SUMMARY
Lipoxins (LXs) are potent endogenous counter-regulatory lipid mediators that dampen acute inflammation and promote its resolution. Here, we present our investigation of a new class of thermally and metabolically stable benzo-LXA4 analogs that are potently anti-inflammatory and easier to synthesize. Replacement of the tetraene unit of native LXA4 with a benzo-fused ring system not only increases the thermal stability but also enables highly convergent and efficient syntheses of these analogs. In addition, they resist rapid catalysis and inactivation by eicosanoid oxidoreductase. Like native LXs, o-[9, 12]-benzo-ω6-epi-LXA4, o-[9, 12]-benzo-deoxy-LXA4, m-[9, 12]-benzo-ω6-epi-LXA4 and [9, 14]-benzo-ω6-(R/S)-LXA4 demonstrated potent time-dependent reduction, at nanogram dosages, of PMN infiltration and pro-inflammatory cytokine generation in vivo in murine peritonitis and were organ protective in hind limb ischemia-reperfusion injury of the lung. The o-[9, 12]-benzo-ω6-epi-LXA4 and m-[9, 12]-benzo-ω6-epi-LXA4 were most potent in nanogram doses; both decreased PMN infiltration by ~32%, while o-[9, 12]-benzo-deoxy-LXA4 and [9, 15]-ω6-(R/S)-LXA4 were less potent. The [9,12]- benzo-ω6-epi-LXA4 also activated a lipoxin A4 GPCR and increased macrophage phagocytic activity. Taken together, these findings demonstrate a new generation of LXA4 stable analogs that are easy to synthesize and anti-inflammatory. These benzo-LXA4 analogs are promising tools for new therapeutic approaches as well as assessing endogenous mechanisms in anti-inflammation and resolution.
INTRODUCTION
The acute inflammatory response is mainly a protective mechanism; it destroys and/or separates the injurious agent, removes damaged tissue and repairs the area as best possible [1]. While inflammation is a fundamental component of normal host defense and wound repair, it is also implicated in a broad range of diseases [reviewed in ref. 2]. Elucidation of the relationship between such major diseases and inflammation has led to the targeting of inflammatory mediators, particularly those derived from membrane lipids, as potential therapeutics. While the classical eicosanoids (20-carbon essential fatty acid derivatives) such as prostaglandins and leukotrienes amplify inflammation, lipoxins dampen the process in addition to promoting its resolution [see 3]. Pro-resolving compounds such as lipoxins have the potential to simultaneously reduce the adverse affects of inflammation and promote its resolution, thus providing a new strategy for the development of novel and targeted therapeutics [see refs. 4, 5].
Lipoxins (LXs) are tri-hydroxy derivatives of arachidonic acid that are potently anti-inflammatory, as demonstrated in a variety of pathologic settings such as allergy [6], nephritis [7], asthma [8], gastritis [9] and cystic fibrosis [10]. Formation of both lipoxin A4 (LXA4) and 15-epi-LXA4 (differing only in the stereochemical configuration of the 15-hydroxyl, S and R respectively) occurs via separate transcellular biosynthesis routes demonstrated with isolated human cells (Figure 1) as well as isolated human cells such as macrophages [11]. The biosynthesis of LXA4 can involve a combination of either 15- and 5- or 5- and 12-lipoxygenase (LOX), 15-epi-LXA4 generation is triggered by aspirin-induced acetylation of cyclooxygenase 2 (COX-2), in addition to the activation of 5-LOX [reviewed in ref. 11]. Since treatment with aspirin enhances the biosynthesis of this 15-epimer form of LXA4, it is termed aspirin-triggered LX (ATL). While the biosynthetic pathways of LXA4 and ATL differ [reviewed in ref. 11], both mediators are inactivated via dehydrogenation to form 15-oxo-LXA4 [12]. Interestingly, 15-epi-LXA4 is much less susceptible to enzymatic inactivation by recombinant enzyme than native LXA4. Since LXA4 and ATL exhibit, in most cases, equal potencies in vitro, this finding demonstrates that the 15R configuration appears to increase the half-life and hence overall in vivo potency of ATL over native LXA4 [13]. These findings were confirmed independently, establishing analogs of ATL as benchmarks for LXA4 analogs, resisting inactivation as well as exhibiting protective actions in several animal models of inflammation [14]. Despite the potent activity of the 16-para-fluoro-phenoxy analogs of ATL [reviewed in ref. 11], it is cleared quickly in vivo and subject to β-oxidation [15]. In light of this, a second generation of β-oxidation-resistant LX analogs was introduced that retain both the anti-inflammatory and immunomodulatory actions of LXA4 [15] and its protection in colitis [16].
Figure 1. Biosynthetic pathways for lipoxin A4 and 15-epi-LXA4/ATL.
LX and ATL are both biosynthesized from arachidonic acid. Human 15-LOX type I produces mainly 15S-HpETE that is enzymatically reduced to 15S-HETE. When treated with aspirin, endothelial or epithelial cells expressing COX-2 insert molecular oxygen predominantly in the R configuration at carbon 15 in arachidonic acid. These hydroxy products are then converted by 5-LOX from PMNs, which are rapidly transformed into epoxide intermediates. Opening of the epoxide-containing intermediates follows enzymatically to form the tri-hydroxy tetraene-containing mediators [see ref. 11 for further details].
LXA4 and ATL are examples of endogenous, anti-inflammatory autacoids that are also capable of promoting the resolution of acute inflammation [3]. While LX actions are appreciated in several physiologic and pathologic settings, its full spectrum of therapeutic applications has yet to be fully uncovered, in part because of their inherent chemical lability of the conjugated tetraene system present in the native endogenous mediators. An earlier generation of synthetic LX analogs that retain bioactivity and resist rapid metabolic inactivation have been synthesized and studied in our laboratories [13]. These analogs contain several structural elements that contribute to long and complex synthetic routes: mainly, a fatty acid backbone, a conjugated tetraene system and stereochemically defined hydroxyl substituents. More recently, we [17] and O’Sullivan et al. [18] introduced new generations of lipoxin analogs that can be produced via simplified total synthetic routes, which display lipoxin anti-inflammatory actions. Here, we report the detailed actions of this benzo class of chemically and biologically stable LXA4 analogs and show that they share the anti-inflammatory and pro-resolving properties of native LXA4 and its aspirin-triggered form.
MATERIALS AND METHODS
LXA4 analogs
The total organic synthesis of the LXA4 and analogs used in the present experiments and their physical properties were recently reported and prepared as in [17].
Enzymatic stability
Activity of 15-prostaglandin dehydrogenase/eicosanoid oxidoreductase (abbreviated here and throughout as EOR) was monitored by the formation of NADH from NAD+ spectrophotometrically at 340 nm as in [12]. Substrates in ethanol were taken to dryness under N2 stream and suspended in buffer containing Tris-HCl (0.1 M, pH=9.0) (Sigma, Saint Louis, MO, USA) and NAD+ (1.0 mM) (Sigma, Saint Louis, MO, USA) to a final concentration of 18 μM (100 μL total volume). Reactions were initiated with the addition of partially purified EOR (0.05 μg/incubation) and absorptions were read every 30 seconds for 25 minutes at 37 °C. Initial reaction velocities were determined using linear regressions calculated during the linear phase of conversion.
Acute inflammation: peritonitis
Murine peritonitis was performed using 6-8-wk-old FVB male mice (Charles River Laboratories, Wilmington, MA, USA) that were fed laboratory Rodent Diet 5001 (Purina Mills, Richmond, IN, USA). After anesthetization with isoflurane, compounds were administered in 100 μL phosphate-buffered saline (PBS) intravenously through a tail vein (2 hour dose response and side by side rank order) or in 200 μL of PBS via intraperitoneal injection (time-course studies). Zymosan A (1 mg/1 ml of PBS, Sigma, Saint Louis, MO, USA) or murine TNF-α (100 ng/500 μL, Roche) was injected intraperitoneally immediately following compound administration. In accordance with the Harvard Medical Area Standing Committee on Animals protocol no. 02570, mice were sacrificed (after 2, 4, 12, 24 or 48 hours) and peritoneal lavages were rapidly collected in Dulbecco’s PBS (minus Mg2+ and Ca2+). Aliquots of the lavage were stained with trypan blue and enumerated by light microscopy. For differential leukocyte counts, 300 μL of the lavage was added to 300 μL of 15% bovine serum albumin and centrifuged onto microscope slides at 2200 rpm for 4 min. using a Cytofuge (StatSpin, Norwood, MA). The slides were allowed to air dry and cells were visualized using a modified Wright-Giemsa stain (Sigma, Saint Louis, MO, USA). For chemokine and cytokine determination, aliquots (120 μL) taken from the supernatants of the peritoneal lavages were analyzed using the SearchLight Proteome Array custom-designed by Pierce Boston Technology Center (Woburn, MA, USA).
Hind-limb ischemia-reperfusion-induced second-organ lung injury
Mice were anesthetized by intraperitoneal injection of pentobarbital, 50 mg kg−1 (Nembutal sodium solution, NDC 0074-3778-04). Hind-limb ischemia was induced by placing a rubber band (no. 30, 2 × 1/32 × 1/8 inches (length gauge width: 1800 count pound 1, Pure rubber bands, Plymouth Office Products, Muscatine, IA, U.S.A.) on each hind limb using small clamps (Harvard Apparatus). After 1 h of ischemia, the tourniquets were removed and reperfusion followed for 1 h. At the end of the reperfusion period, the mice were sacrificed with an overdose of pentobarbital (400 μl 10 mg ml 1 i.p.), and the left lung lobes quickly excised and frozen in liquid nitrogen. o-[9, 12]-benzo-LXA4 (20 μg kg−1 in 200 μL sterile saline) or vehicle (0.5% ethanol in 200 μL sterile saline) were injected intravenously 5 min before the start of the ischemic period. A second dose was administered 5 min prior to reperfusion. The increases in lung myeloperoxidase activity were monitored as in [19], and taken to numerate PMN accumulation in the lung.
Phagocytosis of zymosan A
Resident peritoneal macrophages were collected from 6-to 8-week-old naïve male FVB mice (~2 ×106 cells/mouse), plated into 24 well plates (at 1 ×105/well) in PBS with calcium and magnesium, and allowed to adhere for 1h at 37 °C as in [20]. LXA4 and analogs were then added (15 min, 37 °C) followed by fluorescein isothiocyanate (FITC)-labeled zymosan A (Sigma, St Luis, MO) (30 min, 37°C). Wells were gently aspirated and Trypan blue (0.03 % vol/vol) was added (~ 60 sec) to quench any extracellular FITC-zymosan particles. Phagocytosis was quantified using a fluorescent plate reader Victor3 (Perkin Elmer).
Recombinant human ALX-FPR2 GPCR β-arrestin reporter construct
Experiments to assess the activation of lipoxin A4 receptor ALX-FPR2 [21, 22] were carried out using the new G protein coupled receptor (GPCR) PathHunter β-arrestin system (DiscoveRx, Fremont, CA) [23]. In this system, two inactive fragments of β-galactosidase (β-Gal), ProLink and Enzyme Acceptor (EA), are fused with the intracellular domain of GPCR and β-arrestins, respectively. Upon receptor activation following ligand interaction, binding of β-arrestins to GPCR [24] results in complementation of the ProLink and EA fragments. Activation of GPCR, in this case ALX-FPR2, can be then monitored by measuring conversion of a luminogenic substrate of β-Gal. For these experiments, HEK-293 PathHunter β-arrestin cells expressing human ALX-FPR2 (UniProtKB/Swiss-Prot P25090) were custom made (DiscoveRx) and then cultured in Dulbecco’s modified Minimum Eagle’s Medium (DMEM) with heat inactivated fetal calf serum (10%), L-glutamine (2 mM), Penicillin/Streptomycin (1%), G418 (800 μg/mL), and Hygromycin (200 μg/mL). Twenty-four hours prior to experiments, cells were plated (20,000/well/96 well plate) in serum free medium and then incubated with 15-epi-LXA4 or its analogs (1h, 37 °C). The luminogenic substrate for β-Gal (PathHunter EFC Detection kit™, DiscoveRx) was added (1h, 21 °C) and luminescence was measured on a plate reader (EnVision, Perkin Elmer, Waltham, MA). Values obtained after subtraction of background, readings obtained from cells kept in medium alone prior addition of β-Gal substrate, were fitted on SigmaPlot software (SPPS Inc, Chicago, IL) to calculate EC50 values for each tested compound.
Statistical analysis
Results are expressed as the mean ± SEM in different animals. Additional comparative analysis was performed using unpaired Student’s t-tests, assigning significance at p < 0.05.
RESULTS
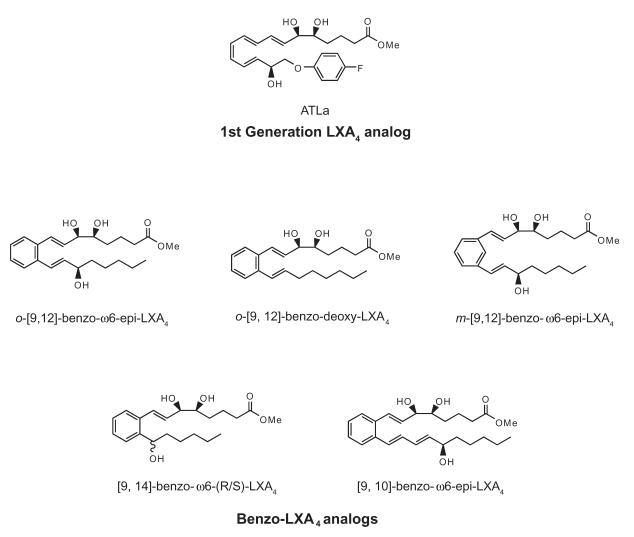
These five analogs studied herein are all structural analogs of LXA4 and specifically 15-epi-LXA4, its aspirin-triggered form (Figure 2). Naming of these analogs, therefore, is with respect to ATL. The location of the benzo ring is denoted with [x, y], where x represents the distance from the α end of the molecule and y is determined by the distance from the ω-proximal hydroxyl group (designated here as position 15, as in native ATL). The ω-proximal hydroxyl group, when present, is located at the ω6 position and is denoted as such. In these analogs, the ω-proximal hydroxyl group (excluding the benzo-ω6-deoxy-LXA4) was synthesized in the R configuration as in ATL since ATL is resistant to rapid enzymatic inactivation [13]. To determine whether these analogs were resistant to rapid enzymatic catalysis, each of these compounds (~ 15 μM) was incubated with recombinant EOR (0.05 μg) and their rates of conversion were calculated by monitoring formation of NADH, an essential cofactor [see ref. 17]. Native LXA4 was converted most readily (3.1 μM/min), while ATL-Me was used here for direct comparison, given its potent actions in vivo [reviewed in refs. 11, 17]. Each of the benzo LXA4 analogs, o-[9,12]-benzo-ω6-epi-LXA4, m-[9,12]-benzo-ω6-epi-LXA4, [9, 14]-benzo-ω6-(R/S)-LXA4, and [9, 10]-benzo-ω6-epi-LXA4 were not readily converted (0.09 - 0.45 μM/min, p < 0.05 when directly compared to LXA4). The initial rate of conversion for o-[9, 12]-benzo-deoxy-LXA4 was the slowest (0.04 μM/min, p < 0.025 when compared to ATL-Me), suggesting a potential for increased metabolic stability in vivo by resisting rapid enzymatic inactivation.
Figure 2. Structures of benzo-LXA4 analogs.
All structures are based upon 15-epi-LXA4 as the biotemplate because of its long bio-half-life. Benzene ring location is designated with respect to the 6 and 15 position hydroxyl groups of 15-epi-LXA4. Note that these analogs were used in their carboxy methyl ester form.
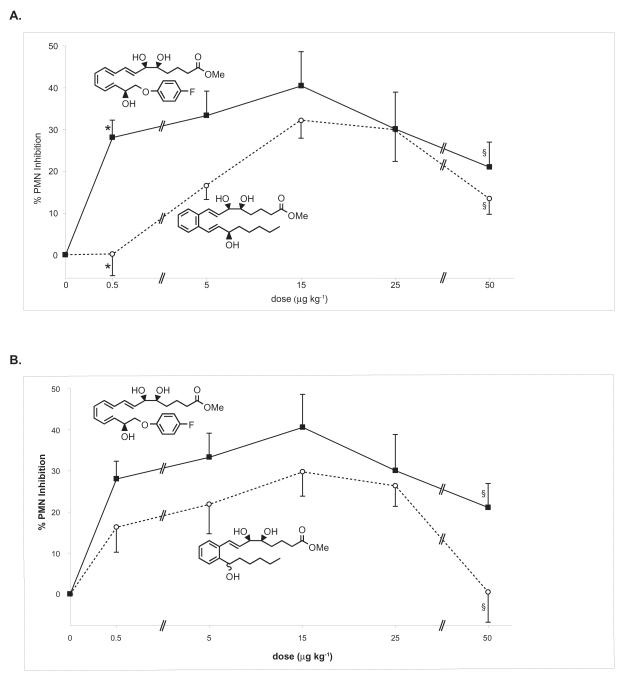
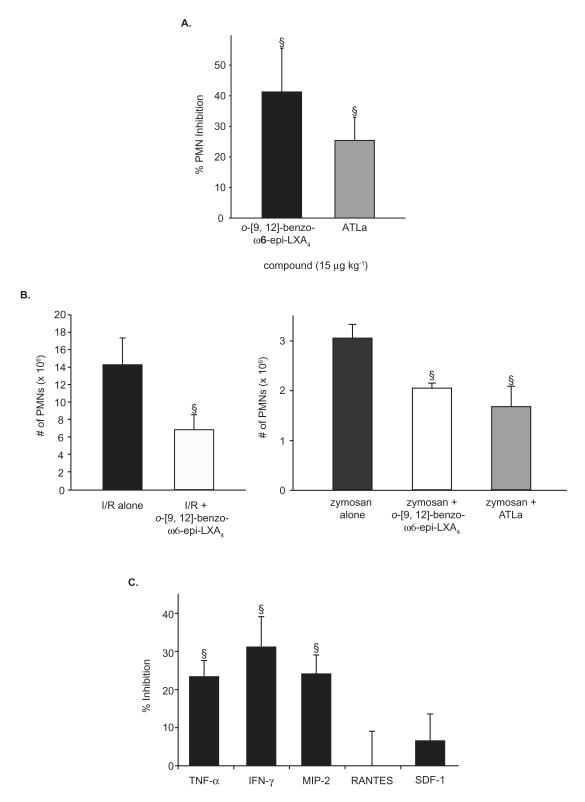
In order to determine the appropriate dose to compare the in vivo anti-inflammatory actions of these analogs, the dose-dependent affects of o-[9,12]-benzo-ω6-LXA4 and [9,14]-benzo-ω6-(R/S)-LXA4 on PMN infiltration were assessed and directly compared (Figure 3a and 3b) with ATLa using a range of doses close to the IC50 reported for ATLa [25]. Administration of o-[9,12]-benzo-ω6-epi-LXA4 and [9,14]-benzo-ω6-(R/S)-LXA4 at five different doses that ranged from 10 ng to 1 μg per mouse (~0.5-50 μg kg−1) resulted in a bell-shaped relationship between dose and reduction in PMN infiltration. In this model, the peak reduction was observed at a dose of 15 μg kg−1. ATLa also showed reduced PMN infiltration at all doses as expected and proved to be statistically superior to o-[9,12]-benzo-ω6-epi-LXA4 at the 0.5 and 5 μg kg−1 doses (p < 0.0001 and p < 0.05 respectively, when compared to o-[9, 12]-benzo-ω6-epi-LXA4). ATLa was also more potent at reducing PMN infiltration above that obtained with [9,14]-benzo-ω6-(R/S)-LXA4. Of interest, this increased efficacy proved to be statistically significant at the highest dose (p < 0.05, when compared to [9, 14]-benzo-ω6-15-R/S-LXA4) in contrast to o-[9, 12]-benzo- epi-LXA4.
Figure 3. Benzo-LXA4 analogs reduce inflammation in vivo in a dose-dependent fashion.
ATLa, o-[9,12]-benzo-ω6-epi-LXA4, or [9,14]-benzo-ω6-(R/S)-LXA4 were injected by intravenous bolus injection (0.5, 5, 15, 25 or 50 μg kg−1 in 100 μL of sterile saline) via the tail vein of 6-8-wk male FVB mice followed by peritoneal injection of zymosan A (1 mg/1 mL). Peritoneal lavages were collected (2 h) and total leukocytes, PMNs and monocytes were enumerated. Reduction in PMN infiltration was determined by comparison to vehicle control (100 μL of sterile saline). A. Dose-response comparison between ATLa (■) and o-[9,12]-benzo-ω6-epi-LXA4(○). B. Dose-response comparison between ATLa (■) and [9,14]-benzo-ω6-(R/S)-LXA4 (○). Values represent mean ± SEM, n=3-5, *P < 0.0001, §P < 0.05.
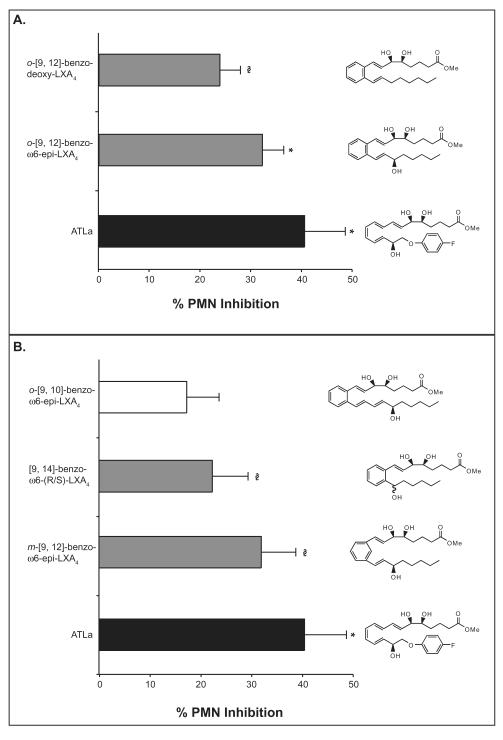
For side-by-side comparison of the anti-inflammatory action of these five analogs, a dose of 15 μg kg−1 or ~300 ng/mouse was used since it was most efficacious in the earlier dose-response experiments in Figure 4. In this rank order comparison (Figure 4), o-[9,12]-benzo-ω6-epi-LXA4 and m-[9, 12]-benzo-ω6-epi-LXA4 were most effective, both exhibiting a statistically significant ~32% reduction in PMN infiltration (p < 0.005 and p < 0.05 respectively compared to control). The analogs o-[9,12]-benzodeoxy-LXA4 and [9,14]-benzo-ω6-(R/S)-LXA4 were least effective, decreasing PMN infiltration by ~24% and ~22% respectively (p < 0.05 and p < 0.01 respectively compared to control, one-tailed t-test). Mice treated with [9,10]-benzo-ω6-epi-LXA4 failed to reduce PMN numbers in a statistically significant manner, indicating the requirement for the overall lipoxin A4 trihydroxy structure.
Figure 4. Rank order for benzo-LXA4 analog anti-inflammatory action.
Compounds were injected by intravenous bolus injection (15 μg kg−1/100 μL of sterile saline) via the tail vein of 6-8-week-old male FVB mice followed by peritoneal injection of zymosan A (1 mg/1 mL). Peritoneal lavages were collected (2 h) and total leukocytes, PMNs and monocytes were enumerated. Reduction in PMN infiltration was determined by comparison to vehicle control (100 μL of sterile saline). Values represent mean ± SEM, n=5-10; *P < 0.005, §P < 0.05.
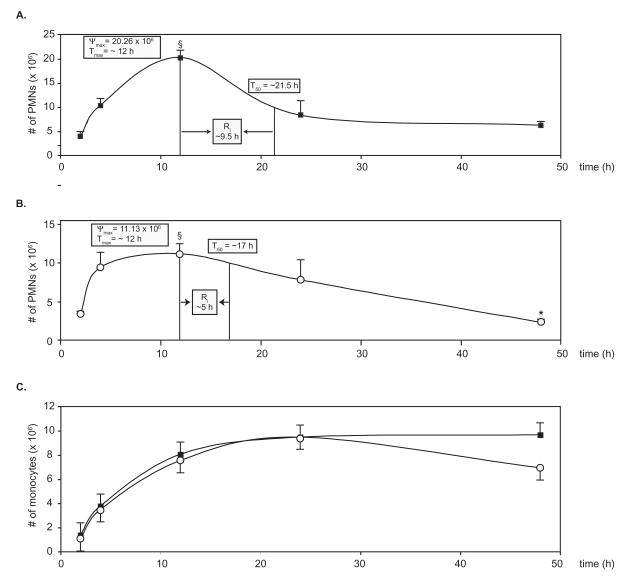
We next assessed the actions of vehicle alone or o-[9, 12]-benzo-ω6-epi-LXA4 in the zymosan A-initiated acute inflammatory response by monitoring the number of PMNs (Figure 5a and 5b respectively) and monocytes (Figure 5c) infiltrating the peritoneum within a 48-hour time period. While intravenous administration of o-[9, 12]-benzo-ω6-epi-LXA4 led to potent reduction in PMN infiltration after 2 hours, intraperitoneal injections of this compound did not have a significant impact on exudate PMN numbers. In order to analyze the actions of o-[9, 12]-benzo-ω6-epi-LXA4 within an acute inflammatory setting, we used the recently introduced resolution indices [26, 27]. To this end, Ψmax (maximal PMN infiltration), Tmax (time of maximal PMN infiltration), Ri (time interval from the recorded maximum PMN infiltration point to the 50% reduction point) and T50 (time required to reach the 50% reduction point) were determined as in [26, 27]. While treatment with o-[9, 12]-benzo-ω6-epi-LXA4 resulted in similar Tmax (~12 h), it gave a dramatic ~50% decrease in Ψmax (20 × 106 PMN to 11 × 106 PMN, p < 0.05), ~ 50% decrease in Ri (9.5 h to 5 h) and a ~20% decrease in the T50 (21.5 h to 17 h). In contrast to the reduction in PMN infiltration, the infiltration of monocytes was relatively unaffected by o-[9, 12]-benzo-ω6-epi-LXA4 treatment throughout the first 24 hours (demonstrated in Figure 5c). At 48 hours, o-[9, 12]-benzo-ω6-epi-LXA4-treated mice gave a slight trend that was not statistically significant, toward decrease in the number of mononuclear cells.
Figure 5. Time course of o-[9,12]-benzo-ω6-epi-LXA4 action in the acute inflammatory response.
Compounds were injected intraperitoneally (15 μg kg−1/200 μL of sterile saline) followed by peritoneal injection of zymosan A (1 mg/1 mL). Mice were sacrificed at indicated time points (2, 4, 12, 24, 48 h), peritoneal lavages were collected, and total leukocytes, PMNs and monocytes were enumerated. A. Time course of PMN infiltration with zymosan A and treated with vehicle (■). B. Time course of PMN infiltration when treated with local o-[9, 12]-benzo-ω6-epi-LXA4 (○). C. Time course of monocyte infiltration in vehicle control (□) and o-[9, 12]-benzo-ω6-epi-LXA4-treated (○) mice. See text for definitions of Ψmax, Tmax, Ri and T50. Values represent mean ± SEM, n=4; *P < 0.005, §P < 0.05.
In order to further assess the in vivo mechanism of action for these analogs, we next examined the actions of o-[9, 12]-benzo-ω6-epi-LXA4 or ATLa in TNF-α-stimulated peritonitis (Figure 6a), since earlier reports demonstrated that LXA4 and ATL can directly counteract the TNF-α pro-inflammatory signal [28, 29]. Again, a direct comparison was made to ATLa since it counteracts TNF-α-stimulated inflammation in vivo [25, 29]. In this case, both LX analogs gave statistically significant reduction in PMN infiltration (~ 41%, p < 0.05 and ~25%, p < 0.05, respectively).
Figure 6. Anti-inflammatory properties of o-[9, 12]-benzo-ω6-epi-LXA4.
Mice were treated with o-[9, 12]-benzo-ω6-epi-LXA4 or vehicle to assess its actions on TNF-α-induced murine peritonitis, ischemia-reperfusion-second organ lung injury, and pro-inflammatory cytokine generation. A. o-[9, 12]-benzo-ω6-epi-LXA4 and ATLa were injected by intravenous bolus injection (15 μg kg−1/100 μL of sterile saline) via the tail vein of 6-8-week-old male FVB mice followed by peritoneal injection of murine TNF-α (100 ng/500 μL). Peritoneal lavages were collected (2 h) and total leukocytes, PMNs and monocytes were enumerated. Reduction of PMN infiltration was determined by comparison to vehicle control (100 μL of sterile saline). Values represent mean ± SEM, n=3-4. §P < 0.05. B. (left panel) o-[9, 12]-benzo-ω6-epi-LXA4 (20 μg kg−1) or vehicle was first administered i.v. 5 min prior to initiation of hind-limb ischemia. A second identical dose was administered i.v. 5 min prior to reperfusion. PMN accumulation in the lung was measured by myeloperoxidase activity in the left lung lobe. See Methods for further details. Values represent mean ± SEM, n=3-4. §P < 0.05. (right panel) o-[9, 12]-benzo-ω6-epi-LXA4 or ATLa was injected by intravenous bolus injection (5 μg kg−1/100 μL of sterile saline) via the tail vein of 6-8-week-old male FVB mice followed by peritoneal injection of zymosan A (1 mg/1 mL). Peritoneal lavages were collected (2 h) and total leukocytes, PMNs and monocytes were enumerated. Values represent mean ± SEM, n=3-8. §P < 0.05. C. Selective reduction in peritonitis cytokines and chemokines. o-[9, 12]-benzo-ω6-epi-LXA4 (15 μg kg−1/100 μL sterile saline) or vehicle was injected intraperitoneally into 6-8-week-old male FVB mice, followed by injection of zymosan A (1 mg/1 mL). Lavage supernatants were collected after 2 hours. Amounts of selected chemokines and cytokines were measured using a custom-designed SearchLight Proteome Array. Values represent mean ± SEM, n=3-4. §P < 0.05.
Since the new LX analogs retained the ability of LX to reduce PMN infiltration in an in vivo setting of local acute inflammation, we next evaluated the ability of the o-[9, 12]-benzo-ω6-epi-LXA4 to protect organs against ischemia-reperfusion (I/R)-initiated second-organ injury of the lung (Figure 6b) since ATLa proved in earlier studies to be very effective in reducing PMN-mediated reflow injury [19]. Intravenous administration of o-[9, 12]-benzo-ω6-epi-LXA4 (20 μg kg−1) resulted in a statistically significant decrease in PMN (14.3 ± 3.07 × 106 in the I/R lungs compared to 6.4 ± 1.61 × 106 when treated with o-[9, 12]-benzo-ω6-epi-LXA4, (p < 0.05)), representing ~ 52% inhibition. In parallel, zymosan-A peritonitis experiments were performed and also gave reduced PMN infiltration to a similar degree (~ 29% and ~44% reduction for o-[9, 12]-benzo-ω6-epi-LXA4 and ATLa respectively, p < 0.05) as in Figure 4.
Cytokines and chemokines are also critical mediators in the regulation of leukocyte trafficking during inflammation [1-3]. Since ATLa significantly modulates their generation during the acute inflammatory response [26, 29], we next sought to determine whether treatment with o-[9, 12]-benzo-ω6-epi-LXA4 could alter inflammatory cytokine expression. To this end, we monitored potential changes in the levels of a select panel of inflammatory cytokines and chemokines after initiating the zymosan-stimulated acute inflammatory response. Mice treated with o-[9, 12]-benzo-ω6-epi-LXA4 gave ~24%, ~31% and ~24% reduction in TNF-α, IFN-γ, and MIP-2 generation respectively (Figure 6c) (p < 0.05, two-tailed t-test) but had essentially no effect with levels of either RANTES or SDF-1 in this system.
The o-[9, 12]-Benzo-ω6-epi-LXA4 enhanced phagocytosis by macrophages and activated ALX
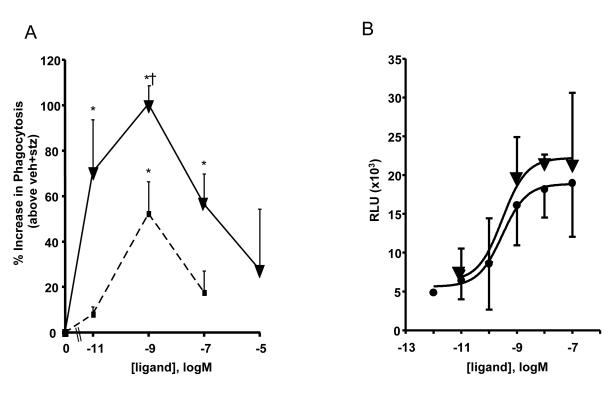
In order to assess potential pro-resolving actions of o-[9,12]-benzo-ω6-epi-LXA4 in these experiments, we investigated its ability to enhance phagocytosis by macrophages compared to LXA4. As shown in Fig. 7A, incubation of murine macrophages with o-[9,12]-benzo-ω6-epi-LXA4 and LXA4 gave significant dose-dependent increases in phagocytosis as monitored by the uptake of FITC zymosan. Of interest, the stable benzo analog was significantly more potent than the native LXA4 in the concentration range tested (from 0.01 nM to 10 μM). Enhanced phagocytosis by macrophages is a hallmark action of pro-resolving mediators [30]. Lipoxin A4 and ATL are ligands for a specific GPCR present on the surface of human leukocytes demoted ALX-FPR2 [for recent reviews, see refs. 21, 22]. LXA4 is a stereoselective agonist of ALX-FPR2, which can also bind a wide range of molecules [22]. To access the pro-resolving agonist actions of LXA4 and related mediators, we recently constructed a new stable cell line expressing human ALX [31]. This system utilizes the new β-arrestin chemiluminescence reporter (see Materials and Methods) thus obviating the need for radioligand binding; also, it is not dependent on intracellular transient second messenger signals. This is important because ALX activation by LXA4 with human PMN does not require Ca2+ mobilization [32] to stop PMN infiltration in vivo [21]. Results in Fig. 7B clearly indicate that the new o-[9, 12]-benzo-LXA4, like LXA4, also activates ALX. In this regard, [9,12]-benzo-LX was essentially equivalent to the efficacy of the native ligand, 15-epi-LXA4.
Figure 7. Benzo-LXA4 activation of the ALX-FPR2 GPCR.
A) Dose dependent increase in phagocytosis. Phagocytosis, determined as indicated in Materials and Methods, from resident murine peritoneal macrophages treated with the indicated concentrations of o-[9,12]-benzo-ω6-epi-LXA4 (▼) or LXA4 (■) (15 min, 37 °C) followed by co-incubation with FITC-labeled zymosan A (30 min, 37°C). Values represent mean ± SEM, n = 3; *, P<0.05 when compared with vehicle; †, P<0.05 when compared with LXA4.
B) Ligand dependent activation of ALX-FPR2 receptor. HEK-ALX β-arrestin cells (see text for details) were exposed to the indicated concentration of 15-epi-LXA4 (●) or o-[9,12]-benzo-ω6-epi-LXA4 (▼) (1h, 37 °C) followed by incubation with the β-galactosidase substrate (1h, 21 °C). Values are relative luminescence unit subtracted from background (mean ± SEM, n = 3-5; RLU, relative luminescence unit).
Since lipoxins exert their action via specific binding and activation of the ALX receptor, these direct comparisons with 15-epi-LXA4 and o-[9,12]-benzo-ω6-epi-LXA4 using the ALX β-arrestin cell system indicated that, in this system, both compounds activated ALX receptor with comparable EC50 ~2.9 ×10−12 and 2.9 ×10−12, respectively (Fig. 7B), which are consistent with the concentration and dose range of their biological actions in in vitro and in vivo systems.
DISCUSSION
In the present study, we prepared and studied a new generation of thermodynamically stable benzo-LXA4 analogs that are resistant to enzymatic catalysis [15], potent anti-inflammatories in vivo that are the products of efficient total synthetic pathways. While the analogs of this series share a common benzo ring system backbone and 5,6-vicinal hydroxyl groups present in lipoxin A4 and ATL, there exist subtle differences among these five analogs. Comparing the impact of these structural differences in both anti-inflammatory and enzymatic catalysis provides a basis to judge structure-function relationships.
Earlier generations of LXA4 analogs resist inactivation by EOR, mainly via the presence of an R hydroxyl group at the 15 position [13]. In view of this, the present generation of lipoxin analogs were synthesized with an ω-6 R hydroxyl group. In addition, a benzene ring was used to replace the labile tetraene backbone present in LXA4 because of the inherent chemical instability of the conjugated tetraene system. This structural modification not only enabled a highly convergent and stereoselective synthesis [17], but also provided conformational rigidity. All five of the benzo analogs examined here demonstrated significant resistance to dehydrogenation at C15 [17], a feature found to enhance bioactivity via prolonging the intact bio-half-life in vivo [as reviewed in 11]. Comparison within our panel of analogs demonstrated that o-[9, 12]-benzo-deoxy-LXA4 was not converted by 15-EOR to an appreciable extent, suggesting that EOR acts primarily at the ω-6 hydroxyl group and not at either of the vicinal diol 5-and 6-hydroxyls of these benzo-LX analogs. While o-[9, 12]-benzo-deoxy LXA4 was essentially inert to enzymatic catalysis, the other four analogs were inefficiently converted but were not more resistant than ATL-Me [17]. These results indicate that the benzene ring does not confer a greater resistance to inactivation. Nonetheless, comparison of ATL-Me and [9, 14]-ω6-(R/S)-benzo-LXA4 may lend some clues to a preferred conformation of LXA4 for the EOR. While these two structures share the distance between the vicinal hydroxyl groups and the ω-proximal hydroxyl, there are two noteworthy differences. First, ATL-Me exists in an enantiomerically pure 15R configuration while [9, 14]-ω6-(R/S)-benzo-LXA4 is racemic with respect to the 15 position. Since the increased metabolic stability of ATL is attributed to its 15R hydroxyl, the finding that [9, 14]-ω6-(R/S)-benzo-LXA4 was converted equally slowly as ATL-Me suggests that the locked conformation dictated by the presence of the benzene ring may interfere with EOR substrate recognition.
ATLa is an established benchmark of LX analogs, demonstrating potent anti-inflammatory actions as well as resistance to inactivation [14]. While other currently available non-steroidal anti-inflammatory drugs (NSAIDs) exhibit similar anti-inflammatory properties, LXs and their analogs are considerably more potent, requiring two to three orders of magnitude lower concentrations/dose to exert similar actions in vivo [33]. Both o-[9, 12]-benzo-ω6-epi-LXA4 and [9,14]-benzo-ω6-(R/S)-LXA4 demonstrated dose-dependent reduction of PMN infiltration. Of interest, when compared to ATLa, o-[9, 12]-benzo-ω6-epi-LXA4 was statistically as effective at the higher doses (15-50 μg kg−1) while [9,14]-benzo-ω6-(R/S)-LXA4 was as effective at the lower doses (0.5-25 μg kg−1) (Figure 3). These relative differences in dose-dependent actions suggest potential alternative sites of action and/or clearance of the 9,14 analog.
In the rank order comparison of these analogs, ATLa, o-[9,12]-benzo-ω6-epi-LXA4, m-[9,12]-benzo-ω6-epi-LXA4, o-[9, 12]-benzo-deoxy-LXA4 and [9,14]-benzo-ω6-(R/S)-LXA4 reduced PMN infiltration in a statistically significant manner (~40%, ~32%, ~32% and 22% respectively). Earlier studies demonstrated that indomethacin, a commonly prescribed NSAID, reduces PMN infiltration by ~35-40% in this murine system [20], providing validation of the potential clinical significance of the degree of reduction observed with these new analogs, since they proved to be in the same potency range as indomethacin. Our results (Figure 4) also demonstrate that both the ortho- and meta- benzo-ω6-epi-LXA4 analogs blocked PMN infiltration to a similar degree. Since these two analogs differ mainly in the angle between the α- and ω-arms, it suggests that LX-receptor interactions exhibit some steric flexibility. In contrast to the other four analogs tested, the 24 carbon [9,10]-benzo-ω6-epi-LXA4 was not statistically different from the vehicle control in zymosan-initiated peritonitis, which suggests the importance of the number of carbons on the ω-proximal chain in ligand-receptor interaction. Along these lines, the finding that o-[9, 12]-benzo-deoxy-LXA4 did decrease PMN entry suggests that, while a misplaced ω-proximal hydroxyl group can inactivate a compound, its presence is not an absolute requirement for partial activity in this system.
In the time course of the zymosan-induced peritonitis, local administration of o-[9, 12]-benzo-ω6-epi-LXA4 also reduced PMN infiltration (Figure 5b). Systemic administration resulted in ~32% decrease in PMNs within 2 hours, while local intraperitoneal injection did not. In conjunction with recent studies showing the potent anti-inflammatory actions of oral LXs [25], these findings suggest the importance of systemic uptake in the LX anti-inflammatory signal. Local treatment with o-[9, 12]-benzo-ω6-epi-LXA4 did result in an ~50% decrease in both Ψmax and Ri (Figure 5). Earlier studies demonstrated that ATLa treatment, in an identical setting, resulted in a dramatic decrease in Ψmax but that the Ri was similar to the untreated acute inflammatory course [26]. Determination of the resolution indices as in refs. [26, 27] helped to assess the potencies and in vivo mechanisms used by the benzo-LXA4 analogs. The dramatic difference between o-[9, 12]-benzo-ω6-epi-LXA4 and ATLa in their Ri suggests increased potency in vivo.
In the consideration of the observed reduction of PMN infiltration in this setting, it is important to distinguish between cytotoxicity and specific prevention of PMN trafficking to the inflamed site. In our model of zymosan-induced peritonitis, ATLa treatment resulted in a bell-shaped dose-dependent inhibition of PMN infiltration (Figure 4). In side-by-side comparisons, o-[9,12]-benzo-ω6-epi-LXA4 and [9,14]-benzo-ω6-(R/S)-LXA4 also demonstrated a similar dose-dependent response suggesting that their inhibition of PMNs is specific and not a result of non-specific cytotoxicity. Of interest, monocytic infiltration remained unaffected for the first 24 hours of the acute inflammatory response (Figure 5c), which further indicates that the decrease in PMN number is not a result of non-specific cytotoxicity.
The immune response to zymosan A includes an increase in TNF-α generation [26] and TNF-α itself can initiate an acute inflammatory response. Our results shown in Figure 6 demonstrate that o-[9, 12]-benzo-ω6-epi-LXA4 can exert its actions downstream of TNF-α activation. Furthermore, the apparent superiority of o-[9, 12]-benzo-ω6-epi-LXA4 to ATLa in this model provides further evidence that these two compounds might have different in vivo bio-half-lives.
In humans, I/R injury following surgical clamping is a clinically significant entity that can lead to second-organ injury and lengthen post-operative recovery [34]. In murine hind-limb ischemia-induced lung injury, a second generation LX analog potently reduced PMN infiltration into the lung by ~ 50% [25]. The present results (Figure 6b) demonstrate a comparable inhibition of PMN infiltration confirming its protective actions and indicating a further retention of bio-activity by this new analog. A side-by-side administration of o-[9, 12]-benzo-ω6-epi-LXA4 in the setting of zymosan-A-induced peritonitis resulted in ~28 % inhibition (Figure 6b). This dramatic reduction is consistent with the findings presented earlier [17] and thus serves to confirm the validity of protection from I/R-induced second organ injury by lipoxin A4 and specifically this new stable LXA4 analog.
Cytokines and chemokines play critical roles in the propagation of the inflammatory response and their generation regulated LXA4, specifically by ATLa [26]. Therefore, the modulation of these soluble protein inflammatory mediators by o-[9, 12]-benzo-ω6-epi-LXA4 provides insight into its anti-inflammatory mechanism. Treatment with o-[9, 12]-benzo-ω6-epi-LXA4 resulted in a marked decrease in the generation of the MIP-2 (Figure 6c), a cytokine that is potently chemotactic for neutrophils but not monocytes, but had no impact on the levels of either RANTES or SDF-1, chemokines that are well appreciated for their chemotactic activity with monocytes [35]. These results provide insights into the in vivo actions and mechanisms by which o-[9, 12]-benzo-ω6-epi-LXA4 is able to reduce PMN infiltration. A decrease in both TNF-α and IFN-γ (Figure 6c) was also noted. Inhibition of TNF-α generation is of particular interest because of the importance of anti-TNF-α therapies in the treatment of chronic inflammatory diseases such as rheumatoid arthritis and Crohn’s disease [36, 37]. This down-regulation of pro-inflammatory cytokines and PMN-specific chemokines identifies them as important downstream effectors of o-[9, 12]-benzo-ω6-epi-LXA4, which begins to elucidate its mechanism of action. The ability of o-[9, 12]-benzo-ω6-epi-LXA4 to inhibit up- and down-stream of TNF-α in the inflammatory cascade demonstrates that, like native LX, anti-inflammatory actions most likely occur at several points within the acute inflammatory response.
A key action of pro-resolving mediators such as lipoxins and resolvins is their ability to stimulate and enhance macrophage uptake of apoptotic neutrophils as well as microbial-derived particles such as zymosan [27, 30]. In this context, lipoxin A4 was the first to show nonphlogistic recruitment actions with mononuclear cells [38], which is a key response in promoting resolution. Lipoxin A4 also stimulates enhanced phagocytosis of apoptotic neutrophils, an obligatory event in the termination and clearance at inflammatory loci in vivo [39]. The benzo-lipoxin A4 analog proved to be a potent mimetic of this key pro-resolving action of lipoxins. Results in Figure 7 clearly demonstrate that the benzo-lipoxin A4 analog is a potent agonist of macrophage phagocytosis. It is noteworthy that mononuclear cells and macrophages rapidly inactivate lipoxin A4 via dehydrogenation [38]. Hence, the stable analog, which resists dehydrogenation, shows increased potency in this in vitro assay, which likely translates to its superiority in in vivo assays.
Another key component of lipoxin’s actions is the ability of the signal to be amplified by lipoxin A4 interaction with G protein-coupled receptors (GPCR). Lipoxin A4, ATL, and their first-generation lipoxin analogs specifically bind to the GPCR denoted ALX-FPR2 [21, 22]. The benzo-lipoxin proved to be a potent mimetic activating this receptor ALX-FPR2 similar to the dose response obtained with 15-epi-LXA4, suggesting that o-[9, 12]-benzo-ω6-epi-LXA4 acts at the same receptor and site of action as lipoxin A4 and its aspirin-triggered 15-epi-LXA4. Recent studies have shown that low-dose aspirin in humans triggers the endogenous production of 15-epi-LXA4, which is directly responsible for its local anti-inflammatory actions in a human model of acute dermal inflammation [40]. Hence, the introduction of a novel benzo-lipoxin A4 analog that can mimic these actions of 15-epi-LXA4 may provide a unique opportunity to investigate the pro-resolving and anti-inflammatory actions of low-dose aspirin that are mediated in humans via the endogenous production of 15-epi-LXA4.
In the present study, a series of benzo-LX analogs was recently designed that are resistant to enzymatic catalysis [17] and demonstrated diverse and potent anti-inflammatory, pro-resolving, and organ protective actions. A related series of benzo-LXA4 analogs have been synthesized earlier [41], but unlike several of the analogs presented here, these earlier analogs had different stereochemistry at the C6-hydroxyl group and also lacked stereospecificity at the ω-proximal hydroxyl group, yet they also showed some anti-inflammatory activity in vitro. Unlike native LXA4, ATL and earlier generations of analogs, this new series of benzo-analogs described herein possess an increased thermal stability and are the products of highly convergent and efficient organic syntheses, characteristics that make them appealing candidates as pharmacological therapeutics. Moreover, the new generation of LX analogs provides novel tools to investigate endogenous anti-inflammatory and pro-resolving mechanisms in vivo.
ACKNOWLEDGEMENTS
We thank Mary Halm Small for expert assistance in the preparation of this manuscript.
This work was supported in part by NIH grant nos. R37-GM038765, P50-DE016191, and R01-DK074448.
Abbreviations used
- ATL
aspirin-triggered lipoxin
- COX-2
cyclooxygenase 2; I/R, ischemia-reperfusion
- LOX
lipoxygenase
- LX
lipoxins
- NSAIDs
non-steroidal anti-inflammatory drugs
- EOR
eicosanoid oxidoreductase
- PMN
polymorphonuclear leukocyte
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Majno G, Joris I. Cells, Tissues and Disease: Principles of General Pathology. Blackwell Science; Cambridge, Mass.: 1996. p. 974. [Google Scholar]
- 2.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 3.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 4.Serhan CN. Resolution phases of inflammation: novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 5.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 6.Bandeira-Melo C, Bozza PT, Diaz BL, et al. Cutting edge: Lipoxin (LX) A4 and aspirin-triggered 15-epi-LXA4 block allergen-induced eosinophil trafficking. J Immunol. 2000;164:2267–2271. doi: 10.4049/jimmunol.164.5.2267. [DOI] [PubMed] [Google Scholar]
- 7.McMahon B, Godson C. Lipoxins: endogenous regulators of inflammation. Am J Physiol Renal Physiol. 2004;286:F189–F201. doi: 10.1152/ajprenal.00224.2003. [DOI] [PubMed] [Google Scholar]
- 8.Levy BD, De Sanctis GT, Devchand PR, et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A4. Nat Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 9.Fiorucci S, De Lima OM, Jr., Mencarelli A, et al. Cyclooxygenase-2-derived lipoxin A4 increases gastric resistance to aspirin-induced damage. Gastroenterology. 2002;123:1598–1606. doi: 10.1053/gast.2002.36558. [DOI] [PubMed] [Google Scholar]
- 10.Karp CL, Flick LM, Park KW, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 11.Serhan CN, editor. Special Issue on Lipoxins and Aspirin-Triggered Lipoxins. Prostaglandins Leukot Essent Fatty Acids. 2005;73(34):139–321. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Clish CB, Levy BD, Chiang N, Tai H-H, Serhan CN. Oxidoreductases in lipoxin A4 metabolic inactivation. J Biol Chem. 2000;275:25372–25380. doi: 10.1074/jbc.M002863200. [DOI] [PubMed] [Google Scholar]
- 13.Serhan CN, Maddox JF, Petasis NA, et al. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- 14.Schottelius AJ, Giesen C, Asadullah K, et al. An aspirin-triggered lipoxin A4 stable analog displays a unique topical anti-inflammatory profile. J Immunol. 2002;169:7063–7070. doi: 10.4049/jimmunol.169.12.7063. [DOI] [PubMed] [Google Scholar]
- 15.Guilford WJ, Bauman JG, Skuballa W, et al. Novel 3-oxa lipoxin A4 analogues with enhanced chemical and metabolic stability have anti-inflammatory activity in vivo. J Med Chem. 2004;47:2157–2165. doi: 10.1021/jm030569l. [DOI] [PubMed] [Google Scholar]
- 16.Fiorucci S, Wallace JL, Mencarelli A, et al. A beta-oxidation-resistant lipoxin A4 analog treats hapten-induced colitis by attenuating inflammation and immune dysfunction. Proc Natl Acad Sci USA. 2004;101:15736–15741. doi: 10.1073/pnas.0404722101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petasis NA, Keledjian R, Sun Y-P, et al. Design and synthesis of benzo-lipoxin A4 analogs with enhanced stability and potent anti-inflammatory properties. Bioorg Med Chem Lett. 2008;18:1382–1387. doi: 10.1016/j.bmcl.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 18.O’Sullivan TP, Vallin KS, Shah ST, et al. Aromatic lipoxin A4 and lipoxin B4 analogues display potent biological activities. J Med Chem. 2007;50:5894–5902. doi: 10.1021/jm060270d. [DOI] [PubMed] [Google Scholar]
- 19.Chiang N, Gronert K, Clish CB, O’Brien JA, Freeman MW, Serhan CN. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J Clin Invest. 1999;104:309–316. doi: 10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 21.Chiang N, Serhan CN, Dahlén S-E, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 22.Ye RD, Boulay F, Wang JM, et al. International Union of Basic and Clinical Pharmacology LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009 doi: 10.1124/pr.109.001578. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson KR, Eglen RM. Beta galactosidase complementation: a cell-based luminescent assay platform for drug discovery. Assay Drug Dev Technol. 2007;5:137–144. doi: 10.1089/adt.2006.052. [DOI] [PubMed] [Google Scholar]
- 24.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 25.Bannenberg G, Moussignac R-L, Gronert K, et al. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br J Pharmacol. 2004;143:43–52. doi: 10.1038/sj.bjp.0705912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bannenberg GL, Chiang N, Ariel A, et al. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 27.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fierro IM, Colgan SP, Bernasconi G, et al. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit human neutrophil migration: comparisons between synthetic 15 epimers in chemotaxis and transmigration with microvessel endothelial cells and epithelial cells. J Immunol. 2003;170:2688–2694. doi: 10.4049/jimmunol.170.5.2688. [DOI] [PubMed] [Google Scholar]
- 29.Hachicha M, Pouliot M, Petasis NA, Serhan CN. Lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor 1a-initiated neutrophil responses and trafficking: regulators of a cytokine-chemokine axis. J Exp Med. 1999;189:1923–1929. doi: 10.1084/jem.189.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:249–261. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnamoorthy S, Recchiuti A, Chiang N, et al. Resolvin D1 binds human phagocytes: Evidence for pro-resolving receptors. Submitted. [DOI] [PMC free article] [PubMed]
- 32.Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein linked lipoxin A4 receptor. J Biol Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- 33.Clish CB, O’Brien JA, Gronert K, Stahl GL, Petasis NA, Serhan CN. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc Natl Acad Sci USA. 1999;96:8247–8252. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gelman S. The pathophysiology of aortic cross-clamping and unclamping. Anesthesiology. 1995;82:1026–1060. doi: 10.1097/00000542-199504000-00027. [DOI] [PubMed] [Google Scholar]
- 35.Baggiolini, Dewald, Moser, Gallin JI, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. Lippincott Williams and Wilkins; Philadelphia: 1999. pp. 419–431. [Google Scholar]
- 36.Olsen NJ, Stein CM. New drugs for rheumatoid arthritis. N Engl J Med. 2004;350:2167–2179. doi: 10.1056/NEJMra032906. [DOI] [PubMed] [Google Scholar]
- 37.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 38.Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 40.Morris T, Stables M, Hobbs A, et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009 doi: 10.4049/jimmunol.0900477. doi:10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 41.Nokami J, Furukawa A, Okuda Y, Hazato A, Kurozumi S. Palladium-catalyzed coupling reactions of bromobenzaldehydes with 3,4-di(tert-butyldimethylsilyloxy)-1-alkene to (3,4-dihydroxyalkenyl)benzaldehydes in the synthesis of lipoxin analogues. Tetrahedron Lett. 1998;39:1005–1008. [Google Scholar]