Abstract
Several lines of evidence indicate that tachykinin neuropeptides [substance P (SP), substance K (SK), and neuromedin K (NK)] play a role in regulating the inflammatory and immune responses. To test this hypothesis in a human inflammatory disease, quantitative receptor autoradiography was used to examine possible abnormalities in tachykinin binding sites in surgical specimens from patients with inflammatory bowel disease. Surgical specimens of colon were obtained from patients with ulcerative colitis (n = 4) and Crohn disease (n = 4). Normal tissue was obtained from uninvolved areas of extensive resections for carcinoma (n = 6). In all cases, specimens were obtained less than 5 min after removal to minimize influences associated with degradation artifacts and were processed for quantitative receptor autoradiography by using 125I-labeled Bolton-Hunter conjugates of NK, SK, and SP. In the normal colon a low concentration of SP receptor binding sites is expressed by submucosal arterioles and venules and a moderate concentration is expressed by the external circular muscle, whereas SK receptor binding sites are expressed in low concentrations by the external circular and longitudinal muscle. In contrast, specific NK binding sites were not observed in any area of the human colon. In colon tissue obtained from ulcerative colitis and Crohn disease patients, however, very high concentrations of SP receptor binding sites are expressed by arterioles and venules located in the submucosa, muscularis mucosa, external circular muscle, external longitudinal muscle, and serosa. In addition, very high concentrations of SP receptor binding sites are expressed within the germinal center of lymph nodules, whereas the concentrations of SP and SK binding sites expressed by the external muscle layers are not altered significantly. These results demonstrate that receptor binding sites for SP, but not SK or NK, are ectopically expressed in high concentrations (1000-2000 times normal) by cells involved in mediating inflammatory and immune responses. These data suggest that SP may be involved in the pathophysiology of inflammatory bowel disease and might provide some insight into the interaction between the nervous system and the regulation of inflammation and the immune response in human inflammatory disease.
Full text
PDF

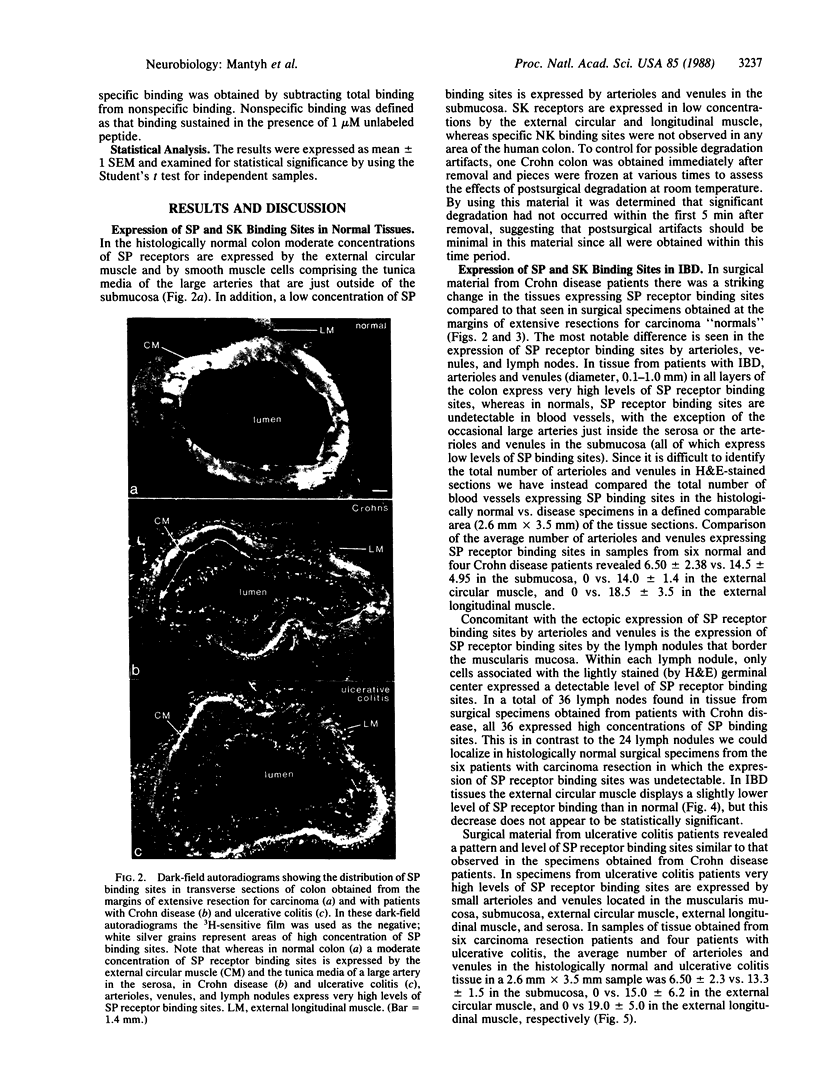

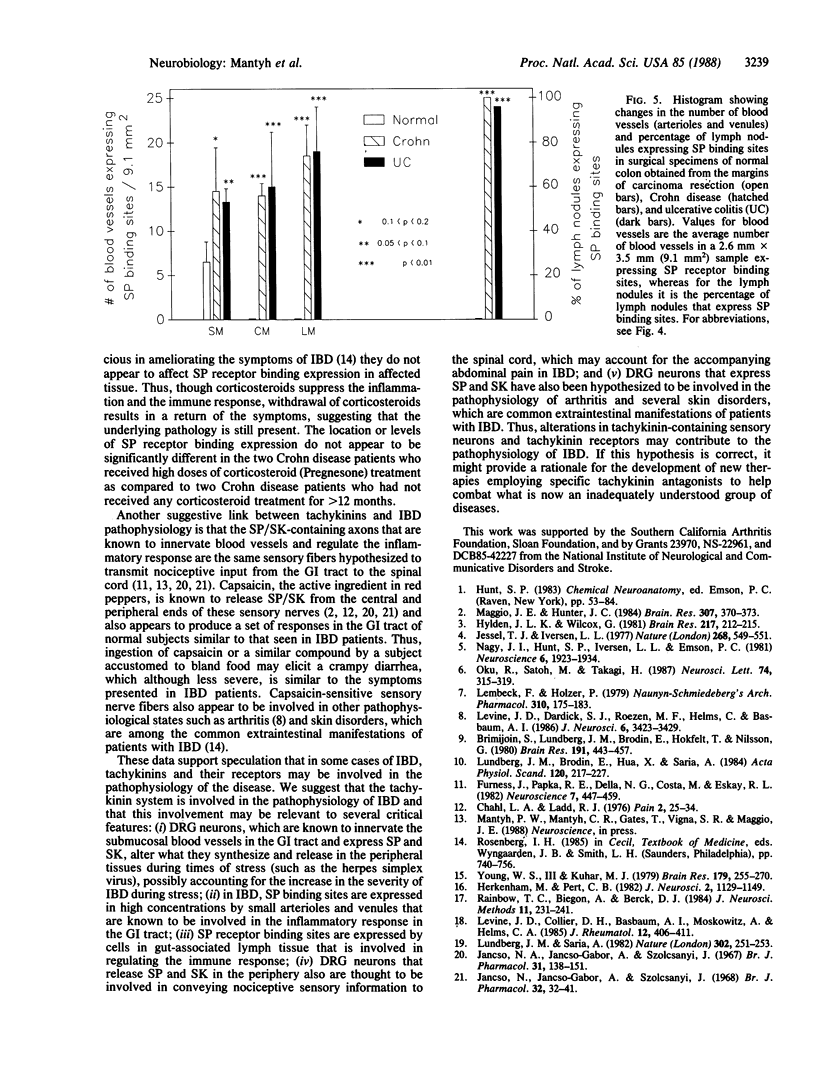
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brimijoin S., Lundberg J. M., Brodin E., Hökfelt T., Nilsson G. Axonal transport of substance P in the vagus and sciatic nerves of the guinea pig. Brain Res. 1980 Jun 9;191(2):443–457. doi: 10.1016/0006-8993(80)91293-7. [DOI] [PubMed] [Google Scholar]
- Chahl L. A., Ladd R. J. Local oedema and general excitation of cutaneous sensory receptors produced by electrical stimulation of the saphenous nerve in the rat. Pain. 1976 Mar;2(1):25–34. doi: 10.1016/0304-3959(76)90043-9. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Papka R. E., Della N. G., Costa M., Eskay R. L. Substance P-like immunoreactivity in nerves associated with the vascular system of guinea-pigs. Neuroscience. 1982 Feb;7(2):447–459. doi: 10.1016/0306-4522(82)90279-2. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Pert C. B. Light microscopic localization of brain opiate receptors: a general autoradiographic method which preserves tissue quality. J Neurosci. 1982 Aug;2(8):1129–1149. doi: 10.1523/JNEUROSCI.02-08-01129.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden J. L., Wilcox G. L. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res. 1981 Jul 27;217(1):212–215. doi: 10.1016/0006-8993(81)90203-1. [DOI] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967 Sep;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. The role of sensory nerve endings in neurogenic inflammation induced in human skin and in the eye and paw of the rat. Br J Pharmacol Chemother. 1968 May;33(1):32–41. doi: 10.1111/j.1476-5381.1968.tb00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Iversen L. L. Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature. 1977 Aug 11;268(5620):549–551. doi: 10.1038/268549a0. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Collier D. H., Basbaum A. I., Moskowitz M. A., Helms C. A. Hypothesis: the nervous system may contribute to the pathophysiology of rheumatoid arthritis. J Rheumatol. 1985 Jun;12(3):406–411. [PubMed] [Google Scholar]
- Levine J. D., Dardick S. J., Roizen M. F., Helms C., Basbaum A. I. Contribution of sensory afferents and sympathetic efferents to joint injury in experimental arthritis. J Neurosci. 1986 Dec;6(12):3423–3429. doi: 10.1523/JNEUROSCI.06-12-03423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Brodin E., Hua X., Saria A. Vascular permeability changes and smooth muscle contraction in relation to capsaicin-sensitive substance P afferents in the guinea-pig. Acta Physiol Scand. 1984 Feb;120(2):217–227. doi: 10.1111/j.1748-1716.1984.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Capsaicin-induced desensitization of airway mucosa to cigarette smoke, mechanical and chemical irritants. Nature. 1983 Mar 17;302(5905):251–253. doi: 10.1038/302251a0. [DOI] [PubMed] [Google Scholar]
- Maggio J. E., Hunter J. C. Regional distribution of kassinin-like immunoreactivity in rat central and peripheral tissues and the effect of capsaicin. Brain Res. 1984 Jul 30;307(1-2):370–373. doi: 10.1016/0006-8993(84)90498-0. [DOI] [PubMed] [Google Scholar]
- Nagy J. I., Hunt S. P., Iversen L. L., Emson P. C. Biochemical and anatomical observations on the degeneration of peptide-containing primary afferent neurons after neonatal capsaicin. Neuroscience. 1981;6(10):1923–1934. doi: 10.1016/0306-4522(81)90032-4. [DOI] [PubMed] [Google Scholar]
- Oku R., Satoh M., Takagi H. Release of substance P from the spinal dorsal horn is enhanced in polyarthritic rats. Neurosci Lett. 1987 Mar 9;74(3):315–319. doi: 10.1016/0304-3940(87)90316-8. [DOI] [PubMed] [Google Scholar]
- Rainbow T. C., Biegon A., Berck D. J. Quantitative receptor autoradiography with tritium-labeled ligands: comparison of biochemical and densitometric measurements. J Neurosci Methods. 1984 Sep;11(4):231–241. doi: 10.1016/0165-0270(84)90085-2. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Kuhar M. J. A new method for receptor autoradiography: [3H]opioid receptors in rat brain. Brain Res. 1979 Dec 28;179(2):255–270. doi: 10.1016/0006-8993(79)90442-6. [DOI] [PubMed] [Google Scholar]