sa307) | 100* (94) | 100* (82) | 100* (96) | 100* (87) | 100* (47) |
| hif-1(ia04); vhl-1(ok161) | 100* (58) | 0* (58) | |||
| hif-1(ia04); egl-9(sa307) | 100* (61) | 2 ± 3 (62) | |||
| Wild type (H2S acclimated) | 100* (49) | 100* (68) | 100* (47) | 100* (38) | |
L4 worms were exposed to H2S for 24 h and scored for growth to adulthood. All surviving worms were identical to room air controls.
* No deviation was observed.
We wondered whether animals with elevated HIF-1 activity would be resistant to H2S. HIF-1 protein is a transcription factor that is negatively regulated by EGL-9 and VHL-1 (Semenza, 2007); thus, mutations in either egl-9 or vhl-1 result in an overabundance of HIF-1. We found that animals with a null mutation either in vhl-1(ok161) or egl-9(sa307) were unaffected by 150 ppm H2S, whereas wild-type worms are incapable of surviving (Table 1). Animals with the egl-9(sa307) tolerated up to 500 ppm, whereas vhl-1(ok161) null animals were only able to tolerate 150 ppm H2S. To confirm that the H2S resistance in these mutant animals is dependent on hif-1, we tested these mutant alleles in combination with hif-1 (ia04). Both the egl-9; hif-1 double mutant and the vhl-l; hif-1 double mutant are sensitive to H2S. Thus, an increase in HIF-1 creates resistance to H2S.
We hypothesized, given that hif-1 is required for wild-type worms to survive in H2S, that exposure to H2S may stabilize and increase HIF-1 activity. In this case, HIF-1 activation may allow worms to survive subsequent exposure to higher levels of H2S. To test this idea, we raised wild-type worms in low-level H2S (50 ppm) and then transferred them to higher concentrations (150 and 500 ppm). Consistent with our hypothesis, we found that all naïve worms died in as little as 150 ppm H2S, whereas acclimated worms survived exposure to 150 ppm and even 500 ppm H2S (Table 1). We conclude from the above-mentioned information that H2S may activate HIF-1.
Hydrogen Sulfide Exposure Results in an Increase of HIF-1 Protein Levels and HIF-1 Nuclear Localization
To further explore the possibility that H2S can activate HIF-1 in vivo, we developed a mAb specific for C. elegans HIF-1. HIF-1 protein is detected at high levels in nuclei of egl-9(sa307) and vhl-1(ok161) mutant animals and is absent in hif-1(ia04) worms (Supplemental Figure 1). The large gut nuclei are most easily visible, but HIF-1 protein is accumulated in nuclei throughout the animal. This is in agreement with previous reports showing that the HIF-1 promoter region is ubiquitously active throughout the worm (Jiang et al., 2001). Western blot analysis of total protein detects two bands (molecular masses 106 and 113 kDa) present in extract prepared from egl-9(sa307) but absent in hif-1(ia04) worm extract, indicating that this antibody is specific for HIF-1 (Supplemental Figure 2).
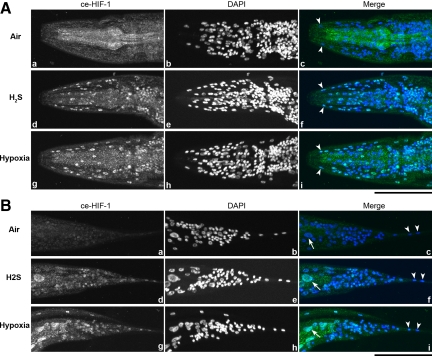
HIF-1 protein levels increase during exposure to H2S. As with hypoxia, H2S exposure results in increased protein concentration in as little as 45 min (Figure 1). We also examined whether H2S treatment alters the cytological location of HIF-1. Immunostaining reveals HIF-1 accumulation in the nuclei throughout the H2S-treated worms, compared with untreated controls (Figure 2). We also exposed nematodes to hypoxia and determined that this causes a pattern of nuclear localization indistinguishable from H2S exposure.
Figure 1.
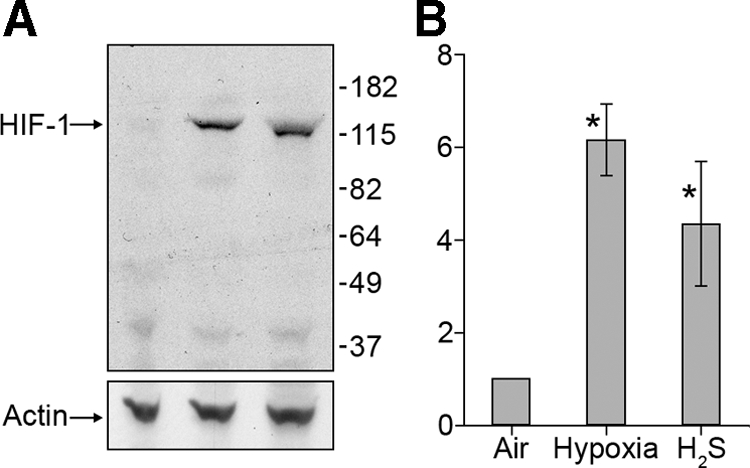
HIF-1 protein levels increase when worms are exposed to H2S. (A) A representative Western blot analysis shows HIF-1 protein increases when worms are exposed to 50 ppm H2S or 0.5% O2. The blots were stripped and reprobed with an anti-actin antibody to confirm equivalent amounts of total protein. The size markers are shown in kilodaltons. (B) Average increase in signal relative to control ± SD of three independent experiments. *p < 0.05 (Student's t test) compared with air-treated animals.
Figure 2.
H2S exposure causes HIF-1 nuclear localization. Both H2S and hypoxia (0.5% O2) treatment induce HIF-1 nuclear localization throughout the animal. Images are stacks of all nuclei visible with DAPI staining. Animals were stained with anti-Ce-Hif-1 antibody (a, d, and g) and DAPI (b, e, and h) to visualize nuclei. Hypodermal nuclei (tailless arrows) and intestinal nuclei (tailed arrows) are clearly observed in the merged images (c, f, and i). (A) Anterior of the worm. (B) Posterior of the worm. Bars, 50 μm.
Hydrogen Sulfide and Hypoxia Treatments Result in Different Patterns of HIF-1 Activity
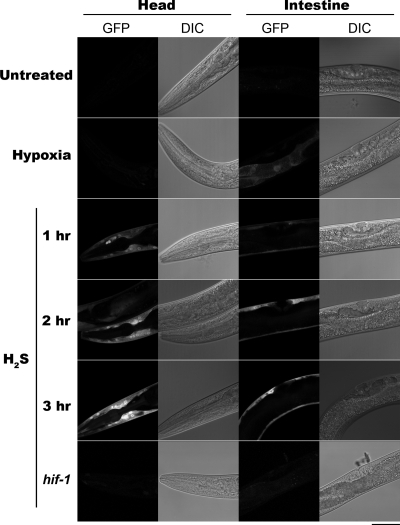
To determine whether nuclear localization of HIF-1 in H2S is associated with transcription of hif-1–responsive genes, we examined expression of nhr-57::gfp(iaIs07), which has HIF-1–dependent expression (Shen et al., 2006). After exposure to hypoxia, NHR-57::GFP was observed in the gut, as has been reported previously (Shen et al., 2006) (Figure 3). In contrast, H2S treatment resulted in expression of NHR-57::GFP in the hypodermis but not in the gut. We tested various H2S exposure lengths (1–3 h) to verify that this expression incongruity was unrelated to the amount of time that worms were exposed to H2S. We found that reporter activity is transient, with maximal fluorescence experienced at 3 h of continuous exposure. We observed bright fluorescence in 100 of 100 worms exposed to H2S, and we observed fluorescence in 0 of 100 untreated worms. We never observed gut fluorescence upon H2S exposure, and we conclude that the pattern of HIF-1 activity in hypoxia and H2S are mutually exclusive. In addition, hif-1(ia04); nhr-57::gfp(iaIs07) worms do not fluoresce when exposed to H2S, showing that hif-1 is required for H2S-induced expression.
Figure 3.
H2S exposure increases expression of NHR-57::GFP. A midfocal plane of a fluorescent confocal photomicrograph and Nomarski micrograph. Fluorescence is not apparent in untreated L4 nhr-57::gfp(iaIs07) transgenic animals. An nhr-57::gfp(iaIs07) animal exposed to hypoxia exhibits HIF-1 activity in the in the gut but absent in the hypodermis. At 1 h into H2S treatment, fluorescence is apparent in the hypodermis throughout the animal but undetectable in the gut. Fluorescence becomes more intense until 3 h into exposure. nhr-57::gfp(iaIs07); hif-1(ia04) animals fail to show any fluorescence, regardless of H2S treatment. Bar, 40 μm.
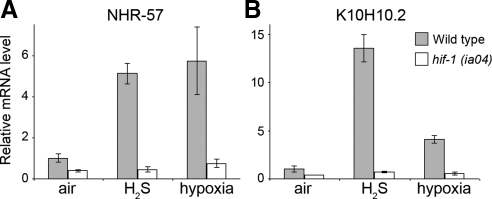
To confirm that HIF-1 activity increase is not an artifact of the transgene, we assayed the relative amounts of known HIF-1 target transcripts in wild-type worms. As with the transgene, H2S exposure increases nhr-57 mRNA levels (Figure 4A), suggesting that the transgene represents a transcriptional activation present in wild-type worms. To confirm that H2S-dependent transcription is not limited to only one gene, we tested another known HIF-1 target, K10H10.2 (Figure 4B), and we found that it was also increased during H2S exposure. Neither of these mRNAs are increased when assayed in a hif-1(ia04) mutant background, showing that hif-1 is required for the induction. These results show that H2S exposure increases transcription of HIF-1 targets in wild-type C. elegans.
Figure 4.
H2S exposure increases mRNA levels of HIF-1 target genes. Real-time reverse transcription-PCR was used to quantitate mRNA levels of HIF-1 target genes in wild-type or hif-1(ia04) worms exposed to H2S or hypoxia. The cDNA of nhr-57 (A) or K10H10.2 (B) is shown as the average increase ± SD relative to sir-2.1 cDNA levels, which do not change during H2S exposure.
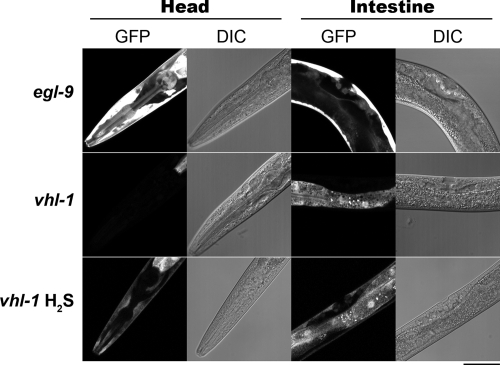
Our previous results demonstrated that egl-9 and vhl-1 have distinct effects on survival, suggesting that they may have distinct effects on HIF-1 activation. To more critically define the relationship between vhl-1 and hif-1, we examined the ability of these mutant worms to express NHR-57::GFP in the presence of H2S (Figure 5). As expected, egl-9(sa307); nhr-57::gfp(iaIs07) mutant worms exhibit constitutive expression in both the gut and in the hypodermis. In contrast, H2S exposure of vhl-1(ok161); nhr-57::gfp(iaIs07) worms results in hypodermal fluorescence that is absent in untreated worms. Thus, this H2S-dependent regulation of HIF-1 is dependent on egl-9 but independent of vhl-l. These results suggest that the activation of HIF-1 by H2S is necessary and sufficient for survival and is distinct from hypoxia.
Figure 5.
H2S dependent HIF-1 activity is independent of VHL-1. nhr-57::gfp(iaIs07); egl-9(sa307) exhibits intense fluorescence is apparent throughout the animal, including in the hypodermis and the gut. 100 Untreated nhr-57::gfp(iaIs07); vhl-1(ok161) animals show basal GFP expression in the gut and H2S treatment induces hypodermal expression. These results are representative of 100 of 100 worms examined for each panel. Bar, 40 μm.
DISCUSSION
Increased H2S or decreased oxygen both constitute a net change in the redox balance to which cells are exposed. We have shown that nematodes rapidly respond to changes in H2S by HIF-1 activation. HIF-1 is also activated by decreased oxygen, which suggests that HIF-1 is important in coordinating the response to changes in environmental and organismal redox balance. Our results demonstrate that vhl-1 is not required for H2S-dependent increase in HIF-1 target gene expression. Previous studies in mammals show that the hypoxic HIF-1 response does require VHL (Iliopoulos et al., 1996), suggesting that cells have multiple ways of activating the HIF responses.
Increased HIF-1 activity is positively correlated with survival of nematodes exposed to H2S. C. elegans exposed to low levels of H2S absolutely require hif-1, and increased levels of HIF-1 facilitate quantal survival in otherwise lethal concentrations of H2S. Hif-1 mutants are sensitive to H2S concentrations that have no effect on wild type (15 ppm). Furthermore, egl-9 and vhl-1 mutants have increased HIF-1 levels and can survive higher concentrations of hydrogen sulfide than wild type. Egl-9 mutant animals show elevated HIF-1 reporter activity compared with vhl-1 mutants worms, consistent with the finding that egl-9 mutant worms can tolerate higher H2S concentrations than vhl-1 mutant worms. The increased survival of egl-9 mutant worms in H2S is reminiscent of previous work which showed that a mutation in egl-9 protects nematodes from cyanide toxicity (Darby et al., 1999; Gallagher and Manoil, 2001). Both sulfide and cyanide disrupt cellular respiration as noncompetitive inhibitors of cytochrome oxidase (Cooper and Brown, 2008). Thus, it is plausible that HIF-1 helps overcome the toxicity by relieving the stress of impeded cytochrome oxidase activity. Indeed, HIF-1 has been shown to increase expression of genes encoding glycolytic enzymes (Chen et al., 2001; Minchenko et al., 2002; Obach et al., 2004) as well as to down-regulate tricarboxylic acid cycle entry and oxygen consumption in mammals (Papandreou et al., 2006).
The H2S-induced expression of a reporter for HIF-1 activity does not require vhl-1 but does require egl-9. This is in contrast to the increase of HIF-1 protein levels observed in both mutant backgrounds. This suggests that H2S may be involved in two steps of HIF-1 activation: protein stabilization and transcriptional activation. A recent article demonstrated the existence of an alternative HIF-1 regulatory pathway in C. elegans (Shen et al., 2006). This pathway does not require vhl-1 but does require egl-9 and a novel protein, regulator of hypoxia-inducible factor (rhy-1). The exact function of rhy-1 remains obscure, but perhaps it is important for responding to nonhypoxic impediments of the respiratory chain. In addition, VHL-independent, EGLN1-dependent HIF regulation has been noted in mammalian cells (Ozer et al., 2005; To and Huang, 2005); thus, it will be interesting to investigate the potential for H2S induction of HIF-1 activity in higher organisms.
We observed that hypoxia and sulfide treatment result in different expression patterns of expression of a reporter for HIF-1 activity. One possibility for the differences observed is that the gut is more easily made hypoxic and the hypodermis is more easily exposed to H2S. This model would predict that increasing the severity of hypoxia should increase the tissue with detectable HIF-1 activity. We were unable to find any concentrations of O2, including anoxia, which would result in HIF-1 activity detected in more tissues. Likewise, increasing the concentration of H2S also failed to increase HIF-1 activity in additional tissues. Also, the near perfect overlap of HIF-1 activity in hypoxia and vhl-1 null animals argues that the observed differences reflect something intrinsic to the tissue, perhaps involving additional transcription factors.
Organisms survive transitions to new environments by changing their physiology to be better suited to the new environment, referred to here as acclimation. Acclimation to nonlethal environmental changes is known to improve the ability to survive otherwise lethal exposure to the same agent. For example, acclimation of nematodes to low concentrations of salt allows them survive subsequent exposure to high salt concentrations that are lethal for naïve animals (Lamitina et al., 2004). We suggest that HIF-1 may be involved in acclimation to H2S containing environments. Wild-type nematodes acclimated to a sub-lethal concentration of H2S (50 ppm), are able to withstand a 10-fold increase in H2S concentration (500 ppm) that would kill all naïve animals. This is concordant with the increase in HIF-1 activity observed and suggests that HIF-1 activity may be essential for the acclimation to H2S.
Here, we have shown that H2S exposure increases HIF-1 activity and increases tolerance of further H2S exposure. This is reminiscent of investigations into hypoxia preconditioning, in which brief exposure to hypoxia increases HIF-1 activity and protects against subsequent hypoxia. We now know that animals produce H2S in a regulated manner and that the resultant sulfide is physiologically important (Kamoun, 2004). In fact, endogenous H2S production has been suggested to be important during hypoxia preconditioning (Bian et al., 2006). In addition, several recent studies have described a protective role of H2S with respect to hypoxia (Blackstone and Roth, 2007), hemorrhage (Morrison et al., 2008), and reperfusion injury (Elrod et al., 2007). It has been shown that overexpression of a sulfide-generating enzyme, cystathionine gamma lyase, results in cardiac protection, suggesting a protective effect of endogenous H2S (Elrod et al., 2007). Although all of these studies implicate H2S as a potentially useful therapeutic, the mechanism of action remains ill defined. Other studies have shown that increased HIF-1 levels also provide protection against reperfusion injury, and the data provided here provide a possible link between these phenomena.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the M.B.R. laboratory for helpful discussions; K. Chan, H. Frazier, D. Miller, and S. Lockett for critical reading; J. Priess for discussing worm anatomy; J. Powell-Coffman for strains and the recombinant ceHIF-1 construct; and the C. elegans Genetics Center for providing strains. This work was supported by supported by the National Institutes of Health grant R01 GM-48435 (to M.B.R.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-03-0199) on November 4, 2009.
REFERENCES
- Arany Z., Huang L. E., Eckner R., Bhattacharya S., Jiang C., Goldberg M. A., Bunn H. F., Livingston D. M. An essential role for p300/CBP in the cellular response to hypoxia. Proc. Natl. Acad. Sci. USA. 1996;23:12969–12973. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian J. S., Yong Q. C., Pan T. T., Feng Z. N., Ali M. Y., Zhou S., Moore P. K. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J. Pharmacol. Exp. Ther. 2006;2:670–678. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- Blackstone E., Morrison M., Roth M. B. H2S induces a suspended animation-like state in mice. Science. 2005;5721:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- Blackstone E., Roth M. B. Suspended animation-like state protects mice from lethal hypoxia. Shock. 2007;4:370–372. doi: 10.1097/SHK.0b013e31802e27a0. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;1:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Pore N., Behrooz A., Ismail-Beigi F., Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J. Biol. Chem. 2001;12:9519–9525. doi: 10.1074/jbc.M010144200. [DOI] [PubMed] [Google Scholar]
- Cooper C. E., Brown G. C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008;40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- Darby C., Cosma C. L., Thomas J. H., Manoil C. Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 1999;26:15202–15207. doi: 10.1073/pnas.96.26.15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod J. W., et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA. 2007;39:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein A. C., et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;1:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Fenchel T., Finlay B. J. Ecology and Evolution in Anoxic Worlds. Oxford, United Kingdom: Oxford University Press; 1995. [Google Scholar]
- Gallagher L. A., Manoil C. Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning. J. Bacteriol. 2001;21:6207–6214. doi: 10.1128/JB.183.21.6207-6214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. E., Gu J., Schau M., Bunn H. F. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA. 1998;14:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos O., Levy A. P., Jiang C., Kaelin W. G., Jr, Goldberg M. A. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc. Natl. Acad. Sci. USA. 1996;20:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Guo R., Powell-Coffman J. A. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc. Natl. Acad. Sci. USA. 2001;14:7916–7921. doi: 10.1073/pnas.141234698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;3:243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- Lamitina S. T., Morrison R., Moeckel G. W., Strange K. Adaptation of the nematode Caenorhabditis elegans to extreme osmotic stress. Am. J. Physiol. Cell. Physiol. 2004;4:C785–C791. doi: 10.1152/ajpcell.00381.2003. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Roth M. B. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2007;51:20618–20622. doi: 10.1073/pnas.0710191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchenko A., Leshchinsky I., Opentanova I., Sang N., Srinivas V., Armstead V., Caro J. Hypoxia-inducible factor-1-mediated expression of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) gene. Its possible role in the Warburg effect. J. Biol. Chem. 2002;8:6183–6187. doi: 10.1074/jbc.M110978200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L. L., Morrison M., Roth M. B. HCP-1, a protein involved in chromosome segregation, is localized to the centromere of mitotic chromosomes in Caenorhabditis elegans. J. Cell Biol. 1999;3:471–480. doi: 10.1083/jcb.147.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison M. L., Blackwood J. E., Lockett S. L., Iwata A., Winn R. K., Roth M. B. Surviving blood loss using hydrogen sulfide. J. Trauma. 2008;1:183–188. doi: 10.1097/TA.0b013e3181507579. [DOI] [PubMed] [Google Scholar]
- Nystul T. G., Goldmark J. P., Padilla P. A., Roth M. B. Suspended animation in C. elegans requires the spindle checkpoint. Science. 2003;5647:1038–1041. doi: 10.1126/science.1089705. [DOI] [PubMed] [Google Scholar]
- Nystul T. G., Roth M. B. Carbon monoxide-induced suspended animation protects against hypoxic damage in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2004;24:9133–9136. doi: 10.1073/pnas.0403312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obach M., Navarro-Sabate A., Caro J., Kong X., Duran J., Gomez M., Perales J. C., Ventura F., Rosa J. L., Bartrons R. 6-Phosphofructo-2-kinase (pfkfb3) gene promoter contains hypoxia-inducible factor-1 binding sites necessary for transactivation in response to hypoxia. J. Biol. Chem. 2004;51:53562–53570. doi: 10.1074/jbc.M406096200. [DOI] [PubMed] [Google Scholar]
- Ozer A., Wu L. C., Bruick R. K. The candidate tumor suppressor ING4 represses activation of the hypoxia inducible factor (HIF) Proc. Natl. Acad. Sci. USA. 2005;21:7481–7486. doi: 10.1073/pnas.0502716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla P. A., Nystul T. G., Zager R. A., Johnson A. C., Roth M. B. Dephosphorylation of cell cycle-regulated proteins correlates with anoxia-induced suspended animation in Caenorhabditis elegans. Mol. Biol. Cell. 2002;5:1473–1483. doi: 10.1091/mbc.01-12-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou I., Cairns R. A., Fontana L., Lim A. L., Denko N. C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell. Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;9:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. STKE. 2007:407–cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- Semenza G. L. Hydroxylation of HIF-1, oxygen sensing at the molecular level. Physiology. 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- Shen C., Shao Z., Powell-Coffman J. A. The Caenorhabditis elegans rhy-1 gene inhibits HIF-1 hypoxia-inducible factor activity in a negative feedback loop that does not include vhl-1. Genetics. 2006;3:1205–1214. doi: 10.1534/genetics.106.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon F., et al. Hemodynamic and metabolic effects of hydrogen sulfide during porcine ischemia/reperfusion injury. Shock. 2008;30:359–364. doi: 10.1097/SHK.0b013e3181674185. [DOI] [PubMed] [Google Scholar]
- Stipanuk M. H., Beck P. W. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 1982;2:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K. K., Huang L. E. Suppression of hypoxia-inducible factor 1alpha (HIF-1alpha) transcriptional activity by the HIF prolyl hydroxylase EGLN1. J. Biol. Chem. 2005;45:38102–38107. doi: 10.1074/jbc.M504342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. L., Semenza G. L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. USA. 1993;9:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J. Cell Biol. 1987;4:1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.