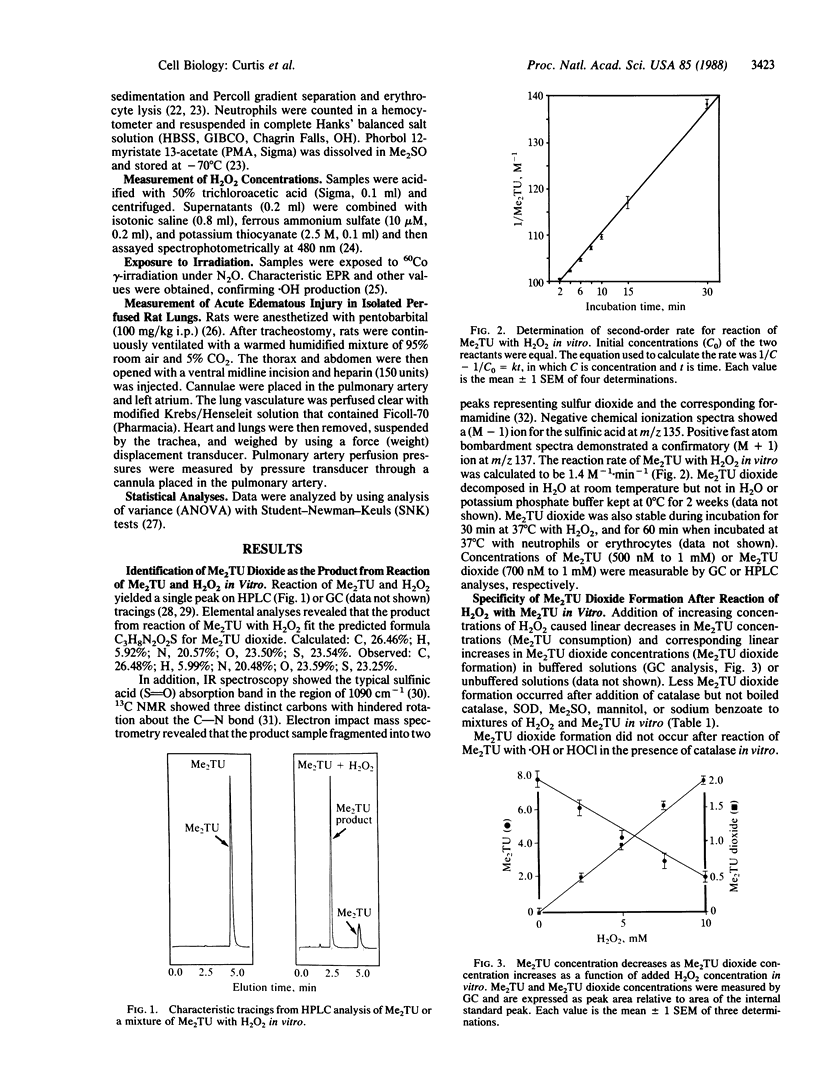
Abstract
We hypothesized that measurement of a specific product from reaction of N,N'-dimethylthiourea (Me2TU) and H2O2 would provide a good indication of the H2O2 scavenging and protection seen after addition of Me2TU to biological systems. We found that addition of H2O2 to Me2TU yielded a single stable product, Me2TU dioxide. Me2TU dioxide formation correlated with Me2TU consumption as a function of added H2O2 concentration and was prevented by simultaneous addition of catalase (but not boiled catalase), superoxide dismutase, dimethyl sulfoxide, mannitol, or sodium benzoate. Me2TU dioxide formation, Me2TU consumption, and H2O2 concentration increases occurred in mixtures containing phorbol 12-myristate 13-acetate (PMA) and normal human neutrophils but not in mixtures containing PMA and neutrophils from patients with chronic granulomatous disease or in mixtures containing PMA and normal neutrophils and catalase. Me2TU dioxide formation also occurred in isolated rat lungs perfused with Me2TU and H2O2 but not in lungs perfused with Me2TU and elastase, histamine, or oleic acid. In contrast, Me2TU dioxide formation did not occur after exposure of Me2TU to 60Co-generated hydroxyl radical or hypochlorous acid in the presence of catalase. The results indicate that reaction of Me2TU with H2O2 selectively forms Me2TU dioxide and that measuring Me2TU dioxide formation from Me2TU may be useful for assessing the presence and significance of H2O2 in biological systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolli R., Zhu W. X., Hartley C. J., Michael L. H., Repine J. E., Hess M. L., Kukreja R. C., Roberts R. Attenuation of dysfunction in the postischemic 'stunned' myocardium by dimethylthiourea. Circulation. 1987 Aug;76(2):458–468. doi: 10.1161/01.cir.76.2.458. [DOI] [PubMed] [Google Scholar]
- Fox R. B. Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J Clin Invest. 1984 Oct;74(4):1456–1464. doi: 10.1172/JCI111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Grootveld M. The measurement of free radical reactions in humans. Some thoughts for future experimentation. FEBS Lett. 1987 Mar 9;213(1):9–14. doi: 10.1016/0014-5793(87)81455-2. [DOI] [PubMed] [Google Scholar]
- Harada R. N., Vatter A. E., Repine J. E. Oxygen radical scavengers protect alveolar macrophages from hyperoxic injury in vitro. Am Rev Respir Dis. 1983 Oct;128(4):761–762. doi: 10.1164/arrd.1983.128.4.761. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Johnston R. B., Jr Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J Clin Invest. 1987 Mar;79(3):669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. H., White C. W., Parker N. B., Ryan J. W., Repine J. E. Dimethylthiourea consumption reflects H2O2 concentrations and severity of acute lung injury. J Appl Physiol (1985) 1985 Dec;59(6):1995–1998. doi: 10.1152/jappl.1985.59.6.1995. [DOI] [PubMed] [Google Scholar]
- Katz M. A. The expanding role of oxygen free radicals in clinical medicine. West J Med. 1986 Apr;144(4):441–446. [PMC free article] [PubMed] [Google Scholar]
- Linas S. L., Whittenburg D., Repine J. E. O2 metabolites cause reperfusion injury after short but not prolonged renal ischemia. Am J Physiol. 1987 Oct;253(4 Pt 2):F685–F691. doi: 10.1152/ajprenal.1987.253.4.F685. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McDonald R. J., Berger E. M., Repine J. E. Neutrophil-derived oxygen metabolites stimulate thromboxane release, pulmonary artery pressure increases, and weight gains in isolated perfused rat lungs. Am Rev Respir Dis. 1987 Apr;135(4):957–959. doi: 10.1164/arrd.1987.135.4.957. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Hoidal J. R., Ferris T. F. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984 Oct;74(4):1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker N. B., Berger E. M., Curtis W. E., Muldrow M. E., Linas S. L., Repine J. E. Hydrogen peroxide causes dimethylthiourea consumption while hydroxyl radical causes dimethyl sulfoxide consumption in vitro. J Free Radic Biol Med. 1985;1(5-6):415–419. doi: 10.1016/0748-5514(85)90155-2. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Clawson C. C., Friend P. S. Influence of a deficiency of the second component of complement on the bactericidal activity of neutrophils in vitro. J Clin Invest. 1977 May;59(5):802–809. doi: 10.1172/JCI108702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Pfenninger O. W., Talmage D. W., Berger E. M., Pettijohn D. E. Dimethyl sulfoxide prevents DNA nicking mediated by ionizing radiation or iron/hydrogen peroxide-generated hydroxyl radical. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1001–1003. doi: 10.1073/pnas.78.2.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., White J. G., Clawson C. C., Holmes B. M. Effects of phorbol myristate acetate on the metabolism and ultrastructure of neutrophils in chronic granulomatous disease. J Clin Invest. 1974 Jul;54(1):83–90. doi: 10.1172/JCI107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate R. M., Morris H. G., Schroeder W. R., Repine J. E. Oxygen metabolites stimulate thromboxane production and vasoconstriction in isolated saline-perfused rabbit lungs. J Clin Invest. 1984 Aug;74(2):608–613. doi: 10.1172/JCI111458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K. M., Clifford D. P., Berger E. M., White C. W., Repine J. E. Intact human erythrocytes prevent hydrogen peroxide-mediated damage to isolated perfused rat lungs and cultured bovine pulmonary artery endothelial cells. J Clin Invest. 1984 Jul;74(1):292–295. doi: 10.1172/JCI111414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasil M., Halliwell B., Grootveld M., Moorhouse C. P., Hutchison D. C., Baum H. The specificity of thiourea, dimethylthiourea and dimethyl sulphoxide as scavengers of hydroxyl radicals. Their protection of alpha 1-antiproteinase against inactivation by hypochlorous acid. Biochem J. 1987 May 1;243(3):867–870. doi: 10.1042/bj2430867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcester J. The statistical method. N Engl J Med. 1966 Jan 6;274(1):27–36. doi: 10.1056/NEJM196601062740106. [DOI] [PubMed] [Google Scholar]


