Abstract
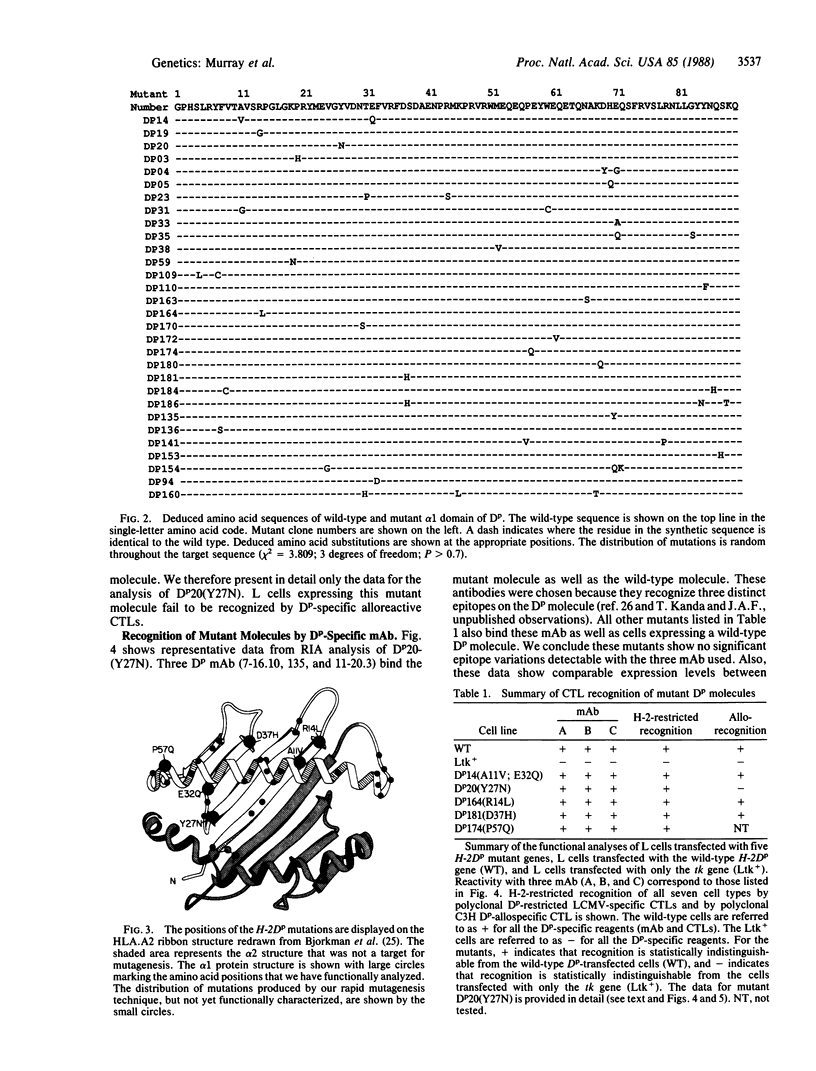
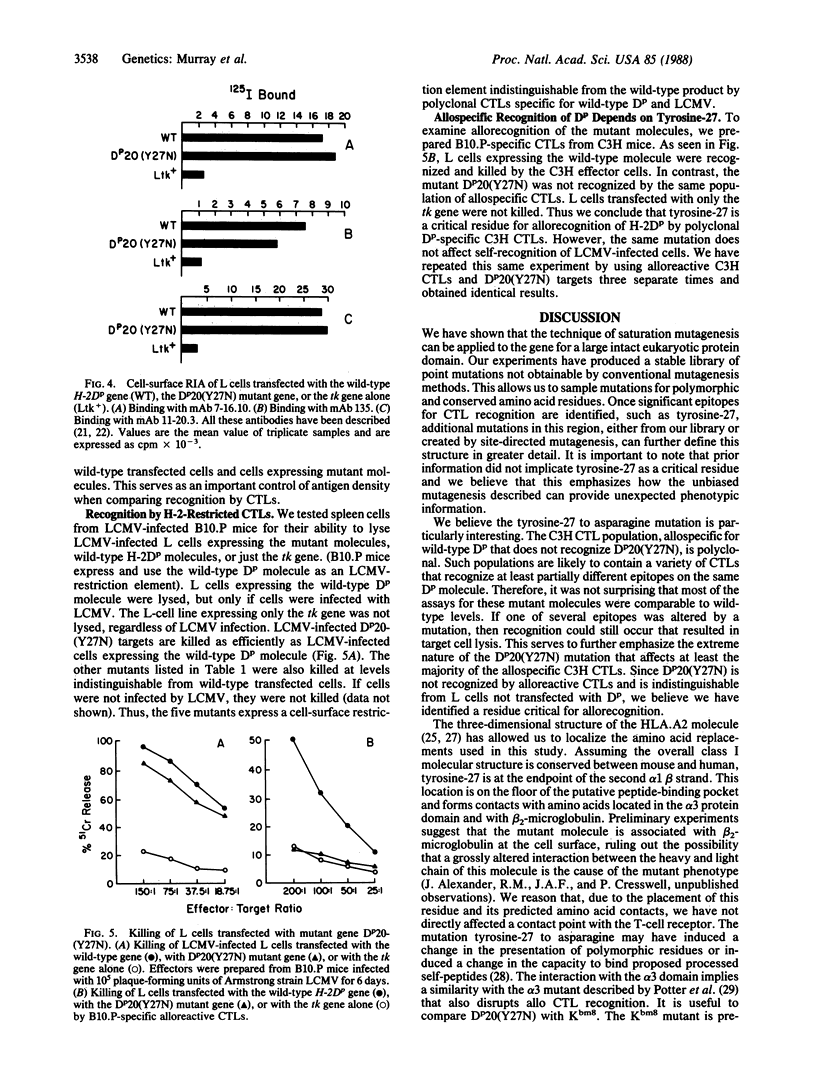
The interactive association between T lymphocytes and their target cells is an important system of cell-cell interactions. Major histocompatibility complex class I molecules are the cell surface structures recognized by cytolytic T lymphocytes. To define the molecular structures recognized by cytotoxic T lymphocytes, we have saturated the 270-base-pair alpha 1 exon of the H-2Dp gene with point mutations, rapidly producing a "library" of 2.5 x 10(3) independent mutants. The library contains enough recombinant clones (each clone encoding approximately one amino acid replacement mutation) to predict a mutation at each nucleotide position of the alpha 1 exon. The functional analysis of the first five transfected gene products tested has shown that mutation of a conserved tyrosine at position 27 to asparagine destroys recognition of the H-2Dp gene product by polyclonal alloreactive cytotoxic T lymphocytes. Recognition of the same mutant molecule by three monoclonal antibodies and H-2-restricted lymphocytic choriomenengitis virus-specific cytotoxic T lymphocytes is unaffected.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold B., Horstmann U., Kuon W., Burgert H. G., Hämmerling G. J., Kvist S. Alloreactive cytolytic T-cell clones preferentially recognize conformational determinants on histocompatibility antigens: analysis with genetically engineered hybrid antigens. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7030–7034. doi: 10.1073/pnas.82.20.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Benzer S. FINE STRUCTURE OF A GENETIC REGION IN BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):344–354. doi: 10.1073/pnas.41.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Darsley M. J., Takahashi H., Macchi M. J., Frelinger J. A., Ozato K., Appella E. New family of exon-shuffled recombinant genes reveals extensive interdomain interactions in class I histocompatibility antigens and identifies residues involved. J Exp Med. 1987 Jan 1;165(1):211–222. doi: 10.1084/jem.165.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Shykind B., Seidman J. G., Ozato K. Exon shuffling: mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982 Dec 23;300(5894):755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- Geier S. S., Zeff R. A., McGovern D. M., Rajan T. V., Nathenson S. G. An approach to the study of structure-function relationships of MHC class I molecules: isolation and serologic characterization of H-2Kb somatic cell variants. J Immunol. 1986 Aug 15;137(4):1239–1243. [PubMed] [Google Scholar]
- Guillet J. G., Lai M. Z., Briner T. J., Buus S., Sette A., Grey H. M., Smith J. A., Gefter M. L. Immunological self, nonself discrimination. Science. 1987 Feb 20;235(4791):865–870. doi: 10.1126/science.2433769. [DOI] [PubMed] [Google Scholar]
- Harmon R. C., Stein N., Frelinger J. A. Monoclonal antibodies reactive with H-2 determinants. Immunogenetics. 1983;18(5):541–545. doi: 10.1007/BF00364395. [DOI] [PubMed] [Google Scholar]
- Hasenkrug K. J., Cory J. M., Stimpfling J. H. Monoclonal antibodies defining mouse tissue antigens encoded by the H-2 region. Immunogenetics. 1987;25(2):136–139. doi: 10.1007/BF00364282. [DOI] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Nordeen S. K., Vogt K., Edgell M. H. A complete library of point substitution mutations in the glucocorticoid response element of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1986 Feb;83(3):710–714. doi: 10.1073/pnas.83.3.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd B. H., Young M. W. An examination of the one cistron: one chromomere concept. Cold Spring Harb Symp Quant Biol. 1974;38:573–579. doi: 10.1101/sqb.1974.038.01.061. [DOI] [PubMed] [Google Scholar]
- Kanda T., LaPan K., Takahashi H., Appella E., Frelinger J. A. The alpha 1 and alpha 2 domains of H-2 class I molecules interact to form unique epitopes. Immunogenetics. 1987;25(2):110–115. doi: 10.1007/BF00364276. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Glasebrook A. L., Bron C., Kelso A., Cerottini J. C. Clonal heterogeneity in the functional requirement for Lyt-2/3 molecules on cytolytic T lymphocytes (CTL): possible implications for the affinity of CTL antigen receptors. Immunol Rev. 1982;68:89–115. doi: 10.1111/j.1600-065x.1982.tb01061.x. [DOI] [PubMed] [Google Scholar]
- Macchi M. J., Woodward J. G., McLaughlin-Taylor E., Griffin J., Hood L., Frelinger J. A. Cloning and identification of the H-2Dp gene. Immunogenetics. 1984;19(3):195–204. doi: 10.1007/BF00364763. [DOI] [PubMed] [Google Scholar]
- McLaughlin-Taylor E., Woodward J. G., McMillan M., Frelinger J. A. Distinct epitopes are recognized by cytolytic T lymphocyte clones on the same class I molecule: direct demonstration using DNA-transfected targets and long-term cytolytic T cell clones. Eur J Immunol. 1984 Nov;14(11):969–974. doi: 10.1002/eji.1830141102. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Geliebter J., Pfaffenbach G. M., Zeff R. A. Murine major histocompatibility complex class-I mutants: molecular analysis and structure-function implications. Annu Rev Immunol. 1986;4:471–502. doi: 10.1146/annurev.iy.04.040186.002351. [DOI] [PubMed] [Google Scholar]
- Orn A., Goodenow R. S., Hood L., Brayton P. R., Woodward J. G., Harmon R. C., Frelinger J. A. Product of a transferred H-2Ld gene acts as restriction element for LCMV-specific killer T cells. Nature. 1982 Jun 3;297(5865):415–417. doi: 10.1038/297415a0. [DOI] [PubMed] [Google Scholar]
- Pious D., Krangel M. S., Dixon L. L., Parham P., Strominger J. L. HLA antigen structural gene mutants selected with an allospecific monoclonal antibody. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7832–7836. doi: 10.1073/pnas.79.24.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter S. D., Smith M. Homoeo-domain homology in yeast MAT alpha 2 is essential for repressor activity. Nature. 1986 Apr 24;320(6064):766–768. doi: 10.1038/320766a0. [DOI] [PubMed] [Google Scholar]
- Potter T. A., Bluestone J. A., Rajan T. V. A single amino acid substitution in the alpha 3 domain of an H-2 class I molecule abrogates reactivity with CTL. J Exp Med. 1987 Oct 1;166(4):956–966. doi: 10.1084/jem.166.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter R. D., Clayberger C., Lomen C. E., Krensky A. M., Parham P. In vitro mutagenesis at a single residue introduces B and T cell epitopes into a class I HLA molecule. J Exp Med. 1987 Jul 1;166(1):283–288. doi: 10.1084/jem.166.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Santos-Aguado J., Biro P. A., Fuhrmann U., Strominger J. L., Barbosa J. A. Amino acid sequences in the alpha 1 domain and not glycosylation are important in HLA-A2/beta 2-microglobulin association and cell surface expression. Mol Cell Biol. 1987 Mar;7(3):982–990. doi: 10.1128/mcb.7.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepart B. S., Takahashi H., Cozad K. M., Murray R., Ozato K., Appella E., Frelinger J. A. The nucleotide sequence and comparative analysis of the H-2Dp class I H-2 gene. J Immunol. 1986 May 1;136(9):3489–3495. [PubMed] [Google Scholar]
- Shiroishi T., Evans G. A., Appella E., Ozato K. In vitro mutagenesis of a mouse MHC class I gene for the examination of structure-function relationships. J Immunol. 1985 Jan;134(1):623–629. [PubMed] [Google Scholar]
- Stroynowski I., Orn A., Goodenow R. S., McMillan M., Forman J., Brayton P. R., Frelinger J., Hood L. Cytotoxic T lymphocytes recognize determinants on the BALB/c-H-2Ld molecule controlled by alpha 1 and alpha 2 but not alpha 3 external domains. Immunogenetics. 1984;20(2):141–154. doi: 10.1007/BF00364486. [DOI] [PubMed] [Google Scholar]
- Stuart G. W., Searle P. F., Chen H. Y., Brinster R. L., Palmiter R. D. A 12-base-pair DNA motif that is repeated several times in metallothionein gene promoters confers metal regulation to a heterologous gene. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7318–7322. doi: 10.1073/pnas.81.23.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Woodward J. G., Orn A., Harmon R. C., Goodenow R. S., Hood L., Frelinger J. A. Specific recognition of the product of a transferred major histocompatibility complex gene by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3613–3617. doi: 10.1073/pnas.79.11.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis E. J., Amos D. B. Three closely linked genetic systems relevant to transplantation. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3031–3035. doi: 10.1073/pnas.68.12.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]



