Abstract
Despite our advanced understanding of primary cancer development and progression, metastasis and the systemic spread of the disease to secondary sites remains the leading cause of cancer-associated death. The metastatic process is therefore a major potential therapeutic target area for cancer researchers and elucidating the key steps that are susceptible to therapeutic intervention will be critical to improve our treatment strategies. Recent advances in intravital imaging are rapidly improving our insight into this process and are helping in the design of stage-specific drug regimes for the treatment of metastatic cancer. Here we discuss current developments in intravital imaging and our recent use of photobleaching and photoactivation in the analysis of dynamic biomarkers in living animals to assess the efficacy of therapeutic intervention on early stages of tumor cell metastasis.
Key words: in vivo imaging, photobleaching, photoactivation, biomarkers
Metastasis is a complex process consisting of interactions between cancer cells and their surrounding extra-cellular matrix and stroma. To give rise to a secondary tumor, a primary tumor cell undergoes alterations to its cell-cell and cell-ECM contacts, allowing it to breach the basement membrane and intravasate into the vasculature or the lymphatic system. A tumor cell must survive in the circulation before extravasating at a secondary site and initiating new tumor growth and the development of its own blood supply. Imaging this process in live animals under native physiological conditions is inherently difficult due to poor sample stability, tissue penetration and autofluorescence of the tissue. However, new advances in fluorescent imaging, including the continued development of green fluorescent protein (GFP) and its variants, have facilitated the observation of this process and shed light on some key mechanisms that determine how and why cells metastasise. The use of fluorescent probes for in vivo imaging can be divided into two types (1) ‘passive’ markers or reporters used for direct visualization and tracking of cell movement in relation to extracellular structures and (2) more complex, ‘active’ reporters or biosensors for monitoring detailed processes such as biochemical activity or protein-protein interactions during metastasis.1,2 In some cases there can be overlap between both types of imaging which will be addressed here.
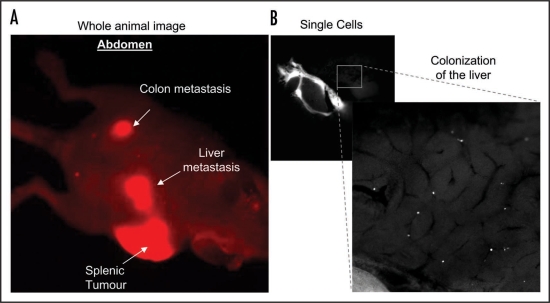
The majority of early intravital imaging studies focused on the stages of metastasis that occur after dissemination from the primary tumor and predominantly used a ‘passive’ reporter approach to assess tumor cell behavior. Models of circulating tumor cells have allowed for analysis at the single cell level of tumor cell velocity, persistence, shape change and interactions with the ECM and stroma in secondary tissue.3–5 The use of fluorescently-labelled cells has also revealed some limiting factors that cause the arrest of cancer cells in target tissue such as trapping in small capillary networks due to tumor cell size or adhesion to surrounding vessel walls.6,7 Furthermore, experimental models of metastasis such as intra-splenic, intra-cardial and tail vein injections in combination with fluorescently-tagged tumor cells has provided information on the colonisation, extravasation and dormancy of tumor cells in secondary sites (Fig. 1 and refs. 5, 8 and 9). Collectively, along with the rapid increase in tissue specific expression of GFP in mouse cancer models,10 a wealth of information on different steps of the metastatic process has begun to emerge.
Figure 1.
(A) Whole body optical imaging of mCherry-expressing SW 620 colon cancer cell metastases after approximately six weeks post intra-splenic injection. Images were obtained using the Olympus OV100 whole body imaging system with an Olympus MT10, 150 w, Xenon light source, using a low magnification objective (macro lens) with a magnification of 0.14× and numerical aperture of 0.04. (B) mCherry expressing SW 620 colon cancer cells colonizing the liver 30 mins after intra-splenic injection. 1 × 106 cells were injected into the spleen of an anesthetised CD-1 nude mouse and the incision sealed using ‘Clay Adams’ vetinary clips (VetTec). The mouse was placed on a heat pad for 30 mins then sacrificed. An incision was made in the abdomen to expose the liver and images of fluorescent cells within the liver were obtained using a 0.8× (0.22 NA) objective lens with variable zoom on the Olympus OV100.
The departure of individual cells away from solid primary tumors into the blood stream has been a more difficult process to study using intravital imaging. It is a rare, sporadic event, requiring long acquisition and the inherent density and complex nature of the tumor tissue poses problems for imaging. Overcoming autofluorescence and light scattering has recently been improved due to advances in fluorophores1,11 and the combined use of long-term multiphoton microscopy12 has allowed greater resolution and tissue penetration than before. Multiphoton imaging can also provide additional detail regarding the interaction between cells and the surrounding extracellular environment using second harmonic signal generation (SHG) from collagen, elastin and other matrix proteins found in connective tissue.13,14 In this regard, imaging the interaction of cancer cells with extracellular matrix has revealed distinct modes of cell locomotion adopted by cancer cells in vivo, such as ameboid or mesenchymal invasion, that depend upon the topography or density of the surrounding matrix.3,13,15 A greater understanding of the initial cell movement and interaction with the extracellular environment will enhance our ability to pin-point cell-ECM targets that may be of clinical relevance in the future.
Concurrent with the use of GFP as a ‘passive’ marker, a number of techniques have been developed that facilitate the visualization and localisation of GFP-tagged fusion proteins to quantify changes in protein expression, mobility and sub-cellular interactions during various processes in vitro. These include photobleaching (PB), photoactivation (PA), fluorescence resonance energy transfer (FRET) and fluorescent life time imaging microscopy (FLIM).2,16,17 The adaptation of these techniques for in vivo imaging to examine the activity of key molecules will provide new ‘active’ markers or reporters that can be correlated with biological processes important in disease progression such as migration, proliferation and cell death. Other fluorescent probes such as MMPsense or Apotrace that measure ‘active’ processes such as metalloproteinase activity or apoptosis have also recently been used in animals.18,19 In this way we can get closer to understanding how subcellular components or signal transduction pathways interact in real-time. The improved spatial and temporal detail will facilitate the ‘when and where’ we should target metastatic cancer cells for therapy.12
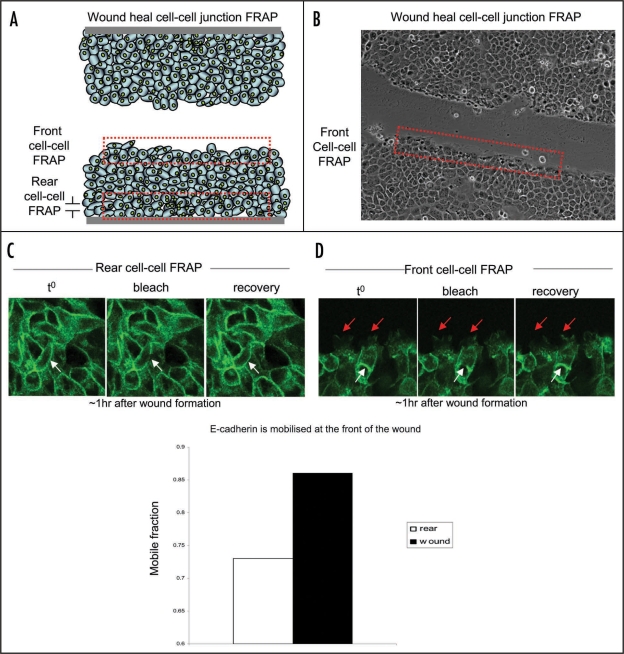
In our recent paper we have adapted two techniques, photobleaching and photoactivation, for in vivo imaging and used them to assess the potential of E-cadherin as a molecular biosensor for cell migration in live tumors.20 E-cadherin-based cell-cell contacts are prominent sites of remodelling during early stages of epithelial to mesenchymal transition (EMT). The disruption or deregulation of E-cadherin-based adhesions leads to the collapse of normal epithelial architecture that precedes the initial intravasation of cells from tumors.21–23 In vitro photobleaching analysis of E-cadherin can be used as an ‘active’ molecular read-out of cell migration, as cells within a stationary colony show significantly reduced E-cadherin mobility compared to collectively migrating cells.20 Moreover, as demonstrated in Figure 2 (reviewed in ref. 20), E-cadherin mobility can also be spatially regulated within a population of tumor cells, as cells at the rear of a wound show impaired E-cadherin mobilisation compared to cells at the leading edge of the wound. This suggests a gradient of E-cadherin mobilisation within the local environment of a tumor may exist and could potentially be used in the future to map areas of weakened cell-cell adhesion from which cells are more likely to migrate. In vivo analysis of E-cadherin dynamics showed that changes in the mobility of E-cadherin can also be used as an ‘active’ marker of cell behavior in live animals, and may be useful in predicting cell mobilisation from primary tumors.20
Figure 2.
FRAP of GFP-E-cadherin at the rear or front of a wound heal assay. (A and B) Schematic and representative images of a wound heal assay depicting the area of cells selected for E-cadherin-based cell-cell junction FRAP analysis (red broken line). (C and D) Representative images of FRAP experiments performed at the rear or front of a wound heal assay respectively. White solid arrows represent area of photobleaching at the rear and white broken arrows represent area of photobleaching at the front of the wound. Red arrows indicate dynamics of cells at the front of the wound. Cells were classed to be at the front of the wound within the first three cells from the wound border (reviewed in ref. 20).
We also demonstrated the subcellular tracking of plasma membrane dynamics in vivo using the membrane-targeting sequence of H-Ras fused to photoactivatable-GFP.24,25 Importantly, both the dynamics of cell-cell junctions, as visualised using E-cadherin:GFP, and the dynamics of the plasma membrane, which also plays a fundamental role in cell invasion and metastasis, are significantly different in vivo than in vitro.20 Critically, this raises the possibility that many signalling axes and networks may function differently in vivo and therefore care must be taken when correlating in vitro information to the live setting. Lastly, we demonstrated the benefits of in vivo imaging in the assessment of molecular-based targeted therapeutics by using the Src inhibitor dasatinib, which impaired E-cadherin cell mobility in vivo but not in vitro.20,26
In the context of previous intravital imaging studies, our work suggests that we are at the beginning of a new stage of intravital imaging in which ‘active’ probes can help predict the efficacy of novel therapeutic treatments and also provide a context dependent read-out of oncogene-induced biological behavior in live animals. Importantly, not all molecules are adaptable for this type of in vivo imaging. Careful selection of candidate molecular markers that demonstrate clear changes attributable to a biological function, for example, subcellular relocalization or compartmentalisation, will be ideally suited for this type of intravital examination in the future.
Here we have adopted two key fluorescent imaging techniques typically used in vitro and combined them with a fundamental biological question in vivo. The adaptation of other techniques for in vivo imaging such as FRET or FLIM-FRET probes will provide a detailed pixel by pixel map of the activity and behavior of key signalling proteins in live animals.2,27 The use of these ‘active’ probes in vivo may hold further surprises concerning differences in molecular behavior in live animals compared to the traditional ‘snap-shot’ approach in vitro. Finally, one of the major challenges of in vivo imaging during drug discovery is the need for repeated imaging of the same animal in the presence or absence of drugs. The continued development of optical windows and observation chambers for non-invasive real-time imaging will facilitate this and allow for the assessment of drug response at the single cell level.28 This, when combined with the subcellular optical techniques described here, will prove very useful in the future for in vivo imaging when evaluating the aetiology of the disease or during the drug discovery process.
Acknowledgements
Grant support: Cancer Research UK core grant (K. Anderson), a fellowship from AstraZeneca (P. Timpson), Cancer Research UK Program grant C157/A9148 (A. Serrels, M. Canel, M. Frame and V. Brunton), and by Fundación Española para la Ciencia y la Tecnología (M. Canel). The authors wish to thank Haley L. Bennett for critical reading of the manuscript.
Abbreviations
- ECM
extra-cellular matrix
- GFP
green fluorescent protein
- PB
photobleaching
- PA
photoactivation
- FRET
fluorescence resonance energy transfer
- FLIM
fluorescence lifetime imaging microscopy
- SHG
second harmonic generation
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/9460
References
- 1.Zhang J, Campbell RE, Ting AY, Tsien RY. Creating new fluorescent probes for cell biology. Nat Rev Mol Cell Biol. 2002;3:906–918. doi: 10.1038/nrm976. [DOI] [PubMed] [Google Scholar]
- 2.Wouters FS, Verveer PJ, Bastiaens PI. Imaging biochemistry inside cells. Trends Cell Biol. 2001;11:203–211. doi: 10.1016/s0962-8924(01)01982-1. [DOI] [PubMed] [Google Scholar]
- 3.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 4.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 5.Condeelis J, Segall JE. Intravital imaging of cell movement in tumors. Nat Rev Cancer. 2003;3:921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 6.Naumov GN, Wilson SM, MacDonald IC, Schmidt EE, Morris VL, Groom AC, et al. Cellular expression of green fluorescent protein, coupled with high-resolution in vivo videomicroscopy, to monitor steps in tumor metastasis. J Cell Sci. 1999;112:1835–1842. doi: 10.1242/jcs.112.12.1835. [DOI] [PubMed] [Google Scholar]
- 7.Yang M, Chishima T, Wang X, Baranov E, Shimada H, Moossa AR, et al. Multi-organ metastatic capability of Chinese hamster ovary cells revealed by green fluorescent protein (GFP) expression. Clin Exp Metastasis. 1999;17:417–422. doi: 10.1023/a:1006665112147. [DOI] [PubMed] [Google Scholar]
- 8.Goodison S, Kawai K, Hihara J, Jiang P, Yang M, Urquidi V, et al. Prolonged dormancy and site-specific growth potential of cancer cells spontaneously disseminated from non-metastatic breast tumors as revealed by labeling with green fluorescent protein. Clin Cancer Res. 2003;9:3808–3814. [PubMed] [Google Scholar]
- 9.Sturm JW, Keese MA, Petruch B, Bonninghoff RG, Zhang H, Gretz N, et al. Enhanced green fluorescent protein-transfection of murine colon carcinoma cells: key for early tumor detection and quantification. Clin Exp Metastasis. 2003;20:395–405. doi: 10.1023/a:1025470312074. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed F, Wyckoff J, Lin EY, Wang W, Wang Y, Hennighausen L, et al. GFP expression in the mammary gland for imaging of mammary tumor cells in transgenic mice. Cancer Res. 2002;62:7166–7169. [PubMed] [Google Scholar]
- 11.Giepmans BN, Adams SR, Ellisman MH, Tsien RY. The fluorescent toolbox for assessing protein location and function. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- 12.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, et al. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 14.Alexander S, Koehl GE, Hirschberg M, Geissler EK, Friedl P. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem Cell Biol. 2008;130:1147–1154. doi: 10.1007/s00418-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 15.Kikkawa H, Kaihou M, Horaguchi N, Uchida T, Imafuku H, Takiguchi A, et al. Role of integrin alpha(v)beta3 in the early phase of liver metastasis: PET and IVM analyses. Clin Exp Metastasis. 2002;19:717–725. doi: 10.1023/a:1021356019563. [DOI] [PubMed] [Google Scholar]
- 16.Lippincott-Schwartz J, Altan-Bonnet N, Patterson GH. Photobleaching and photoactivation: following protein dynamics in living cells. Nat Cell Biol. 2003:7–14. [PubMed] [Google Scholar]
- 17.Lippincott-Schwartz J, Snapp E, Kenworthy A. Studying protein dynamics in living cells. Nat Rev Mol Cell Biol. 2001;2:444–456. doi: 10.1038/35073068. [DOI] [PubMed] [Google Scholar]
- 18.Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med. 2001;7:743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- 19.Damianovich M, Ziv I, Heyman SN, Rosen S, Shina A, Kidron D, et al. ApoSense: a novel technology for functional molecular imaging of cell death in models of acute renal tubular necrosis. Eur J Nucl Med Mol Imaging. 2006;33:281–291. doi: 10.1007/s00259-005-1905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serrels A, Timpson P, Canel M, Schwarz JP, Carragher NO, Frame MC, et al. Real-time study of E-cadherin and membrane dynamics in living animals: implications for disease modeling and drug development. Cancer Res. 2009;69:2714–2719. doi: 10.1158/0008-5472.CAN-08-4308. [DOI] [PubMed] [Google Scholar]
- 21.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Yap AS, Crampton MS, Hardin J. Making and breaking contacts: the cellular biology of cadherin regulation. Curr Opin Cell Biol. 2007;19:508–514. doi: 10.1016/j.ceb.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avizienyte E, Wyke AW, Jones RJ, McLean GW, Westhoff MA, Brunton VG, et al. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat Cell Biol. 2002;4:632–638. doi: 10.1038/ncb829. [DOI] [PubMed] [Google Scholar]
- 24.Patterson GH, Lippincott-Schwartz J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science. 2002;297:1873–1877. doi: 10.1126/science.1074952. [DOI] [PubMed] [Google Scholar]
- 25.Ashery U, Yizhar O, Rotblat B, Kloog Y. Nonconventional trafficking of Ras associated with Ras signal organization. Traffic. 2006;7:119–126. doi: 10.1111/j.1600-0854.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- 26.Serrels A, Macpherson IR, Evans TR, Lee FY, Clark EA, Sansom OJ, et al. Identification of potential biomarkers for measuring inhibition of Src kinase activity in colon cancer cells following treatment with dasatinib. Mol Cancer Ther. 2006;5:3014–3022. doi: 10.1158/1535-7163.MCT-06-0382. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, et al. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- 28.Kedrin D, Gligorijevic B, Wyckoff J, Verkhusha VV, Condeelis J, Segall JE, et al. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods. 2008;5:1019–1021. doi: 10.1038/nmeth.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]