Abstract
The Tgs proteins are structurally homologous AdoMet-dependent eukaryal enzymes that methylate the N2 atom of 7-methyl guanosine nucleotides. They have an imputed role in the synthesis of the 2,2,7-trimethylguanosine (TMG) RNA cap. Here we exploit a collection of cap-like substrates to probe the repertoire of three exemplary Tgs enzymes, from mammalian, protozoan, and viral sources, respectively. We find that human Tgs (hTgs1) is a bona fide TMG synthase adept at two separable transmethylation steps: (1) conversion of m7G to m2,7G, and (2) conversion of m2,7G to m2,2,7G. hTgs1 is unable to methylate G or m2G, signifying that both steps require an m7G cap. hTgs1 utilizes a broad range of m7G nucleotides, including mono-, di-, tri-, and tetraphosphate derivatives as well as cap dinucleotides with triphosphate or tetraphosphate bridges. In contrast, Giardia lamblia Tgs (GlaTgs2) exemplifies a different clade of guanine-N2 methyltransferase that synthesizes only a dimethylguanosine (DMG) cap structure and cannot per se convert DMG to TMG under any conditions tested. Methylation of benzyl7G and ethyl7G nucleotides by hTgs1 and GlaTgs2 underscored the importance of guanine N7 alkylation in providing a key π-cation interaction in the methyl acceptor site. Mimivirus Tgs (MimiTgs) shares with the Giardia homolog the ability to catalyze only a single round of methyl addition at guanine-N2, but is distinguished by its capacity for guanine-N2 methylation in the absence of prior N7 methylation. The relaxed cap specificity of MimiTgs is revealed at alkaline pH. Our findings highlight both stark and subtle differences in acceptor specificity and reaction outcomes among Tgs family members.
Keywords: Giardia, cap hypermethylation, dimethylguanosine, mimivirus
INTRODUCTION
A 2,2,7-trimethylguanosine (TMG) cap is found on noncoding eukaryal RNAs such as small nuclear (sn) and small nucleolar (sno) RNAs and telomerase RNA (Busch et al. 1982; Seto et al. 1999). The enzyme Tgs1 is required for TMG capping in budding and fission yeasts (Mouaikel et al. 2002; Hausmann et al. 2007; Franke et al. 2008; Gallardo et al. 2008), Drosophila (Komonyi et al. 2005), and human somatic cells (Lemm et al. 2006). Tgs homologs are distributed widely among primitive and higher eukarya. Initial biochemical characterization of Schizosaccharomyces pombe Tgs1 (SpoTgs1) indicated that it catalyzes two successive methyltransfer reactions from AdoMet to the N2 atom of guanosine via a distributive mechanism (Hausmann and Shuman 2005a). SpoTgs1 activity in vitro was strictly dependent on guanine-N7 methylation, signifying that its activity in vivo is likely confined to RNAs that already have an m7G cap. Cap-specific guanine-N2 methyltransferase activity has since been reported for human Tgs1 (Hausmann et al. 2008) and several protozoan Tgs homologs, including those of Giardia lamblia, Trichomonas vaginalis, Entamoeba histolytica, and Trypanosoma brucei (Hausmann and Shuman 2005b; Simoes-Barbosa et al. 2008). A recently characterized Tgs homolog encoded by mimivirus is the first example of a viral cap-specific guanine-N2 methyltransferase (Benarroch et al. 2009).
The fungal, protozoal, mammalian, and viral Tgs enzymes comprise a distinct branch of the AdoMet-dependent methyltransferase superfamily. Mutational studies of the fungal, Giardia, human, Trichomonas, and mimivirus proteins highlighted a constellation of conserved essential amino acid side chains that were surmised to form the binding pockets for the AdoMet donor and m7G acceptor (Mouaikel et al. 2003; Hausmann et al. 2007, 2008; Simoes-Barbosa et al. 2008; Benarroch et al. 2009). The recent crystal structure of human Tgs1 (hTgs1) in a ternary complex with m7GTP and AdoHcy (Monecke et al. 2009) confirmed the substrate interactions inferred from mutational studies and modeling exercises, including an essential tryptophan that forms a π-cation stack on the m7G base (Supplemental Fig. S1). Most notably, the structure revealed an extensive network of hTgs1 contacts to the triphosphate moiety of m7GTP that presumably contribute to its cap specificity (Supplemental Fig. S1; Monecke et al. 2009).
All of the recombinant Tgs enzymes studied to date are able to convert simple substrates such as m7G nucleotides or m7G cap dinucleotides to their respective 2,7-dimethylguanosine (DMG) derivatives, as gauged by transfer of a tritiated methyl group from [3H-CH3]-AdoMet to an m7G nucleotide acceptor (Hausmann and Shuman 2005a; Hausmann et al. 2008). This result signifies that there is no inherent requirement for an RNA polynucleotide for cap binding or catalysis. The capacity of SpoTgs1 and hTgs1 to convert a DMG cap to a TMG cap was surmised from a pulse–chase experiment, whereby the 3H-labeled m2,7G nucleotide formed during a pulse reaction could be chased into a 3H-labeled m2,2,7G product in the presence of excess cold AdoMet and fresh Tgs enzyme (Hausmann and Shuman 2005a; Hausmann et al. 2008). Thus, SpoTgs1 and hTgs1 are plausible single agents for TMG capping in S. pombe and human cells, respectively. In contrast, the G. lamblia Tgs (GlaTgs2), T. vaginalis Tgs (TvTgs), and mimivirus Tgs (MimiTgs) enzymes were unable to perform the second methylation reaction (DMG to TMG) when tested using the same pulse–chase procedures (Hausmann and Shuman 2005b; Simoes-Barbosa et al. 2008; Benarroch et al. 2009). This disparity in reaction outcomes between, for example, SpoTgs1 and GlaTgs2, was not an artifact of reliance on m7G nucleotides as methyl acceptors. When similar experiments were performed using m7[α32P]GpppN cap-labeled RNA as the methyl acceptor, it was again noted that the Tgs proteins fell into two functional classes: (1) those that generated only a DMG-capped RNA product (GlaTgs2 and TvTgs) that could be subsequently converted to TMG during a chase reaction catalyzed by SpoTgs1; and (2) those that synthesized per se a TMG-capped product (SpoTgs1, Entamoeba and Trypanosoma Tgs) (Simoes-Barbosa et al. 2008).
To better understand and classify the substrate specificities of the Tgs enzymes implicated in cap hypermethylation, it is critical to be able to assay the first and second guanine-N2 methylation reactions separately, so that detection of DMG conversion to TMG is not contingent on prior DMG formation in the same reaction mixture. Here, we exploited a collection of differentially methylated guanine nucleotides and cap-like dinucleotides to achieve this aim. We report that hTgs1 performs the second methylation reaction (DMG to TMG) and that both guanine-N2 methylation steps are strictly dependent on prior guanine-N7 methylation. In contrast, GlaTgs2 and MimiTgs cannot add a second methyl group to an N2-monomethylated guanine nucleotide under any conditions tested, signifying that these enzymes exemplify a distinct subfamily of DMG-only synthases. We proceeded to evaluate the effects of the cap 5′-phosphates, guanine-N7 substituent, and cap-proximal nucleoside on methyltransferase activity. The findings emphasize the importance of guanine N7 alkylation, rather than a specific requirement for an N7 methyl group, in sustaining the π-cation interaction in the methyl acceptor site. The two obligate DMG synthases are distinguished by their stringency of cap specificity. Whereas GlaTgs2 is strictly dependent on guanine-N7 methylation under all conditions tested, we find that MimiTgs can convert G to m2G with good yield at alkaline pH.
RESULTS
New insights to the methyl acceptor specificity of human Tgs1
We surveyed a collection of cap-like guanine nucleotides for their ability to serve as methyl acceptors for Tgs enzymes, by incubating the proteins with 100 μM [3H-CH3]-AdoMet and 1 mM guanine nucleotide substrate for 60 min at 37°C. The reaction products were then analyzed by polyethyleneimine (PEI)-cellulose thin layer chromatography (TLC), and the 3H-labeled material was visualized by autoradiography. Note that the ionic strength of the TLC buffer was varied for each set of substrates assayed in parallel in order to attain adequate separation of the guanine nucleotide substrates and 3H-labeled guanine nucleotide products from each other (to provide convincing evidence that different substrates yielded different products) and from the 3H-AdoMet donor. In general, the methyl-labeled products migrated immediately ahead of the unlabeled guanine nucleotide substrates, which were visualized by UV illumination of the chromatogram.
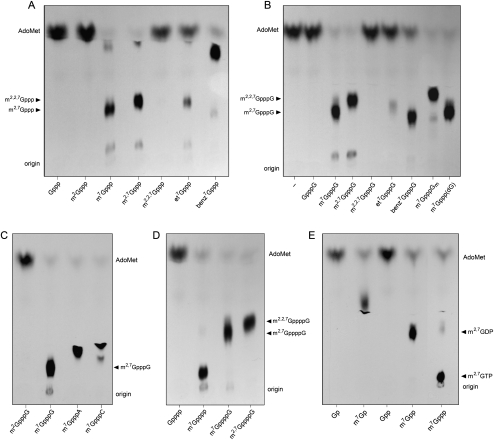
An exemplary product analysis in Figure 1 shows that 1 μg of hTgs1 sufficed to transfer nearly all of the input 3H-CH3 from 0.1 mM AdoMet to 1 mM m7GpppG to form the DMG cap product m2,7GpppG (Fig. 1B). In contrast, hTgs1 was unreactive with the unmethylated dinucleotide GpppG (Fig. 1B). Similarly, hTgs1 catalyzed methyl transfer to the mononucleotide m7GTP to yield m2,7GTP (Fig. 1A). (Note that the minor labeled product migrating just below AdoMet corresponds to m2,7GDP formed by reaction with m7GDP in the m7GTP preparation) (Hausmann and Shuman 2005a). hTgs1 was unreactive with unmethylated GTP (Fig. 1A). These results underscore the requirement for an N7 methyl group for the first guanine-N2 methylation reaction of the two-step TMG synthetic pathway.
FIGURE 1.
Methyl acceptor specificity of human Tgs1. Reaction mixtures (10 μL) containing 50 mM Tris-HCl (pH 8.0), 5 mM DTT, 50 mM NaCl, 100 μM [3H-CH3]AdoMet, 1 mM guanine nucleotide methyl acceptor as specified, and 1 μg of hTgs1(631–853) were incubated for 60 min at 37°C. Aliquots (6 μL) were spotted on PEI-cellulose TLC plates, which were developed either with 0.2 M (NH4)2SO4 (A,D) or 0.05 M (NH4)2SO4 (B,C,E). A control reaction mixture containing enzyme but no guanine nucleotide was included in panel B (lane –). The chromatograms were treated with Enhance, and 3H-labeled material was visualized by autoradiography. (Arrowheads) Positions of unlabeled markers corresponding to methyltransferase reaction products.
Catalysis by hTgs1 of the second methylation step was evinced by the efficient transfer of 3H-CH3 from AdoMet to either the DMG cap dinucleotide m2,7GpppG to form a TMG cap product m2,2,7GpppG (Fig. 1B) or to the DMG mononucleotide m2,7GTP to form m2,2,7GTP (Fig. 1A). hTgs1 titration experiments indicated that the methyltransferase specific activity with either cap dinucleotide- or guanosine mononucleotide-based methyl acceptors (1 mM each) was similar for the DMG synthase and TMG synthase steps (Supplemental Fig. S2A,B). hTgs1 effected no label transfer from AdoMet to m2,2,7GpppG (Fig. 1B) or m2,2,7GTP (Fig. 1A), signifying that a TMG cap is the final product of this enzyme's activity.
By titrating the cap dinucleotide substrates m7GpppG and m2,7GpppG, we derived Km values for the first and second methylation steps of 134 μM m7GpppG and 135 μM m2,7GpppG, respectively (Table 1). The kcat values for the first (0.73 min−1) and second (0.80 min−1) methylation steps were also similar. Thus, the catalytic efficiencies (kcat/Km) of the DMG and TMG synthase reactions of hTgs1 with a cap dinucleotide methyl acceptor were virtually the same.
TABLE 1.
Cap dinucleotide methyl acceptors for human Tgs1
An important mechanistic question is whether the requirement for the guanine-N7 methyl group is maintained during the second catalytic step, it being conceivable that monomethylation at guanine-N2 allows for effective addition of a second N2 methyl group, whether or not N7 is methylated. We approached the issue by testing m2G nucleotides as methyl acceptors. The instructive findings were that 1 μg of hTgs1 displayed no detectable label transfer from 0.1 mM AdoMet to either 1 mM m2GpppG (Fig. 1C) or m2GTP (Fig. 1A) under the same conditions that allowed for nearly complete label transfer to the control substrates m7GpppG and m7GTP. We conclude that hTgs1 enforces m7G cap specificity at both methyl transfer steps. Because the guanine-N7 moiety makes only a single van der Waals contact to protein in the hTgs1 structure (to the Gly770 Cα atom) (Supplemental Fig. S1), we surmise that its contribution to the methyltransferase reaction derives from the positive charge it imparts to the purine ring, resulting in π-cation stacking of m7G on the Trp766 side chain (Supplemental Fig. S1), which is essential for hTgs1 activity (Hausmann et al. 2008).
To probe further the role of the guanine-N7 alkyl group, we tested N7-ethyl and N7-benzyl derivatives of GpppG and GTP for their ability to serve as methyl acceptors at 1 mM concentration. hTgs1 was adept at efficient label transfer from AdoMet to benzyl7GTP to form a product with distinctive TLC migration that we presume is benzyl7m2GTP (Fig. 1A). The enzyme also methylated the cap dinucleotide benzyl7GpppG (Fig. 1B). In contrast, the extents of methyl transfer to ethyl7GTP and ethyl7GpppG were conspicuously lower (Fig. 1A,B). A protein titration experiment indicated that the specific activity in methylating the ethyl7GpppG substrate was about one-tenth the activity with m7GpppG (Supplemental Fig. S2C). It appears that hTgs1 methyltransferase activity does not correlate with the size of the N7 substituent, insofar as the larger benzyl moiety is superior to the smaller ethyl group (Supplemental Fig. S2C). This trend was confirmed by titrations of the N7 alkylated cap dinucleotides. The N7-ethyl substitution increased Km to 576 μM and elicited a significant (10-fold) decrement in kcat (Table 1). The N7-benzyl group also increased Km (to 319 μM) but had little impact on kcat (Table 1). Manual superposition of an isolated benzyl group over the N7–CH3 of the m7GTP in the hTgs1 crystal structure indicated that the benzyl moiety could be accommodated without gross steric hindrance, such that the benzene ring packs against Pro769, Phe670, and Ser671 (model not shown).
Whereas the m7G base is buried deep within a hydrophobic pocket of hTgs1, the triphosphate bridge projects outward to the enzyme's surface, on which the γ phosphate of m7GTP is exposed (Supplemental Fig. S3). It is not yet clear whether or how the N nucleoside of the m7GpppN cap substrate might interact with hTgs1 on its surface adjacent to the γ phosphate. In order to probe the influence (if any) of the N nucleoside on guanine-N2 methylation, we tested a variety of m7GpppN cap analogs (at 1 mM concentration) that differed with respect to the base or sugar at the N position. First, we noted that hTgs1 efficiently methylated m7Gppp(dG) and m7GpppGm dinucleotides containing 2′-deoxy and 2′-OCH3 sugar modifications, respectively (Fig. 1B). Second, hTgs1 generated similar high yields of dimethylated products with m7GpppG, m7GpppA, and m7GpppC acceptors (Fig. 1C); note that the three m2,7GpppN products migrated differently during TLC according to the identity of the N base. Protein titration experiments revealed similar specific activities for the various m7GpppN substrates (Supplemental Fig. S2), suggesting that hTgs1 does not strongly discriminate the base (Supplemental Fig. S2A) or sugar (Supplemental Fig. S2D) of the nucleoside adjacent to the cap. Substrate titrations confirmed that hTgs1 is relatively indifferent to the cap-flanking nucleoside. Changing the N ribonucleoside to A or C or substituting dG for G had little effect on Km or kcat (Table 1).
In light of the interactions of hTgs1 with the m7G triphosphate bridge (Supplemental Fig. S1), we tested a series of m7G nucleotides that differed in the number of 5′-phosphate groups. hTgs1 efficiently methylated m7GMP, m7GDP, and m7GTP to yield dimethylated products m2,7GMP, m2,7GDP, and m2,7GTP that were well resolved by PEI-cellulose TLC according to their relative negative charge (Fig. 1E). hTgs1 also efficiently methylated the tetraphosphate derivative m7G(5′)pppp to generate a DMG product m2,7G(5′)pppp (Fig. 1D). No label transfer was detected to the unmethylated substrates GMP, GDP, GTP, or G(5′)pppp (Fig. 1D,E), again indicative of stringent cap specificity. Protein titration experiments revealed similar specific activities for the m7G nucleoside mono-, di-, tri-, and tetraphosphates (Supplemental Fig. S2E,F). These experiments implicated m7GMP as a minimal effective substrate for guanine-N2 methylation. By titrating this minimal substrate, we derived a Km of 109 ± 21 μM m7GMP and a kcat of 0.73 ± 0.08 min−1 (data not shown).
Having seen that hTgs1 can accommodate an extra phosphate in the context of a mononucleotide acceptor for the DMG synthase reaction, we proceeded to test whether the enzyme could also tolerate a tetraphosphate bridge in the context of a cap dinucleotide. We found that hTgs1 efficiently catalyzed methylation of m7GppppG to yield m2,7GppppG and of m2,7GppppG to yield m2,2,7GppppG (Fig. 1D; Supplemental Fig. S2F). Thus, hTgs1 is not constrained by a longer phosphoanhydride bridge for either the first or second methylation reactions of the TMG synthesis pathway.
Giardia Tgs2 is a DMG synthase only
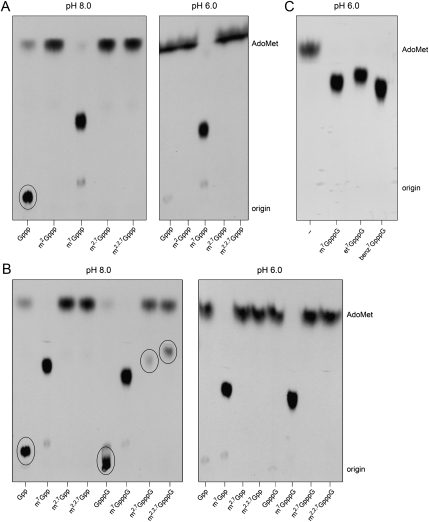
Giardia Tgs2 catalyzed label transfer from AdoMet to the cap dinucleotide m7GpppG and the mononucleotides m7GTP and m7GDP to form 3H-labeled dimethylated products that were resolved from AdoMet by PEI-cellulose TLC (Fig. 2A,B). GlaTgs2 formed no labeled product when reacted with any of the corresponding dimethylated m2,7G nucleotides: m2,7GpppG, m2,7GTP, or m2,7GDP (Fig. 2A,B). Thus, GlaTgs2 is apparently unable to transfer a second methyl group to m2,7G, a property that sharply distinguishes the Giardia enzyme from its human homolog hTgs1 (vide supra). GlaTgs2 failed to methylate GTP (Fig. 2A) or GpppG (data not shown), verifying its reliance on an N7-methylated cap. The possibility that the N7-methyl group might somehow mask the capacity for a second guanine-N2 methylation reaction by GlaTgs2 was ruled out by the finding that m2GTP was also unreactive (Fig. 2A). An N7 alkyl group, rather than a methyl moiety per se, was critical for GlaTgs2 activity, insofar as the enzyme was as effective in label transfer from AdoMet to the N7-substituted cap analogs benzyl7GpppG and ethyl7GpppG as to the standard m7GpppG cap (Fig. 2C). GlaTgs2 readily methylated tetraphosphate cap analogs m7G(5′)pppp and m7GppppG, but was unreactive with G(5′)pppp or m2,7GppppG (Supplemental Fig. S4A).
FIGURE 2.
Methyl acceptor specificity of Giardia Tgs2. Reaction mixtures (10 μL) containing 50 mM Tris-HCl (pH 8.0), 5 mM DTT, 50 mM NaCl, 100 μM [3H-CH3]AdoMet, 1 mM guanine nucleotide methyl acceptor as specified, and 0.5 μg of GlaTgs2 were incubated for 60 min at 37°C. Aliquots (6 μL) were spotted on PEI-cellulose TLC plates, which were developed either with 0.2 M (NH4)2SO4 (A) or 0.05 M (NH4)2SO4 (B,C). 3H-labeled material was visualized by autoradiography.
Mimivirus Tgs is a DMG synthase with less stringent requirements for N7 methylation
Surprising findings emerged when we surveyed the substrate specificity of mimivirus Tgs under the same reaction conditions used to assay the human and Giardia enzymes. MimiTgs catalyzed label transfer from AdoMet to 1 mM m7GTP to form the 3H-labeled dimethylated m2,7GTP, but formed no methylated product when presented with either 1 mM m2,7GTP or m2GTP (Fig. 3A, left panel), signifying that MimiTgs, like Giardia Tgs2, performs only a single step of guanine-N2 methylation. The distinctive and unexpected property of MimiTgs was that it catalyzed methyl transfer to GTP to generate, in high yield, a product migrating just ahead of the chromatographic origin, at the same position as unlabeled m2GTP (highlighted by the oval in Fig. 3A, left panel). The apparent capacity of MimiTgs for guanine-N2 methylation in the absence of N7 methylation contrasted with our initial finding that MimiTgs methyltransferase activity was cap-specific (Benarroch et al. 2009). A potential explanation for this disparity was that, in the initial study, activity was measured in reaction mixtures containing 50 mM Tris-acetate buffer at pH 6.0, whereas all of the present experiments were performed in 50 mM Tris-HCl at pH 8.0. Indeed, when MimiTgs activity was assayed in parallel at pH 6.0, little or no methyl transfer was detected from AdoMet to GTP, though the enzyme catalyzed near quantitative label transfer to m7GTP under the same conditions (Fig. 3A, right panel).
FIGURE 3.
Mimivirus Tgs catalyzes a single methyl addition at cap guanine-N2 and has relaxed cap specificity at alkaline pH. Reaction mixtures (10 μL) containing either 50 mM Tris-HCl (pH 8.0) or 50 mM Tris-acetate (pH 6.0) as indicated; 5 mM DTT, 50 mM NaCl, 100 μM [3H-CH3]AdoMet, 1 mM methyl acceptor as specified; and 1 μg (A,B) or 0.5 μg (C) of MimiTgs were incubated for 60 min at 37°C. Aliquots (6 μL) were spotted on PEI-cellulose TLC plates, which were developed with 0.2 M (NH4)2SO4 (A) or 0.05 M (NH4)2SO4 (B,C). 3H-labeled material was visualized by autoradiography.
These findings were fortified by parallel assays with other guanine nucleotides. MimiTgs catalyzed high-yield methylation of m7GDP and m7GpppG at either pH 8.0 or 6.0 (Fig. 3B), while no methylation of m2,7GDP was seen at either pH. Activity at pH 6.0 was contingent on N7 alkylation with either a methyl, ethyl, or benzyl group (Fig. 3B,C). MimiTgs also methylated tetraphosphate cap analogs m7Gpppp and m7GppppG at pH 6.0, while showing little or no activity with G(5′)pppp or m2,7GppppG (Supplemental Fig. S4B). In contrast, at pH 8.0 we readily detected methyl transfer to GDP and GpppG (highlighted by ovals in Fig. 3B, left panel). We also noted a lower extent of label transfer to the cap dinucleotides m2,7GpppG and m2,2,7GpppG at pH 8.0 (also highlighted by ovals in Fig. 3B, left panel), but not at pH 6.0. Because no such methylation occurred when MimiTgs was presented with the corresponding DMG and TMG mononucleotides m2,7GDP and m2,2,7GDP or m2,7GTP and m2,2,7GTP (Fig. 3A,B), we surmised that the enzyme had added a methyl group at the N2 position of the cap-flanking guanosine, thereby forming m2,7Gppp(m2G) and m2,2,7Gppp(m2G) products, respectively.
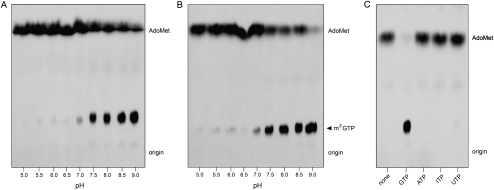
The relaxed cap-dependence of MimiTgs activity as a function of increasing pH was confirmed with either 1 mM GDP (Fig. 4A) or 1 mM GTP (Fig. 4B) as the methyl acceptor. Quantification of results from three independent experiments showed a sharp increase in the yield of m2G product beginning at pH 7.5 and continuing to pH 9.0 (Supplemental Fig. S5). (Higher pH values were not tested). In contrast, MimiTgs catalyzed high-yield methylation m7GDP and m7GTP at all pH values over the same pH range (Supplemental Fig. S6).
FIGURE 4.
Guanine-N2 methylation without N7 methylation. (A,B) Reaction mixtures (10 μL) containing 50 mM Tris buffer (either Tris-acetate pH 5.0–6.5 or Tris-HCl pH 7.0–9.0), 5 mM DTT, 50 mM NaCl, 100 μM [3H-CH3]AdoMet, 1 mM GDP (A) or 1 mM GTP (B), and 1 μg of MimiTgs were incubated for 60 min at 37°C. Aliquots (6 μL) were spotted on PEI-cellulose TLC plates, which were developed either with 0.2 M (NH4)2SO4 (A) or 0.4 M (NH4)2SO4 (B). (C) Reaction mixtures (10 μL) containing 50 mM Tris-HCl (pH 9.0), 5 mM DTT, 100 μM [3H-CH3]AdoMet, 1 mM GTP, ATP, ITP, or UTP as specified, and 1 μg of MimiTgs were incubated for 60 min at 37°C. Aliquots (6 μL) were spotted on PEI-cellulose TLC plates, which were developed with 0.4 M (NH4)2SO4. 3H-labeled material was visualized by autoradiography.
The acceptor specificity of cap-independent methylation by MimiTgs at pH 9.0 was evaluated in the experiment shown in Figure 4C. The enzyme reacted with 1 mM GTP to yield a single labeled product (m2GTP), but was unreactive with ATP, ITP, or UTP. That MimiTgs failed to react with ITP (which lacks an N2 atom but is otherwise identical to GTP) provides further evidence that the guanine-N2 atom is the target of cap-independent methyl transfer.
In light of these results, we retested the ability of hTgs1 and GlaTgs2 to methylate GTP or GDP over the same pH range and found no detectable cap-independent activity even at pH 9.0 (data not shown). Taken together, the distinctive biochemical properties of MimiTgs—DMG synthase only, but relaxed cap-dependence at alkaline pH—define the viral enzyme as a third functional clade within the Tgs family of guanine-N2 methyltransferases.
The Km and kcat values of MimiTgs for m7GDP and GDP were derived from substrate titration experiments at pH 9.0 (Table 2). The salient findings were that the guanine-N7 methyl moiety is a key determinant of substrate binding, eliciting a 175-fold increase in affinity for m7GDP (Km of 5 μM) compared with GDP (Km of 874 μM). In contrast, the N7 methyl group has only a 6.6-fold impact on kcat. The salutary effects of the N7 methyl (comprising a 1100-fold increase in catalytic efficiency for m7GDP versus GDP) are likely because of the positive charge imparted to the guanine base.
TABLE 2.
N7 methyl effect on mimivirus Tgs
DISCUSSION
The present study sharpens the distinctions between cap-specific guanine-N2 methyltransferases, exemplified by human Tgs1, that catalyze two sequential methylation steps to yield a TMG cap, and a functionally separate clade of enzymes—including Giardia Tgs2 and mimivirus Tgs—that can only perform the first step of DMG cap synthesis in vitro. Our findings implicate hTgs1 as sufficient for catalysis of TMG capping in human cells. In contrast, the properties of GlaTgs2 and MimiTgs suggest that: (1) DMG-capped RNAs might exist naturally in protozoa (mimivirus being a pathogen of amoebae); and (2) if the DMG-only synthases function in TMG capping, they do so with help from other proteins, for example, a separate cap guanine-N2 methyltransferase enzyme that specifically converts DMG to TMG or a regulatory protein or subunit that enables GlaTgs2 or MimiTgs to catalyze the second guanine-N2 methylation step.
By using synthetic DMG cap analogs, we were able to study the second hTgs1 cap guanine-N2 methylation reaction in isolation and thereby compare the specificity determinants and kinetic parameters for the TMG synthase reaction of hTgs1 to those of the preceding DMG synthase step. The key findings were that: (1) both guanine-N2 methylation reactions were dependent on prior guanine-N7 methylation; and (2) the catalytic efficiencies of the DMG and TMG synthase steps were similar for a cap dinucleotide substrate. These results reinforce the initial inferences that hTgs1 and SpoTgs1 generate TMG caps in vitro via a distributive mechanism (Hausmann and Shuman 2005a; Hausmann et al. 2008), entailing dissociation of the m2,7G and AdoHcy products of the first step prior to binding of m2,7G and AdoMet substrates for the second step.
From the structure of the hTgs1-m7GTP-AdoHcy ternary complex (Monecke et al. 2009), we can safely surmise that the N2 methyl group must change position during the transition from first step product, where it will be situated “in-line” to the AdoHcy Sδ leaving atom, to second step substrate, whence it must rotate about the C2–N2 bond to free up space for the methyl group of the incoming AdoMet donor. In a distributive mechanism, the newly bound m2,7G could be accommodated directly and specifically in the second step substrate-binding mode by either or both of the following mechanisms: (1) provision of a vicinal docking site for the N2-methyl, oriented away from the AdoMet methyl, that steers the reactive N2 orbital for in-line attack; or (2) adoption of an ordered bi–bi kinetic mechanism in which AdoMet binds first and thereby precludes binding of m2,7G in the first-step product mode. Note that a processive mechanism for TMG capping would involve retention of the m2,7G first step product in the cap-binding pocket as the AdoHcy is released from the donor site, followed by rotation of the N2–CH3, and then binding of fresh AdoMet donor for the second transmethylation step. If the processive mechanism applied to hTgs1, the reaction with m7G nucleotides (in 10-fold molar excess over AdoMet) ought to yield predominantly TMG products, not the DMG products that are observed.
The available hTgs1 crystal structure captures an inhibitor complex of the first methylation reaction. Absent a true substrate or product ternary complex for either step, we cannot surmise the exact position or contacts of an N2 methyl group bound as the second step substrate, although it does appear that the human Tgs1 structure has room to accommodate an N2 methyl facing away from the bound AdoHcy within a hydrophobic pocket lined by Ser763, Pro764, Trp766, and Phe804. A parsimonious explanation for the failure of Giardia Tgs2 and MimiTgs to perform the second methylation reaction is that they cannot accommodate the guanine-N2 methyl in an orientation compatible with AdoMet binding to the donor site. Consistent with this model, we observed that the extent of methyl transfer from 100 μM [3H-CH3]-AdoMet to 100 μM m7GTP by either GlaTgs2 or MimiTgs was undiminished by inclusion of up to 1 mM m2,7GTP in the reaction mixtures (data not shown). The lack of inhibition by a 10-fold excess of the DMG product suggests that DMG does not rebind to the enzyme in the presence of AdoMet to compete with the m7G substrate. It is noteworthy that the Phe804 side chain in the putative N2-methyl pocket of hTgs1 is conserved in Schizosaccharomyces, Entamoeba, and Trypanosoma Tgs1 proteins (each of which has biochemically documented TMG synthase activity), but is substituted by a lysine in Giardia Tgs2 and MimiTgs, which are DMG-only synthases. A simple speculation is that the hydrophobic-to-charged substitution might disrupt the putative m2,7G substrate docking site for the second transmethylation reaction. However, this cannot be the only means to limit action to a single step, insofar as the Trichomonas vaginalis Tgs (a DMG-only synthase) (Simoes-Barbosa et al. 2008) retains a phenylalanine in the position corresponding to Phe804 in human Tgs1. Needless to say, a fuller appreciation of the basis for the different repertoires of the cap guanine-N2 methyltransferase clades depends on obtaining an atomic structure of one or several of the DMG-only synthases.
Here, we used a collection of alkylated guanosine nucleotides to probe the methyl acceptor specificity of the DMG synthase reaction. The relative indifference of hTgs1 to the N nucleoside base or sugar of a m7GpppN dinucleotide substrate jibes with the putative exposure of this moiety above the enzyme's surface. An especially instructive finding was that the cap specificity of the hTgs1 and GlaTgs2 DMG synthase reactions is enforced by guanine-N7 alkylation, rather than a specific requirement for a methyl group. Thus, it appears that productive hTgs1 binding to the methyl acceptor is driven in large part by a π-cation interaction with the tryptophan side chain in the Tgs active site. In this respect, Tgs enzymes echo the primacy of π-cation interactions for cap recognition by vaccinia virus cap-specific nucleoside-2′O-methyltransferase (Hu et al. 1999, 2002) and the cap-binding translation initiation factor eIF4E (Niedzwiecka et al. 2002).
Mimivirus Tgs differs from the other enzymes studied here with its relaxed stringency of cap dependence at alkaline pH, whereby it methylates guanine nucleotides that lack the defining N7-methyl group of the RNA cap. The unmethylated GDP substrate has significantly lower affinity for MimiTgs than m7GDP, presumably reflecting the loss of the stabilizing cationic component of the π stack of guanosine and tryptophan in the methyl acceptor pocket. It is not immediately clear why the cap-independent methyltransferase of MimiTgs is unmasked at alkaline pH. It is possible that increased pH caused deprotonation of an amino acid on the enzyme, which then enables productive binding to unmethylated guanine. Alternatively, the switch might be related to deprotonation of the guanine-N1 atom (pKa ∼9.4), which might favor the enolate tautomer and allow productive binding without an N7 alkyl group.
MATERIALS AND METHODS
Materials
[3H-CH3]AdoMet and Enhance were purchased from Perkin Elmer Life Sciences. AdoMet, GTP, GDP, ATP, ITP, and UTP were purchased from Sigma. Protein concentrations were determined by using the Bio-Rad dye reagent with bovine serum albumin (BSA) as the standard.
Cap analogs
Syntheses of dinucleotide cap analogs GpppG, m2GpppG, m7GpppG, m7GpppA, m7GpppC, m7Gppp(dG), m7GpppGm, ethyl7GpppG, benzyl7GpppG, m2,7GpppG, m2,2,7GpppG, m2,7GpppA, m7GppppG, m2,7GppppG, and m2,2,7GppppG were performed as described previously (Darzynkiewicz et al. 1990; Stepinski et al. 1995; Jankowska et al.1996; Niedzwiecka et al. 2007). Mononucleotides m7G(5′)p, m2,7G(5′)p, m7G(5′)pp, m2,7G(5′)pp, m2,2,7G(5′)pp, m2G(5′)ppp, m7G(5′)ppp, m2,7G(5′)ppp, m2,2,7G(5′)ppp, ethyl7G(5′)ppp, benzyl7G(5′)ppp, G(5′)pppp, and m7G(5′)pppp were prepared according to the methods reported previously (Darzynkiewicz et al. 1985; Jankowska et al. 1993; Zuberek et al. 2004; Niedzwiecka et al. 2007).
Human Tgs1
Plasmid pET28b-hTgs1(631-853) encoding the C-terminal catalytic domain of hTgs1 (Hausmann et al. 2008) fused to an N-terminal His10Smt3 tag was transformed into Escherichia coli BL21(DE3)pRIL. A 4-L culture derived from a single transformant was grown at 37°C in Luria–Bertani medium containing 50 μg/mL kanamycin and 125 μg/mL chloramphenicol until the A600 reached 0.6. The culture were adjusted to 0.2 mM isopropyl β-D-thiogalactopyranoside and 2% ethanol and incubation was continued for 17 h at 17°C with constant shaking. The cells were harvested by centrifugation and stored at −80°C. All subsequent steps were performed at 4°C. Thawed bacteria were resuspended in 200 mL of buffer A (50 mM Tris-HCl, pH 8.0, 1 M NaCl, 10% glycerol). Lysozyme and Triton X-100 were added to final concentrations of 1 mg/mL and 0.1%, respectively. The lysate was sonicated to reduce viscosity, and insoluble material was removed by centrifugation for 45 min at 16,000 rpm in a Sorvall SS34 rotor. Aliquots (50 mL) of the supernatant were mixed with 2 mL of Ni2+-nitrilotriacetic acid-agarose (Qiagen) that had been equilibrated with buffer A. Protein and resin were incubated for 30 min at 4°C and then poured into columns. The columns were washed with 100 mL of 50 mM imidazole in buffer A and then eluted with 500 mM imidazole in buffer A. The polypeptide compositions of the column fractions were monitored by SDS-PAGE. The peak 500 mM imidazole eluate fractions containing recombinant His10Smt3-hTgs1(631-853) were combined (14 mg protein in 7 mL), supplemented with 10 μg of purified Ulp1 (a Smt3-specific protease) (Mossessova and Lima 2000), and then dialyzed overnight against 100 mM NaCl in buffer A. The dialysate was applied to mixed for 30 min with 1 mL of phosphocellulose that had been equilibrated with buffer A containing 100 mM NaCl. The resin was poured into a column, and the tag-free hTgs1(631-853) protein (7 mg) was recovered in the flow-through. The enzyme preparation was stored at −80°C.
Giardia Tgs2
GlaTgs2 was produced in E. coli as an N-terminal His10 fusion and purified from a soluble bacterial lysate by Ni-agarose chromatography, as described previously (Hausmann and Shuman 2005b), except that the enzyme buffer contained 500 mM NaCl instead of 200 mM NaCl.
Mimivirus Tgs
MimiTgs was produced in E. coli as a His10Smt3 fusion and recovered from a soluble bacterial lysate by Ni-agarose chromatography as described previously (Benarroch et al. 2009). The tag was removed by treatment with Ulp1, and the tag-free MimTgs was recovered in the flow-through during a second round of Ni-agarose chromatography.
Methyltransferase assay
Reaction mixtures (10 μL) containing 50 mM Tris-HCl (pH 8.0) or Tris-Acetate (pH 6.0), 5 mM DTT, 50 mM NaCl, 100 μM [3H-CH3]AdoMet, and guanine nucleotides and enzyme as specified were incubated at 37°C. Aliquots (6 μL) were withdrawn at the times specified and then spotted on polyethyleneimine cellulose TLC plates, which were developed with 0.05 M, 0.2 M, or 0.4 M (NH4)2SO4 as specified. The dried TLC plates were sprayed with Enhance, and radiolabeled material was visualized by autoradiography after exposing the plates to X-ray film for 2 d at −80°C. Alternatively, the AdoMet- and 3H product-containing portions of the lanes were cut out, and the radioactivity in each was quantified by liquid scintillation counting.
SUPPLEMENTAL DATA
Supplemental material can be found at http://www.rnajournal.org.
ACKNOWLEDGMENTS
This work was supported by NIH grants GM42498 and GM52470 (to S.S.) and Polish National Science Support Project 2008-2010 PBZMNiSW-07/I/2007 (to E.D.). S.S. is an American Cancer Society Research Professor. E.D. is a Howard Hughes Medical Institute International Scholar (HHMI grant 55005604).
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.1872110.
REFERENCES
- Benarroch D, Qiu ZR, Schwer B, Shuman S. Characterization of a mimivirus RNA cap guanine-N2 methyltransferase. RNA. 2009;15:666–674. doi: 10.1261/rna.1462109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch H, Reddy R, Rothblum L, Choi YC. SnRNAs, SnRNPs, and RNA processing. Annu Rev Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz E, Ekiel I, Tahara SM, Seliger LS, Shatkin AJ. Chemical synthesis and characterization of 7-methylguanosine cap analogues. Biochemistry. 1985;24:1701–1707. [Google Scholar]
- Darzynkiewicz E, Stepinski J, Tahara SM, Stolarski R, Ekiel I, Haber D, Neuvonen K, Lehikoinen P, Labadi I, Lönnberg H. Synthesis, conformation, and hydrolytic stability of P1, P3-dinucleoside triphosphates related to mRNA 5′-cap, and comparative kinetic studies on their nucleoside and nucleoside monophosphate analogs. Nucleosides Nucleotides. 1990;9:599–618. [Google Scholar]
- Franke J, Gehlen J, Ehrenhofer-Murray AE. Hypermethylation of yeast telomerase RNA by the snRNA and snoRNA methyltransferase Tgs1. J Cell Sci. 2008;121:3553–3560. doi: 10.1242/jcs.033308. [DOI] [PubMed] [Google Scholar]
- Gallardo F, Olivier C, Dandjinoud AT, Welinger RJ, Chartrand P. TLC1 RNA nucleo-cytoplasmic trafficking links telomerase biogenesis to its recruitment to telomeres. EMBO J. 2008;27:748–757. doi: 10.1038/emboj.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann S, Shuman S. Specificity and mechanism of RNA cap guanine-N2 methyltransferase (Tgs1) J Biol Chem. 2005a;280:4021–4024. doi: 10.1074/jbc.C400554200. [DOI] [PubMed] [Google Scholar]
- Hausmann S, Shuman S. Giardia lamblia RNA cap guanine-N2 methyltransferase (Tgs2) J Biol Chem. 2005b;280:32101–32106. doi: 10.1074/jbc.M506438200. [DOI] [PubMed] [Google Scholar]
- Hausmann S, Ramirez A, Schneider S, Schwer B, Shuman S. Biochemical and genetic analysis of RNA cap guanine-N2 methyltransferases from Giardia lamblia and Schizosaccharomyces pombe. Nucleic Acids Res. 2007;35:1411–1420. doi: 10.1093/nar/gkl1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann S, Zheng S, Costanzo M, Brost RL, Garcin D, Boone C, Shuman S, Schwer B. Genetic and biochemical analysis of yeast and human cap trimethylguanosine synthase: Functional overlap of TMG caps, snRNP components, pre-mRNA splicing factors, and RNA decay pathways. J Biol Chem. 2008;283:31706–31718. doi: 10.1074/jbc.M806127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Gershon PD, Hodel AE, Quiocho FA. mRNA cap recognition: Dominant role of enhanced stacking interactions between methylated bases and protein aromatic side chains. Proc Natl Acad Sci. 1999;96:7149–7154. doi: 10.1073/pnas.96.13.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Oguro A, Li C, Gershon PD, Quiocho FA. The ‘cap-binding’ slot of an mRNA cap-binding protein: Quantitative effects of aromatic side chain choice in the double-stacking sandwich with cap. Biochemistry. 2002;41:7677–7687. doi: 10.1021/bi0201926. [DOI] [PubMed] [Google Scholar]
- Jankowska M, Stepinski J, Stolarski R, Temeriusz A, Darzynkiewicz E. Synthesis and properties of new NH2 and N7 substituted GMP and GTP 5′-mRNA cap analogues. Collection Czechoslov. Chem. Commun. Special Issue. 1993;58:138–141. [Google Scholar]
- Jankowska M, Stepinski J, Stolarski R, Wieczorek Z, Temeriusz A, Haber D, Darzynkiewicz E. 1H NMR and fluorescence studies of new mRNA 5′-cap analogues. Collect Czech Chem Commun. 1996;61:S197–S202. [Google Scholar]
- Komonyi O, Papai G, Enunlu I, Muratoglu S, Pankotai T, Kopitova D, Maróy P, Udvarty A, Boros I. DTL, the Drosophila homolog of PIMT/Tgs1 nuclear receptor coactivator-interacting protein/RNA methyltransferase, has an essential role in development. J Biol Chem. 2005;280:12397–12404. doi: 10.1074/jbc.M409251200. [DOI] [PubMed] [Google Scholar]
- Lemm I, Girard C, Kuhn AN, Watkins NJ, Schneider M, Bordonné R, Lührmann R. Ongoing U snRNP biogenesis is required for the integrity of Cajal bodies. Mol Biol Cell. 2006;17:3221–3231. doi: 10.1091/mbc.E06-03-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monecke T, Dickmanns A, Ficner R. Structural basis for m7G-cap hypermethylation of small nuclear, small nucleolar and telomerase RNA by the dimethyltransferase TGS1. Nucleic Acids Res. 2009;37:3865–3877. doi: 10.1093/nar/gkp249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossessova E, Lima CD. Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol Cell. 2000;5:865–876. doi: 10.1016/s1097-2765(00)80326-3. [DOI] [PubMed] [Google Scholar]
- Mouaikel J, Verheggen C, Bertrand E, Tazi J, Bordonné R. Hypermethylation of the cap structure of both yeast snRNAs and snoRNAs requires a conserved methyltransferase that is localized to the nucleolus. Mol Cell. 2002;9:891–901. doi: 10.1016/s1097-2765(02)00484-7. [DOI] [PubMed] [Google Scholar]
- Mouaikel J, Bujnicki JM, Tazi J, Bordonné R. Sequence-structure-function relationships of Tgs1, the yeast snRNA/snoRNA cap hypermethylase. Nucleic Acids Res. 2003;31:4899–4909. doi: 10.1093/nar/gkg656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiecka A, Marcotrigiano J, Stepinski J, Jankowska-Anyszka M, Wyslouch-Cieszynska A, Dadlez M, Gingras AC, Mak P, Darzynkiewicz E, Sonenberg N, et al. Biophysical studies of eIF4E cap-binding protein: Recognition of mRNA 5′ cap structure and synthetic fragments of eIF4G and 4E-BP1 proteins. J Mol Biol. 2002;319:615–635. doi: 10.1016/S0022-2836(02)00328-5. [DOI] [PubMed] [Google Scholar]
- Niedzwiecka A, Stepinski J, Antosiewicz JM, Darzynkiewicz E, Stolarski R. Biophysical approach to studies of cap-eIF4E interaction by synthetic cap analogues. Methods Enzymol. 2007;430:209–246. doi: 10.1016/S0076-6879(07)30009-8. [DOI] [PubMed] [Google Scholar]
- Seto AG, Zaug AJ, Sobel SG, Wolin SL, Cech TR. Saccharomyces cerevisiae telomerase is a small nuclear ribonucleoprotein particle. Nature. 1999;401:177–180. doi: 10.1038/43694. [DOI] [PubMed] [Google Scholar]
- Simoes-Barbosa A, Louly C, Franco OL, Rubio MA, Alfonzo JD, Johnson PJ. The divergent eukaryote Trichomonas vaginalis has an m7G cap methyltransferase capable of a single N2 methylation. Nucleic Acids Res. 2008;36:6848–6858. doi: 10.1093/nar/gkn706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepinski J, Bretner M, Jankowska M, Felczak K, Stolarski R, Wieczorek Z, Cai A-L, Rhoads RE, Temeriusz A, Haber D, et al. Synthesis and properties of P1,P2-, P1,P3-, and P1,P4-dinucleoside di-, tri-, and tetraphosphate mRNA 5′-cap analogues. Nucleosides Nucleotides. 1995;14:717–721. [Google Scholar]
- Zuberek J, Jemielity J, Jablonowska A, Stepinski J, Dadlez M, Stolarski R, Darzynkiewicz E. Influence of electric charge variation at residues 209 and 159 on interaction of eIF4E with the mRNA 5′ terminus. Biochemistry. 2004;43:5370–5379. doi: 10.1021/bi030266t. [DOI] [PubMed] [Google Scholar]