Abstract
Study Objectives:
Although sleep-related attentional bias has been shown to be evident in primary insomnia, the association with objectively measured sleep has not been investigated. In the present study, we used polysomnography (PSG) to fill this void.
Design:
Patients with primary insomnia and healthy controls were studied using a visual dot probe task (VDP) and an emotional Stroop task (EST). Additionally, polysomnography was carried out in a sub-sample (n = 22) of patients in the subsequent night.
Setting:
Department of Psychiatry and Psychotherapy of the University of Freiburg Medical Center.
Participants:
Thirty patients with primary insomnia and 30 matched healthy controls.
Interventions:
N/A
Measurements and Results:
Patients with primary insomnia demonstrated a significant sleep-related attentional bias compared to controls in the EST but no significant group effects were found for the VDP. VDP attentional bias scores were positively correlated with measures of sleep pressure, including total sleep time, sleep efficiency, and the amount of slow wave sleep. EST attentional bias scores were not correlated with subsequent PSG parameters, and we did not observe a correlation between attentional bias scores on the two tasks.
Conclusions:
The unexpected relationship between increased attentional bias, in the VDP task, and improved markers of sleep duration and continuity, may be indicative of a homeostatic craving for sleep in those with high attentional bias. This awaits further testing in multiple night studies, to shed light on the mechanisms and implications of sleep-related attentional bias.
Citation:
Spiegelhalder K; Kyle SD; Feige B; Prem M; Nissen C; Espie CA; Riemann D. The impact of sleep-related attentional bias on polysomnographically measured sleep in primary insomnia. SLEEP 2010;33(1):107-112.
Keywords: Primary insomnia, attentional bias, emotional Stroop task, visual dot probe task, polysomnography
INSOMNIA IS ESTIMATED TO AFFECT APPROXIMATELY 10% OF THE ADULT POPULATION1 AND IS CHARACTERIZED BY DIFFICULTIES WITH INITIATING AND/or maintaining sleep, or non-restorative sleep, accompanied by daytime impairment.2 Primary insomnia (PI), a largely exclusionary diagnosis of poor sleep, ruling out psychiatric, medical and additional sleep-related pathology, is estimated to affect up to 3% of the adult population.3,4
It is widely assumed that PI develops, and is maintained, by an interaction between both psychological and physiological factors,5 and several have been identified, including elevated brain metabolism during peri-sleep onset and NREM sleep,6 increased 24-h metabolic rate,7 heightened cognitive arousal,8 and greater dysfunctional beliefs and attitudes about sleep and consequences of sleep loss.9
Recent cognitive models of PI10–12 emphasize the role of sleep-related cognitive arousal, in particular, selective attention to internal/external sleep-related cues, in the etiology and maintenance of poor sleep. Specifically, it has been argued that the attentional systems of patients with PI become abnormally sensitive to sleep-related information, which in turn triggers a cascade of processes leading the poor sleeper to engage in ruminative processes,13 and direct effort and intention towards sleeping.12 This enhanced focus and preoccupation with sleep, coupled with attempts to directly control sleep initiation, it is argued, has the net effect of inhibiting sleep initiation and maintenance.
Several studies have investigated selective attention to sleep-related cues in PI using a number of computerized tasks, including the emotional Stroop and visual dot probe paradigms.14–21 These hallmark measures of attentional bias are often assumed to be equivalent measures of the same construct. However, most studies that administered both tasks in the same sample came to differing results.22–26 The emotional Stroop task measures the degree to which people are slower in responding to the color of concern-related words than to the color of neutral words. This involves the implicit competition between emotional content of the word and the subsequent naming of color. However, due to partly inconsistent results, it has been questioned whether the emotional Stroop task is a pure measure of preferential attention.27 The visual dot probe task, on the other hand, is a more direct measure of attention allocation, reflecting the scanning of a visual field. In this task, participants are required to indicate the location of a probe stimulus that appears in the location of one of two previously presented images, one concern-related and one neutral.
The majority of studies support the notion that poor sleepers show an attentional bias for sleep-related stimuli relative to normal sleeping controls. There is also some emerging evidence that sleep-related attentional bias is specific to primary insomnia, and not merely an artifact of any sleep disorder17,18,28 or frequency of concept usage.20 Finally, recently presented conference data reveal reductions in attentional bias scores after cognitive behavior therapy for insomnia, in parallel with improvements in sleep, relative to a wait-list control group.29 In sum, these findings suggest that enhanced attentional focus towards sleep-related cues may be involved in the development and maintenance of chronic primary insomnia, though more work is needed to further assess causality and mediating factors.
To date, no study has investigated the relationship between sleep-related attentional bias and objective sleep parameters. Such data may help inform how sleep affects attention allocation to sleep stimuli, as well as the converse relationship (i.e., how sleep-related attentional bias subsequently impacts sleep). For example, patients presenting with an elevated preoccupation/biased processing of sleep stimuli, prior to sleep onset, may subsequently experience a more perturbed sleep; possibly reflecting a primed negative schema, triggering cognitive/emotional arousal, which then interferes with sleep initiation/maintenance.
In the current study we assessed the relationship between sleep-related attentional bias and subsequent sleep quality, measured by objective PSG, in patients with primary insomnia. Although exploratory, our primary aim was to better understand the “feed-forward” impact of attentional bias on sleep parameters, hypothesizing a negative impact on subsequent sleep. Our secondary objectives were to better unpick the well documented poor versus normal-sleeper differential attentional bias using 2 hallmark measures of this effect (the visual dot probe task and the emotional Stroop task) across clinically referred PIs and normal sleepers. We hypothesized that insomnia patients exhibit a preferential attention allocation to sleep-related stimuli in both tasks. One final aim of the study was to assess the degree of relationship between the 2 attention tasks.
METHODS
Participants
Thirty patients meeting diagnostic criteria for PI, according to DSM-IV-TR,2 and 30 good sleeping controls were included in the present study. All patients with primary insomnia who were referred to our sleep disorders clinic by their primary care providers between July 2006 and June 2008, were screened systematically for study participation. Before entering the protocol, all patients underwent our standard physical and psychiatric examination, excluding those with medical, psychiatric or occult sleep disorder pathology. As 8 patients received only ambulant diagnostics and treatment, polysomnographic recordings were performed in a sub-group of 22 PI patients. Healthy controls were recruited from hospital staff or friends/relatives of hospital staff. None of the participants had experience of participating in attentional bias tasks. The study was conducted in accordance with the Declaration of Helsinki. All subjects gave their informed written consent prior to inclusion in the study. The study protocol was approved by the local ethics committee of the University of Freiburg Medical Center.
Attentional Bias Tasks
Two computerized reaction time tasks, a visual dot probe task30 and an emotional Stroop task31 were administered on a 17-inch monitor (43.2 cm) using the Presentation® software (Neurobehavioral systems, http://www.neurobs.com/).
In the visual dot probe task, 2 pictorial stimuli were simultaneously presented for 500 ms on the left and right side of the screen. On disappearance of the pictures, a probe (a white dot) subsequently appeared in the same spatial location as one of the stimuli previously presented. The participants were required to indicate, as quickly as possible, the location of the probe (left or right side of the screen) using designated left and right buttons with the corresponding index finger. Participants completed 80 experimental trials, each commencing with the presentation of a central fixation cross for 500 ms. Stimuli consisted of 40 sleep-related pictures (bedrooms) and 120 control pictures (kitchens, living rooms, and bathrooms) that were matched in terms of brightness and complexity using the GIMP software (GNU Image Manipulation Program, http://www.gimp.org/). The complexity matching was performed by applying an edge-detect filter and quantifying the amount of pixels in edges. The stimulus set was an extension of those pictures that have been used in our previous investigations.20,21 All pictures were resized to the same dimensions (5.6 inches wide, 4.2 inches high). The presentation of the pictures was randomized with the constraint that a sleep-related picture was always coupled with a control picture, resulting in 40 pairs of sleep-neutral, and 40 pairs of neutral-neutral pictures. The presentation of the probes was randomized with the constraint that the probes did not appear more than 4 times in a row on the same side. Prior to the start of the experimental trials, subjects were given 24 practice trials with visual feedback (i.e., “Correct” or “Incorrect” was displayed on the screen for 1000 ms) in which colored boxes were presented instead of pictures.
The emotional Stroop task was identical to one previously used to investigate attentional bias in insomnia, and used the exact same stimuli.21 In each trial, a written word (either a sleep-related or neutral word) was presented in the center of the screen, in one of 4 possible colors (red, green, blue, yellow). Participants were instructed to respond by pressing a button of corresponding color with the index finger of their dominant writing hand. Responses were recorded using a designated button box. Stimuli were composed of 10 sleep-related words and 30 neutral words. Both word sets were matched carefully in terms of word length, syllables, word type and frequency of occurrence in the German language. Words were presented in each of the four colors, resulting in 160 experimental trials. Randomization of word and color meant that the same word or color would not appear more than twice in a row. Similar to the visual dot probe task there was a block of 24 practice trials to familiarize participants with the task.
Polysomnography
Twenty-two participants underwent 2 consecutive nights of PSG sleep monitoring. The first night served as an adaptation and screening night to rule out sleep apnea, PLMS, and occult sleep disorder pathology. The second night was used for the present analysis. A standard laboratory procedure and PSG montage were followed. Sleep was recorded on 24-channel Sagura EEG-polysomnographs for 8 h from “lights out” (23:00) until “lights on” (07:00). All recordings included EEG (C3-A2; C4-A1), EOG (horizontal and vertical), and EMG (submental), and were scored visually by experienced raters according to the criteria of Rechtschaffen and Kales.32 During the adaptation night, all participants were screened for apneas and periodic leg movements by monitoring abdominal and thoracic effort, nasal airflow, oximetry, and bilateral tibialis anterior EMG. No participant was excluded on the basis of PSG evaluation. Sleep recordings were evaluated for the following parameters of sleep continuity: total sleep time; sleep efficiency: ratio of TST to time in bed * 100 %; sleep onset latency: time from lights out until sleep onset (defined as first epoch of stage 2); number of awakenings; arousal index: number of arousals per hour.33 Sleep architecture parameters were amounts of stages 1, 2, slow wave sleep (SWS), and REM sleep as percentages of sleep period time (time from sleep onset until final awakening).
Procedure
The attentional bias tasks were carried out in the evening between 16:15 and 22:15 in a quiet, well-lit room. Directly before commencing the tasks, participants filled in the PSQI to assess the previous month's subjective sleep quality. The primary investigator then instructed participants, verbally, on task order (first visual dot probe task, then emotional Stroop task) and nature of required responses for each task. Participants were asked to respond as quickly as possible without making mistakes. Verbal instructions followed a standardized protocol. Throughout the duration of the experiment, the investigator remained in the room, keeping as quiet as possible, and directed visual attention away from the computer monitor. On completion of the tasks, participants completed the SSS to assess subjective state sleepiness, and were debriefed on the underlying aims of the research. Total task duration was approximately 25 min.
The sub-sample (n = 22) of patients who underwent PSG recordings completed the exact described experimental protocol on the second evening, prior to the experimental sleep laboratory night. All investigations in this sub-sample were carried out between 18:30 and 19:45 to carefully keep the time of measurement constant for the correlation with the subsequent PSG data.
Analysis
All trials with errors were excluded from the analyses. Additionally, response times that exceeded each person's mean by > 3 standard deviations were eliminated as outliers. In the emotional Stroop task, attentional bias scores were calculated for each participant as the mean response latency to sleep-related stimuli minus the mean response latency to neutral stimuli. Positive attentional bias scores indicate vigilance for sleep-related stimuli because performance is expected to be disrupted by processing of concern-related information.
In the visual dot probe task, attentional bias scores were calculated for each participant using the following equation:
Attentional bias score = mean(RT(SL/DR),RT(SR/DL))-mean(RT(SR/DR),RT(SL/DL))
where RT is reaction time, S is the sleep-related picture, D is the dot probe, L is the left side of the screen, and R is the right side of the screen.34 Accordingly, for example, RT(SL/DR) refers to the reaction times of those trials in which the sleep-related picture was presented on the left side and the dot probe appeared on the right side. As in the emotional Stroop task, positive attentional bias scores indicate vigilance for sleep-related stimuli.
Between-group differences in attentional bias scores were analyzed using independent t-tests with statistical thresholds at P < 0.05 (2-tailed). Correlational analyses between attentional bias scores and PSG derived sleep variables as well as between attentional bias scores from both tasks were exploratory in nature with a statistical threshold of P < 0.05 (2-tailed) for each analysis.
RESULTS
Participants
The insomniac group consisted of 20 women and 10 men (age 46.9 ± 14.9 years, range 20-77 years). Two had problems initiating sleep only, 3 had problems maintaining sleep only, and the remaining 25 had problems both initiating and maintaining sleep. Average duration of primary insomnia was 11.3 ± 8.9 years (range 1-35 years). Mean scores on the Pittsburgh Sleep Quality Index (PSQI35) and Stanford Sleepiness Scale (SSS36) were 13.3 ± 3.4 and 2.9 ± 1.0, respectively. The mean body mass index (BMI) in the insomniac group was 22.7 ± 3.7 kg/m2. In terms of secondary school education, 6 patients had the highest school-leaving certificate (German “Abitur”), 13 had the German “Realschulabschluss” (middle level), and 11 had the German “Hauptschulabschluss” (lowest level). All but 3 patients (medication: zopiclone, zolpidem, doxepin) were free of any psychoactive drugs potentially influencing sleep ≥ 2 days prior to the investigation.
Healthy controls (21 women and 9 men, age 48.3 ± 12.9 years, range 22-68 years) had mean PSQI and SSS scores of 3.9 ± 1.7 and 1.8 ± 0.9. Mean BMI was 23.9 ± 3.3 kg/m2. Six had the Abitur, 14 the Realschulabschluss, and 10 the Hauptschulabschluss.
There were no significant group differences for sex distribution (χ2 test, χ21 = 0.08, P = 0.78), age (t-test, t29 = −0.38, P = 0.71), BMI (t-test, t29 = −1.29, P = 0.20) or level of education (Wilcoxon-Test, W29 = 462, P = 0.85). The groups differed significantly in their mean PSQI scores (t-test, t29 = 13.52, P < 0.001) and SSS scores (t-test, t29 = 4.26, P < 0.001), with the PI group demonstrating higher PSQI and SSS scores.
Twenty-two of the 30 PI patients were additionally investigated using polysomnography (age 44.6 ± 14.2 years, range 20–77 years). Average duration of primary insomnia in this sub-sample was 9.5 ± 7.5 years. Their mean PSQI score was 13.5 ± 3.2. SSS scores were 2.9 ± 1.0. Mean BMI was 21.8 ± 3.5 kg/m2. Five of them had an educational level of Abitur, 10 had a Realschulabschluss, and 7 had a Hauptschulabschluss.
Visual Dot Probe Task
The mean reaction time was 501 ± 155 ms across all participants, 492 ± 132 ms in the PI group and 509 ± 174 ms in the control group with no significant group difference (t-test, t29 = −0.59, P = 0.56). Data loss due to errors 1.2%: 1.3% in the PI group and 1.0 % in healthy controls. An additional 1.3% of data was excluded due to outlier identification—1.3% in the PI group and 1.4% in the control group. Neither the number of errors (t-test, t29 = −0.55, P = 0.59) nor the number of outliers (t-test, t29 = 0.55, P = 0.58) differed significantly between groups.
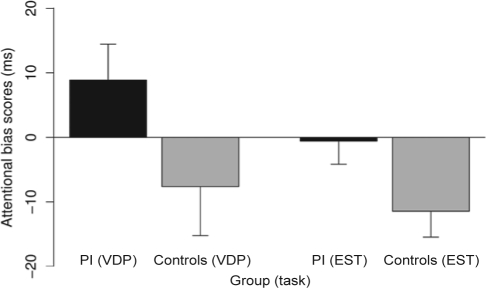
For both groups, the mean attentional bias scores of the visual dot probe task are presented in Figure 1. Attentional bias scores were 8.9 ± 30.5 ms for the PI group and −7.6 ± 41.6 ms for the control group with the group comparison failing to reach statistical significance (t-test, t29 = 1.75, P = 0.085). The effect size of this difference was small to medium (Cohen d = 0.45).
Figure 1.
Attentional bias scores (with standard errors) for patients with primary insomnia (PI, black bars) and healthy controls (grey bars) in the visual dot probe task (VDP) and emotional Stroop task (EST). Between-group t-tests revealed no significance in the visual dot probe task (P = 0.085) and a significance in the emotional Stroop task (P = 0.048).
The association between attentional bias scores in the visual dot probe task and PSG parameters are presented in Table 1. As listed in Table 1, attentional bias scores in the visual dot probe task correlated significantly positive with total sleep time, sleep efficiency and amount of slow wave sleep. A significant negative correlation was observed between attentional bias scores and the number of awakenings.
Table 1.
Results of the correlation analyses between PSG parameters and attentional bias scores in both attentional bias tasks
| mean ± SD | VDP Task correlations |
Stroop Task correlations |
||||
|---|---|---|---|---|---|---|
| r values | P values | r values | P values | |||
| Total sleep time (min) | 371.0 ± 75.4 | 0.47 | 0.027 | −0.22 | 0.331 | |
| Sleep efficiency (%) | 77.3 ± 15.8 | 0.46 | 0.030 | −0.22 | 0.323 | |
| Sleep onset latency (min) | 23.3 ± 24.3 | −0.28 | 0.199 | 0.24 | 0.272 | |
| Number of awakenings | 35.8 ± 20.2 | –0.49 | 0.021 | 0.34 | 0.121 | |
| Arousal index / TST (/h) | 14.2 ± 6.1 | −0.12 | 0.601 | 0.25 | 0.263 | |
| Stage I (%) | 8.8 ± 4.3 | −0.18 | 0.425 | 0.14 | 0.527 | |
| Stage II (%) | 50.3 ± 11.3 | 0.13 | 0.554 | –0.15 | 0.500 | |
| Stage SWS (%) | 5.6 ± 6.2 | 0.56 | 0.006 | −0.11 | 0.626 | |
| Stage REM (%) | 18.8 ± 5.6 | 0.20 | 0.376 | −0.22 | 0.318 | |
Emotional Stroop Task
The mean reaction time was 864 ± 210 ms across all participants: 883 ± 206 ms in PI and 844 ± 211 ms in healthy controls with no significant group difference (t-test, t29 = 1.12, P = 0.27). Data loss included 1.1% of data due to errors—0.7% in the PI group and 1.5% in healthy controls. There was a significant group difference in the number of errors (t-test, t29 = −2.28, P = 0.03). An additional 2.6% of data was excluded due to outlier identification—2.7% in PI and 2.5% in the control group, with no significant group difference (t-test, t29 = 0.62, P = 0.54).
Attentional bias scores were −0.6 ± 19.7 ms for the PI group and −11.4 ± 22.0 ms for the control group (see Figure 1). The between-group difference was significant (t-test, t29 = 2.02, P = 0.048) with a medium effect size (Cohen d = 0.52).
Correlation analyses between the attentional bias scores of the emotional Stroop task and the PSG parameters are presented in Table 1. In these analyses, no significant correlation has been observed. Pearson correlation between the attentional bias scores of the 2 tasks was not significant across all participants (r = 0.05, t58 = 0.35, P = 0.73), in the PI group (r = −0.09, t28 = −0.50, P = 0.62), or in the control group (r = 0.04, t28 = 0.21, P = 0.83).
DISCUSSION
The present study aimed at further understanding sleep-related attentional bias in individuals with primary insomnia by investigating for the first time the association with subsequent PSG defined sleep parameters. In terms of these PSG analyses in the PI group, to our surprise, we found a significant positive correlation between attentional bias scores on the visual dot probe task and total sleep time, sleep efficiency, and percentage of slow wave sleep, as well as a significant negative correlation with the number of awakenings, measured the subsequent night. In contrast, we did not find any significant correlation between attentional bias scores on the emotional Stroop task and PSG defined sleep parameters. Additionally, corroborating previous work,12 we found evidence of significantly higher attentional bias scores in the PI group compared with healthy controls on the emotional Stroop task. Yet, against our prediction, insomnia patients demonstrated only a tendency towards sleep-related attentional bias in the visual dot probe task, indicating medium to small effect sizes. Of final note, there was no significant correlation between the attentional bias scores from both tasks.
This is the first study to assess, and observe, relationships between attentional bias scores and subsequent objective measures of sleep. At the outset of the study, we presumed if any relationships existed between attentional bias and sleep, that these would be negative, i.e., higher sleep-related attentional bias would be associated with poorer sleep. The data of the visual dot probe task, however, are in the opposite direction, so that higher attentional bias looks to be related to markers of improved sleep continuity. These findings pose difficulties for models positing that high levels of attentional bias and related arousal lead to disruptions of subsequent sleep. An alternative interpretation of these data, though still requiring further exploration, is that attentional bias, as measured by the visual dot probe task, is affected by sleep pressure, perhaps associated with an underlying craving for sleep. That is, those who had accumulated a large sleep debt caused by disrupted or non-restorative sleep (over preceding nights) are more likely to show increased attentional bias scores because they are “homeostatically” craving sleep.12 This enhanced build up of sleep pressure then translates into increases in SWS, increased total sleep time and sleep efficiency, and less frequent awakenings, the following night. That is, sleep cues may have some incentive value, acquiring motivational salience in a similar manner as alcohol cues do for subjects with alcohol dependence.37
A valid criticism of this account, of course, is how does one know that the PI group are in fact sleep deprived? Clearly, to fully elucidate a craving explanation for sleep-related attentional bias, relations need to be further investigated in both “feedforward” and “feedback” directions. Our data and interpretation are therefore limited because we only relate attentional bias to subsequent sleep. Nevertheless, given the consistency of direction and strength of associations across several measures of sleep continuity, coupled with intuitive appeal,12 we think it is reasonable to further postulate a role for craving in sleep-related attentional bias, beyond existing published work. In this regard, only two studies have partially contributed to defining relations between sleep/sleep pressure and attentional bias. The first one failed to find within-subject attentional bias for sleep words relative to neutral words, in a sleep-deprived group of students.19 More recently, consistent with the craving model, we found that state sleepiness partly mediated attentional bias in a large sample of university students.21 Importantly, both studies used Stroop paradigms, whereas in the current study it seems likely that the pictorial dot-probe, and particularly the presentation of bed stimuli, directly influenced the attention of those under sleep pressure. Future work should assess dot-probe performance in an experimentally sleep restricted/deprived group, and normal sleeping controls, as well as individuals with insomnia, across good and poor nights, to determine if and how transient motivational state mediates attentional bias effects.
Differing results from the visual dot probe and emotional Stroop paradigms used in the same sample are not an uncommon finding within the psychopathology literature—data from populations with anxiety disorders, chronic pain or alcohol dependence reveal a similar pattern of results.22–26 To our knowledge, very few published studies exist demonstrating significant correlations between these two hallmark measures of attentional bias.38,39 Thus, it seems likely that the tasks tap different dimensions of attention. The visual dot probe task is a more direct measure of attention allocation, reflecting the scanning of a visual field. However, in anxious individuals, a vigilance-avoidance pattern of anxiety-related attentional bias has been observed depending on stimulus exposure durations of the visual dot probe task.34,40 This pattern might also be evident in sleep-related attentional bias, possibly obscuring group differences and correlation analyses; and therefore should be investigated in future studies. The emotional Stroop task, on the other hand, involves the implicit competition between emotional content of the word and the subsequent naming of color; reduced inhibitory functioning towards semantically salient stimuli may thus result in delayed information processing. It is noteworthy though, that the emotional Stroop task may not be a “pure” measure of attention allocation, as cognitive avoidance effects have also been implicated in the mediation of performance.27 An analogy of “spotlight of attention” has been suggested for illustrating the difference between the two tasks, with the visual dot probe task reflecting the scanning of a spotlight and the emotional Stroop task reflecting a stationary spotlight where the information at the focus must be disentangled.22
An alternative explanation for the observed difference is that the results depend on the stimulus material that has been used in the present study. While pictures of beds were used in the visual dot probe task, verbal material was presented in the emotional Stroop task. As the group difference to healthy controls was more pronounced in the emotional Stroop paradigm, it might be that attentional bias in insomnia is linked to ruminative processes that are more likely to be triggered by verbal stimuli, rather than sleep-related objects. This explanation is however at odds with previously published work demonstrating large attentional bias effects using the pictorial flicker paradigm with a pair of slippers and a teddy bear as sleep-related stimuli.16,17 Of note, in our study, the absolute difference between the mean attentional bias scores was greater in the visual dot probe task; this task did however produce a higher variance in scores.
Several limitations of the present study have to be addressed. First, we did not measure polysomnographic parameters in the control group. Therefore, conclusions about the interactions between sleep-related attentional bias and polysomnographic parameters can only be drawn for individuals with insomnia. However, our main intention was to further shed light on the possibility that sleep-related attentional bias may negatively interact with subsequent sleep in primary insomnia. According to this, we focussed on measuring PSG parameters in the patient group specifically.
Second, the PIs in the present study are a heterogeneous group, including patients with medication, different sub-types of insomnia (sleep initiating problems, sleep maintenance problems) and large ranges for age and of duration of disorder. This leads, however, to a higher ecological validity of the results, and it is important to note, that many of the studies concerning sleep-related attentional bias in insomnia have recruited non-clinical samples.16–18 Future studies should address the question whether sleep-related attentional bias differs in sub-groups of insomnia.
As we had the hypothesis that sleep-related attentional bias has a negative effect on subsequent sleep, we restricted the PSG measurements to the night following the attentional bias measures. Accordingly, we cannot draw conclusions about the impact of a preceding good or bad night on attentional bias scores. Future studies are needed to investigate reciprocal effects between sleep-related attentional bias and objectively measured sleep in long-term studies.
In conclusion, this study provides further support for the concept of sleep-related attentional bias in primary insomnia. Specifically, we found that sleep-related attentional bias, as measured with a pictorial dot probe task, correlated with polysomnographic measures of sleep pressure in the subsequent night. In contrast, sleep-related attentional bias in the verbal emotional Stroop task was not correlated with PSG parameters. Further understanding sleep-related attentional bias as a core concept, relevant to the development and maintenance of primary insomnia, might bear the potential to contribute to the development of novel and more specific cognitive interventions to treat this prevalent sleep disorder.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Espie has consulted for Sanofi-Aventis, GlaxoSmithKline, and Actelion and has participated in speaking engagements for Takeda and Lundbeck. Dr. Feige received a travel grant for Lundbeck. Dr. Riemann has received research support from Takeda and has participated in speaking engagements for Sanfi-Synthelabo, Servier, Lundbeck, Cephalon, GlaxoSmithKilne, and Boehringer Ingelheim. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Morin CM, LeBlanc M, Daley M, Gregoire JP, Merette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition, text revision (DSM-IV-TR) Washington, DC: American Psychiatric Publishing; 2000. [Google Scholar]
- 3.Ohayon M. Epidemiological study on insomnia in the general population. Sleep. 1996;19:S7–15. doi: 10.1093/sleep/19.suppl_3.s7. [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 5.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: The behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 6.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–8. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 8.Wicklow A, Espie CA. Intrusive thoughts and their relationship to actigraphic measurement of sleep: towards a cognitive model of insomnia. Behav Res Ther. 2000;38:679–93. doi: 10.1016/s0005-7967(99)00136-9. [DOI] [PubMed] [Google Scholar]
- 9.Morin CM. New York: Guilford Press; 1993. Insomnia: psychological assessment and management. [Google Scholar]
- 10.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 11.Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol. 2002;53:215–43. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- 12.Espie CA, Broomfield NM, Macmahon KM, Macphee LM, Taylor LM. The attention-intention-effort pathway in the development of psychophysiologic insomnia: a theoretical review. Sleep Med Rev. 2006;10:215–45. doi: 10.1016/j.smrv.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Tang NK, Anne Schmidt D, Harvey AG. Sleeping with the enemy: clock monitoring in the maintenance of insomnia. J Behav Ther Exp Psychiatry. 2007;38:40–55. doi: 10.1016/j.jbtep.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Lundh LG, Fr̈oding A, Gyllenhammar L, Broman JE, Hetta J. Cognitive bias and memory performance in patients with persistent insomnia. Scand J Behav Ther. 1997;26:27–35. [Google Scholar]
- 15.Taylor LM, Espie CA, White CA. Attentional bias in people with acute versus persistent insomnia secondary to cancer. Behav Sleep Med. 2003;1:200–12. doi: 10.1207/S15402010BSM0104_3. [DOI] [PubMed] [Google Scholar]
- 16.Jones BT, Macphee LM, Jones BC, Broomfield NM, Espie CA. Sleep-related attentional bias in good, moderate, and poor (primary insomnia) sleepers. J Abnorm Psychol. 2005;114:249–58. doi: 10.1037/0021-843X.114.2.249. [DOI] [PubMed] [Google Scholar]
- 17.Marchetti LM, Biello SM, Broomfield NM, Macmahon KM, Espie CA. Who is pre-occupied with sleep? A comparison of attention bias in people with psychophysiological insomnia, delayed sleep phase syndrome and good sleepers using the induced change blindness paradigm. J Sleep Res. 2006;15:212–21. doi: 10.1111/j.1365-2869.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- 18.MacMahon KAM, Broomfield NM, Marchetti LM, Espie CA. Attention bias for sleep-related stimuli in primary insomnia and delayed sleep phase syndrome using dot-probe task. Sleep. 2006;29:1420–7. doi: 10.1093/sleep/29.11.1420. [DOI] [PubMed] [Google Scholar]
- 19.Sagaspe P, Sanchez-Ortuno M, Charles A, et al. Effects of sleep deprivation on Color-Word, Emotional, and Specific Stroop interference and on self-reported anxiety. Brain Cogn. 2006;60:76–87. doi: 10.1016/j.bandc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Spiegelhalder K, Espie C, Nissen C, Riemann D. Sleep-related attentional bias in patients with primary insomnia compared with sleep experts and healthy controls. J Sleep Res. 2008;17:191–6. doi: 10.1111/j.1365-2869.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 21.Spiegelhalder K, Espie C, Riemann D. Is sleep-related attentional bias due sleepiness or sleeplessness. Cognition Emotion. 2009;23:541–50. [Google Scholar]
- 22.Mogg K, Bradley BP, Dixon C, Fisher S, Twelftree H, McWilliams A. Trait anxiety, defensiveness and selective processing of threat: an investigation using two measures of attentional bias. Pers Indiv Differ. 2000;28:1063–77. [Google Scholar]
- 23.Duka T, Townshend JM. The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology. 2004;176:353–61. doi: 10.1007/s00213-004-1906-7. [DOI] [PubMed] [Google Scholar]
- 24.Asmundson GJ, Wright KD, Hadjistavropoulos HD. Hypervigilance and attentional fixedness in chronic musculoskeletal pain: consistency of findings across modified stroop and dot- probe tasks. J Pain. 2005;6:497–506. doi: 10.1016/j.jpain.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Elsesser K, Heuschen I, Pundt I, Sartory G. Attentional bias and evoked heart-rate response in specific phobia. Cognition Emotion. 2006;20:1092–107. [Google Scholar]
- 26.Heim-Dreger U, Kohlmann CW, Eschenbeck H, Burkhardt U. Attentional biases for threatening faces in children: vigilant and avoidant processes. Emotion. 2006;6:320–5. doi: 10.1037/1528-3542.6.2.320. [DOI] [PubMed] [Google Scholar]
- 27.de Ruiter C, Brosschot JF. The emotional Stroop interference effect in anxiety: attentional bias or cognitive avoidance? Behav Res Ther. 1994;32:315–9. doi: 10.1016/0005-7967(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 28.Woods H, Steele AJ, Biello SM, Espie CA. Selective attention to sleep is not an artefact of sleep complaint in insomnia: a study with pregnant and postpartum women. J Sleep Res. 2008;17:13. [Google Scholar]
- 29.Espie CA, Fleming LM, TL A, Paul J. Cognitive and attentional changes following cognitive behaviour therapy (CBT) for persistent insomnia associated with cancer: A randomized controlled trial (RCT) Sleep. 2008;31:A228–9. [Google Scholar]
- 30.MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. J Abnorm Psychol. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- 31.Williams JM, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychol Bull. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- 32.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Washington DC: Publication 204, National Institutes of Health; 1968. [Google Scholar]
- 33.Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. EEG arousals: scoring rules and examples. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 34.Bradley BP, Mogg K, Falla SJ, Hamilton LR. Attentional bias for threatening facial expressions in anxiety: Manipulations of stimulus duration. Cognition Emotion. 1998;12:737–53. [Google Scholar]
- 35.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 36.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 37.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 38.Brosschot JF, de Ruiter C, Kindt M. Processing bias in anxious subjects and repressors, measured by emotional Stroop interference and attentional allocation. Pers Indiv Differ. 1999;26:777–93. [Google Scholar]
- 39.Egloff B, Hock M. Assessing attention allocation toward threat-related stimuli: a comparison of the emotional Stroop task and the attentional probe task. Pers Indiv Differ. 2003;35:475–83. [Google Scholar]
- 40.Mogg K, Bradley BP, Miles F, Dixon R. Time course of attentional bias for threat scenes: testing the vigilance-avoidance hypothesis. Cognition Emotion. 2004;18:689–70. [Google Scholar]