Abstract
Insights into the molecular basis and the temporal evolution of neurotoxicity in prion disease are increasing, and recent work in mice leads to new avenues for targeting treatment of these disorders. Using lentivirally mediated RNA interference (RNAi) against native prion protein (PrP), White et al. report the first therapeutic intervention that results in neuronal rescue, prevents symptoms and increases survival in mice with established prion disease.1 Both the target and the timing of treatment here are crucial to the effectiveness of this strategy: the formation of the neurotoxic prion agent is prevented at a point when diseased neurons can still be saved from death. But the data also give new insights into the timing of treatment in the context of the pattern of spread of prion infection throughout the brain, with implications for developing the most effective treatments.
Key words: prion, RNA interference, gene therapy, neurodegeneration, synaptic
This perspective considers developments in the field that led to the rationale for targeting endogenous prion protein (PrP) in prion therapeutics and to the discovery of a window of reversibility of early neuronal damage in prion disease. It introduces RNA interference (RNAi) and its therapeutic use in this context and discusses insights into prion pathogenesis and future treatment strategies and goals. A key concept is targeting the critical brain regions for the spread of prion replication. This may have relevance in other neurodegenerative diseases due to protein misfolding, which recent literature suggests may also propagate throughout the brain in disease-specific patterns.
Prion Diseases: Background and Pathogenesis
Prion diseases, or transmissible spongiform encephalopathies, are fatal, neurodegenerative diseases that include Creutzfeldt-Jacob disease (CJD) and kuru in humans, scrapie in sheep and bovine spongiform encephalopathy (BSE) in cattle. They are transmissible within and between mammalian species and are unique in having sporadic, inherited and acquired origins.
Central to prion pathogenesis is the conversion of a host-encoded prion protein, PrPC, into a partially-protease resistant isoform, PrPSc, which accumulates in the brain and is associated with infectivity. The ability of transmission to be effected both naturally and experimentally by disease-associated prion protein, in the absence of associated nucleic acid, led to the ‘protein only hypothesis’ of prion propagation and infectivity.2 The conversion process is self-propagating, with PrPSc acting as a conformational template recruiting PrPC for further conversion. The conversion reaction itself is critical to neurotoxicity in prion diseases: neither loss of PrPC function,3–5 nor deposition of PrPSc is sufficient to cause pathology. However, the precise identity of the neurotoxic prion species and the exact mechanism of neurotoxicity are unknown. Key pathological features of prion disorders, in addition to the aggregation of PrPSc (often in the form of amyloid plaques), are spongiform degeneration of neurons, astrocytosis and neuronal loss.
Prion diseases in humans are rare, with an overall incidence of ∼1/million worldwide, but their unique biology has resulted in their being amongst the best understood of the neurodegenerative disorders at a molecular level. Currently, there is no effective treatment for prion diseases and the recent evidence for prion spread through contaminated blood products,6–8 the identification of a novel type of vCJD with a protracted incubation time,9 and the emergence of atypical strains of BSE in cattle10 re-emphasize the importance of continued research into therapies for this ongoing threat to public health.
Therapeutic Strategies in Prion Disease: Rationale for Targeting PrPC
Any successful therapy in prion disease should prevent the formation, or block the actions, of the neurotoxic species. While pathological changes are characterized by, and infectivity is associated with, PrPSc there is no evidence for in vivo toxicity caused by PrPSc.11,12 The existence of subclinical disease states where high levels of PrPSc are present without clinical symptoms13–15 also argue against its direct toxicity. Further, there are several inherited prion diseases in which PrPSc is not detected in significant amounts,16–18 and the degree of PrPSc accumulation in specific brain regions does not necessarily correlate with clinical features. Expression of host PrPC is, however, essential for both prion propagation and pathogenesis: PrP-null mice are resistant to prion disease and unable to replicate infectivity,3,4 disease incubation time is determined by PrPC expression levels,4,19 and prion neurotoxicity is confined to PrP-expressing neural tissue.20 Thus the expression of host PrPC is necessary for prion-induced neurotoxicity, suggesting the generation of a toxic intermediate form during prion conversion, or possibly the toxicity of early oligomeric forms of PrPSc. Removing PrPC, the substrate for prion conversion, is therefore an appealing therapeutic target.
PrPC is highly conserved across species. Its physiological function is not well understood and ablation of PrPC is surprisingly well tolerated in vivo, at least in laboratory mice. Thus PrP-null mice are essentially normal phenotypically and behaviorally with only very subtle changes, including altered intrinsic properties of specific cell types, detectable electrophysiologically,21 and reported alterations of circadian rhythms.22 Depletion of PrPC in adult mice by transgene mediated deletion of PrP produces no further phenotypic consequences and effectively excludes loss of PrPC function as a central mechanism in prion-mediated neurodegeneration.5
Proof of principle of the validity of strategies targeting PrPC came from the use of the adult onset model of PrPC depletion. Transgenic animals in which PrP was depleted in neurons at nine weeks of age provided a system for testing the effects of PrP depletion in prion infection. PrP-expressing mice were infected with prions at one week of age, eight weeks prior to transgene mediated PrP depletion. Prion infection was allowed to develop over this time, with establishment of early spongiform change, astrocytosis and PrPSc deposition by the time neuronal PrP knockout occurred. This transgene mediated removal of neuronal PrPC during established prion infection allowed the animals to survive long term without symptoms and led to a reversal of early spongiform change. The animals were effectively clinically cured.12 Control animals with equivalent early pathology at eight weeks post infection (wpi) progressed to full prion neurodegeneration and death, just four weeks after the onset of PrP knockout in ‘treated’ animals.
Timing of Treatment: Rescuing Early Neuronal Dysfunction, a Window of Reversibility
Spongiform change and synapse loss precede neuronal loss in mouse scrapie.23 Changes in species-typical behaviors also occur long before end stage motor symptoms24–27 and correlate with early loss of presynaptic terminals in the dorsal hippocampus.28 In other neurodegenerative disorders, such as Alzheimer and Huntington diseases, the loss of synapses precedes neuronal loss. Compromised synaptic function is proposed to underlie early symptoms and cognitive decline in these disorders.
In prion infected mice with PrP depletion early spongiform change was associated with cognitive and behavioral deficits and impaired synaptic transmission in the hippocampus, which recovered just one week after neuronal PrP depletion had occurred.29 The link between spongiosis and synaptic loss is not clear, but may reflect a stage of functional impairment of synapses before they are physically lost. The recovery of early spongiform change supports the concept that the early stage of disease represents a window when prion diseased neurons can be rescued. The early stage of synaptic dysfunction may be the ‘window’ here, which is further supported by the findings of White and co-workers, discussed below.
Using RNAi for Therapeutic Gene Knockdown
Transgene-mediated reduction of PrPC expression does not offer direct therapeutic possibilities in human patients. Potential treatments aimed at removing PrPC must be achievable using extrinsic means. Recent developments in the field of RNA interference (RNAi) offer new opportunities to achieve such therapeutic gene silencing in vivo.
RNAi is a naturally occurring, highly conserved, sequence-specific mechanism for post-transcriptional gene silencing in eukaryotes. It is initiated by the presence of double-stranded RNA (dsRNA), which is exogenously introduced to the cell such as viral RNA, or endogenously encoded such as microRNAs (miRNAs) that regulate gene expression. Exogenously introduced dsRNA is recognized by Dicer, a cytoplasmic ribonuclease that cleaves it into 21–23 nt sequences called short interfering RNAs (siRNAs).30 Both siRNAs and miRNAs interact with a multi-protein RNA-induced silencing complex (RISC) that unwinds the RNA duplex and destroys one of the strands, known as the “passenger” strand.31 The remaining “guide” strand is used as a template to localize cellular mRNAs containing a homologous sequence. The degree of homology between the guide strand and the mRNA determines whether RISC initiates endonucleolytic cleavage or translational arrest of the target mRNA, thereby silencing expression of that gene. Generally, siRNAs mediate destruction of target mRNAs whereas miRNAs silence gene expression through translational repression due to their imperfect complementarity to the target mRNA.32–34
When using RNAi as a biological tool, interfering RNA sequences can be designed to enter the RNAi pathway at various points. siRNA duplexes can be synthesized for direct loading into RISC without requiring further processing.35 This approach has the advantage of bypassing cellular defence mechanisms for recognition of long viral dsRNA, but unmodified siRNAs are typically unstable in vivo and cannot cross the blood-brain-barrier unaided. Targeting of brain structures has been achieved successfully with infusion of naked siRNA duplexes alone36,37 in conjunction with transfection reagents38–42 and conjugated to a peptide derived from the rabies virus glycoprotein.43 Whilst promising results have been attained, current technologies mean clinical translation for treatment of many neurodegenerative diseases would require continuous or repeated long-term infusion of the interfering RNA directly to the CNS.
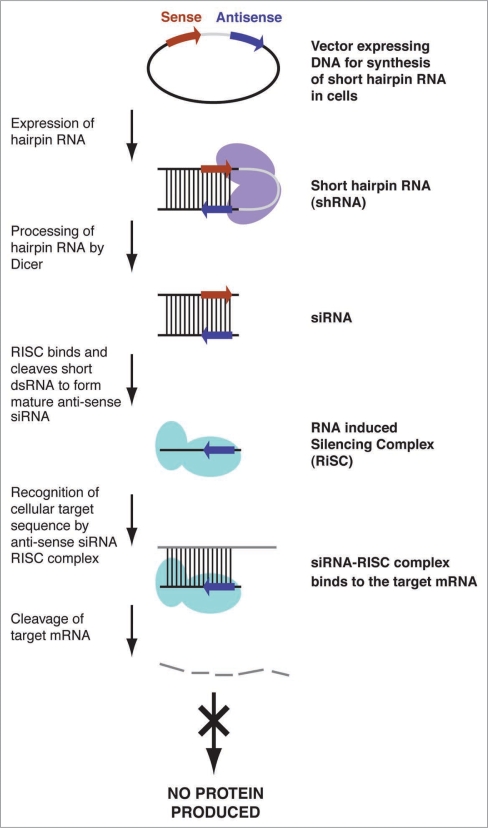
Alternatively, stable, long-term expression of interfering RNA sequences can be achieved through the use of recombinant viral vectors (see schematic in Fig. 1). In this approach, siRNAs are expressed as short hairpin RNA (shRNA) stem-loop structures usually driven by RNA polymerase III promoters.44,45 As the shRNAs are expressed in the nucleus, they mimic pre-miRNAs ready for processing by Dicer.
Figure 1.
Schematic representing virally mediated RNAi. A viral vector expresses shRNA sequences. These are processed by Dicer and the anti-sense or guide strand is loaded into RISC for targeting of a specific mRNA, which is then degraded.
For efficient delivery of shRNA-expressing vectors to neuronal cells in vivo, recombinant viruses, including retroviruses, adenoviruses, adeno-associated viruses and herpes-simplex viruses have all been harnessed. Retroviruses are commonly used due to their relatively low immunogenic potential and their ability to integrate into the host genome to facilitate stable expression. Lentiviruses are of particular use in the CNS as they are able to transduce and integrate into the genome of post-mitotic cells such as neurons and yield long-term expression of shRNAs in neurons when delivered intracerebrally.46,47 Virally mediated RNAi has been used successfully as a therapeutic treatment in models of a number of different neurodegenerative diseases, including mouse models of spinocerebellar ataxia,48 Huntington disease49–51 and Amyotrophic lateral sclerosis (ALS).52,53
Therapeutic Use of RNAi in Prion Disease
The first therapeutic use of lentivirally mediated RNAi against prion protein was demonstrated in mice with established prion infection by White and co-workers.1 Knockdown of PrP by RNAi,54 and resultant inhibition of PrPSc replication in cell culture, have been described55 and RNAi of PrP has been achieved in vivo: virally expressed RNAi has been used to reduce the levels of PrPC in goats, cattle56 and mice.57 Transgenic mice generated by lentiviral transduction of embryos stably express anti-PrP shRNAs and have increased resistance to prion infection due to resultant RNAi of endogenous PrP.57 Here, RNAi of PrP is due to genetic engineering and is not a treatment. Importantly, however, this model confirmed the safety and longevity of stably expressed shRNAs against PrP in vivo, and the increased resistance of the chimeric animals generated to prion infection give additional insights into targeting prion therapies, which is further discussed below.
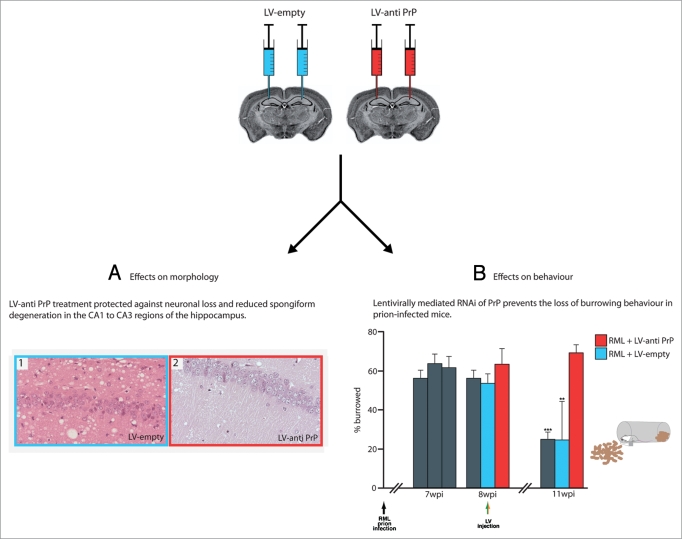
White and colleagues have now used lentivirally mediated RNAi of PrP as an actual treatment. They essentially ‘replicated’ their earlier experiments with the transgene mediated PrP depletion, allowing comparison of therapeutic efficacy of lentiviral mediated RNAi with the effects of adult onset Cre-mediated PrPC depletion in prion infection. Transgenic mice overexpressing PrPC were inoculated with mouse-adapted scrapie and allowed to incubate the disease for 8 weeks until early neuropathology was established. They then received a single injection of lentivirus expressing an shRNA targeting PrPC into each hippocampus. This treatment prevented the onset of early behavioral and cognitive deficits associated with this stage of prion infection, protected hippocampal neurons from degeneration, reduced PrPSc deposition and spongiosis (Fig. 2), and resulted in significantly increased survival of the animals (Fig. 3). Thus RNAi-treated animals were protected against developing the first behavioral deficits associated with early pathology of the CA1 region: loss of burrowing activity and object recognition memory. Morphologically, there was significantly less spongiform degeneration and neuronal loss where anti-PrP lentivirus was delivered compared to mock treated animals, suggesting sustained focal protection against neurotoxicity where PrP knockdown occurred. Interestingly, spongiosis was also reduced beyond the site of lentiviral injection—in thalamus and cortex (reviewed in ref. 1). PrPSc accumulation was lower in animals with virally mediated RNAi of PrP in the hippocampus than in mock treated animals, and again this reduction was seen beyond the hippocampus in thalamus and cortex. These more widespread changes throughout the brain are likely to reflect altered spread of prion infection after hippocampal PrP knockdown. Of note, PrPSc accumulation—albeit at lower levels than in control animals—did not appear to affect neuronal function or cell survival, as reflected in preservation of hippocampal behaviors and structural neuronal integrity. This is consistent with observations in mice with transgene mediated PrP depletion, and has implications for the level of knockdown required for therapeutic effect. Thus simply slowing the rate of prion replication—here by reducing PrPC levels—may be effective for prevention of neurotoxic effects.
Figure 2.
Lentivirally mediated RNAi of PrP expression protects against prion neurodegeneration in vivo. Prion infected mice are treated either with bilateral hippocampal injections of LV-empty (blue) or LV-anti PrP (red) eight weeks post infection. Representative images of neuroprotective effects of virally mediated PrP knockdown: (A) LV-anti PrP treatment protected against neuronal loss and reduced spongiform degeneration in CA1–3 regions (right hand) as compared to LV-empty treatment (left hand), which did not. (The right hippocampus is shown for all mice). (B) Mice were infected with RML prions at one week of age (black arrow) and tested for their ability to actively displace (burrow) food pellets from a tube. Mice were treated with either LV-anti PrP or LV-empty at eight weeks post infection (colored arrows). LV-anti PrP treated mice (red bars) continued to actively burrow, whereas both untreated (grey bars) and LV-empty treated mice (blue bars) lost burrowing behavior, characteristic of prion infection at this stage.
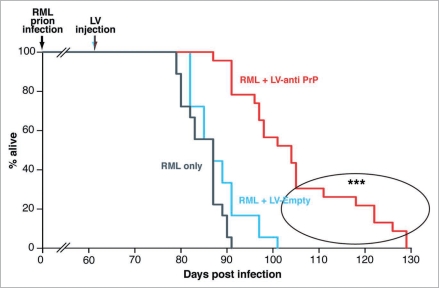
Figure 3.
Treatment with anti-PrP shRNA expressing lentivirus prolongs survival in mice with established prion disease. Mice were infected with RML prions at 1 week of age and were treated with bilateral hippocampal injections of either LV-anti PrP (n = 22) or LV-Empty (n = 18) at 8 wpi or with no virus (n = 18). RML-infected mice treated with no virus or with LV-Empty died within 91 and 101 days post infection (dpi) respectively; mice treated with LV-anti PrP survived longer, living up to 129 dpi. (p < 0.0001 Student's t test, 2 tails, compared to both LV-Empty and untreated mice). The difference in survival among LV-anti PrP treated mice may be the result of timing of lentiviral injection. The earliest treated mice survived up to 50% longer (circled) than those injected later. (Reprinted with permission: White et al. PNAS USA 2008; 105:10238–43).
Additional Insights: Timing of Treatment and Strain-Driven Targets?
The critical advance is the effect of this treatment on survival of prion-infected mice (Fig. 3). A single treatment with focal injection of virus resulted in significantly prolonged survival time of treated animals, compared to mock- or untreated mice, with a mean increase in lifespan of 23.5% compared to untreated animals.
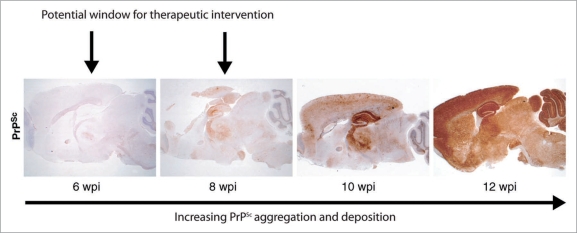
The increased survival in RNAi treated mice is strikingly large with respect to the very small volume of brain targeted. Prion incubation times are inversely proportional to overall levels of PrP expression,4,11 and it is likely that regional variations also affect prion replication rates and incubation periods. Further, prion diseases exist in different strains, which differ in their clinical and pathological profiles, as well as in the biochemical conformation and glycosylation pattern of associated PrPSc. The pattern of PrPSc deposition throughout the brain is generally strain specific.58,59 The hippocampus is a focus of early prion replication and PrPSc deposition for the RML prion strain used here (Fig. 4): this localized knockdown may therefore eliminate a key area for early prion replication in this model.
Figure 4.
Distribution of PrPSc over time in RML-infected tg37 mice. PrPSc staining of RML infected tg37 mice shows that the hippocampus is the main focus of accumulation of PrPSc prion replication at early and mid-stage prion infection. There is widespread deposition of PrP throughout the brain when mice are terminally ill at 12 wpi. (Reprinted with permission: Suppl. data, White et al. PNAS USA 2008; 105:10238–43).
Further, if the survival curve for LV anti-PrP treated mice is examined closely, it is clear the effect of treatment on survival prolongation ranges from 0 to ∼50%. This may in part be due to variation in neuronal transduction by virus in individual mice, but likely also reflects differences in timing of therapy: mice treated earlier survived longest. Analysis of survival times showed that animals surviving longest (survival time up by nearly 50% compared to controls, up to 129 days post inoculation, (dpi)) had been treated a mean of 52 dpi, whereas animals surviving least after treatment (as little as 87 dpi) had been injected a mean of 58 days post inoculation. Anticipating treatment by a few days here has a dramatic effect on survival. This may reflect a critical window for neuronal rescue in terms of a neurons' own program of death, or be dependent on the kinetics of prion spread, reflecting the fact that intervention needs to be accurately timed. This introduces the concept that treatment of prion diseases is likely to be most effective if we understand in detail the patterns of prion spread and replication for particular strains. This idea is supported by the observation that the degree of chimerism in mice transgenic for an shRNA targeting PrPC expression did not always correlate with the survival time following prion infection. Some mice with only 20% chimerism survived as long as others that were 90–95% chimeric (see Pfeifer et al. Suppl. data).57 It would be interesting to correlate the regions in which PrP expression is reduced in the different chimeric models with the effect on incubation time in the shRNA transgenic mice also. If this were to correlate with hippocampal knockdown (Pfeifer and co workers also used RML prions) the concept of therapy targeted to key replication areas would be further strengthened. Further the resistance of different chimeric lines to other prion strains would be interesting to compare.
‘Taking out’ hippocampal prion replication in this model may have disproportionate effects on survival as the focus of replication is removed, or significantly reduced. Interestingly, targeting other brain regions by lentiviral injection in the same model at the same time point had no significant effect on survival, unless the hippocampus was also targeted. Thus thalamic or striatal injections of anti-PrP lentivirus had no therapeutic benefit on survival, but targeting either of these regions along with the hippocampus showed similar increases on survival as targeting the hippocampus alone (data not shown). The findings again speak to the concept of critical areas of prion replication, which likely vary with different prion strains, but may be key in guiding future therapy.
Either earlier intervention is exerting its effects through interference with the kinetics of prion replication, or it is acting at a critical period in neuronal death ‘routines’, or both. Clearly, all animals succumb eventually, presumably due to prion-mediated neurodegeneration in other, critical, brain regions, but the neuroprotective effects seen within the hippocampus and beyond are undoubtedly a desirable effect of therapy. If transduction were to be more widespread, for example by pseudotying lentiviruses with coat proteins that allow retrograde transport,60,61 or by using evolving mechanical techniques for enhanced delivery,62–64 more extensive neuroprotection and longer survival might ensue. Yet given the size of the human brain, perhaps a more focused, localized approach to key areas is a more realistic goal for therapy.
Finally, it appears that the concept of containing ‘spread’ of disease may well apply in protein folding neurodegenerative disorders beyond prion diseases. A recent paper from Clavaguera et al. shows transmission and spread of tauopathy in transgenic mouse brains.65 Mutant tau mouse brain extract injected into wild-type tau expressing mouse brains resulted in a spreading tauopathy in recipient animals. The authors further suggest that different tau mutations and isoforms might represent different strains of tauopathy, with diverse clinical and pathological manifestations. Indeed the evolution or ‘spread’ of tau or Alzheimer pathology in human brains over time and brain region is well recognized.66 Stopping protein propagation in its tracks with localized treatments may be an option worth considering in other human neurodegenerative proteinopathies.
Implications for Therapy in Human Prion Diseases
A significant advantage of the RNAi therapeutic approach in prion disease is its applicability to all known strains of prion disease. Within any species the primary sequence of PrPC, and PrPSc, is the same for all strains: thus RNAi should be an effective treatment for all variants. This is in contrast to many previous candidate treatments for prion disease, which have suffered from inconsistent results dependant upon the prion strain involved. This should also apply to familial prion diseases that arise from a coding mutation in the gene encoding PrPC, PRNP. It is likely that allele-specific silencing strategies to reduce expression of the mutant would be effective here. Genetic testing can identify these patients during the preclinical phase so successful treatment of this category may be possible through preventative silencing of the mutant PRNP allele expression prior to development of pathology.
A further category of prion disease patients that may benefit from RNAi-mediated silencing of PrPC are those individuals known to have been peripherally infected, such as recipients of contaminated blood products. Reduction of PrPC expression in organs that are crucial for prion replication and spread, such as the spleen and bone marrow (reviewed in ref. 68), may significantly prolong the incubation period. This could be achieved through regular systemic infusions of modified siRNA duplexes to lower PrPC levels sufficiently to prevent accumulation of PrPSc above the cellular threshold for clearance, or for longer-term reduction of PrPC, through systemic delivery of VSV-G pseudotyped lentiviruses which can efficiently transduce these organs.69,70 Selective targeting of routes of neuroinvasion such as the sympathetic nerves within the vertebral column may also significantly extend incubation time and perhaps even avert neuroinvasion.
Lowering the amount of PrPC available for conversion may enhance the ability of other agents to inhibit the disease process when administered in combination. For example, by combining RNAi against the prion protein with a drug to increase endogenous clearance of PrPSc it may be possible to delay disease progression indefinitely.
The major obstacle to the use of therapeutic RNAi in neurodegenerative disease remains the problem of delivery to the brain. The blood-brain-barrier (BBB) restricts passive entry of molecules from the peripheral circulation meaning active transport across this barrier, transient disruption of the BBB's impermeability, or direct injection into the brain are currently required for delivery to the CNS. New technologies recently developed offer hope by targeting receptors in the BBB to mediate activate transport of interfering RNA42,71 or utilising viruses capable of transversing the BBB unaided.72
Another caveat to the use of RNAi in vivo is the need to avoid both off-target effects and interference with processing of endogenous miRNAs due to over-loading of the RNAi pathway. Off-target effects can include silencing of unintended genes with limited sequence complementarity to the siRNA guide strand, cytotoxicity or activation of interferon responses. Careful design, in vitro screening and selection of the most potent sequences for RNAi reduces off-target effects and cytotoxicity in vivo,73 and minimizes competition for endogenous miRNA machinery.74 In prion disease, since only partial reduction of PrPC expression is likely to be required for a therapeutic benefit, low doses of RNAi should be sufficient, minimising the potential for unintended side effects.
One drawback of this approach from a public health perspective is that it aims to eliminate neurotoxicity rather than abolish prion replication altogether. While PrPSc continues to be produced, infectivity remains, so the problem of potential transmissibility persists.
Also, whilst the ablation of PrPC expression in adult mice is well tolerated, the consequences of reducing PrPC in humans remain unknown. Initial attempts should proceed with caution and the use of transient silencing through infusion of siRNA duplexes or expression of shRNAs from inducible viral vectors should be considered so that treatment can be halted if unforeseen adverse effects develop. In the end, the balance of possible adverse effects of PrP loss against the benefits of improved survival and protection against neuronal loss in key brain areas, will determine future therapies for prion and other neurodegenerative disorders.
Footnotes
Previously published online: www.landesbioscience.com/journals/prion/article/9289
References
- 1.White MD, Farmer M, Mirabile I, Brandner S, Collinge J, Mallucci GR. Single treatment with RNAi against prion protein rescues early neuronal dysfunction and prolongs survival in mice with prion disease. Proc Natl Acad Sci USA. 2008;105:10238–10243. doi: 10.1073/pnas.0802759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prusiner SB. Molecular biology of prion diseases. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 3.Büeler H, Fischer M, Lang Y, Bluethmann H, Lipp HP, DeArmond SJ, et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 4.Manson JC, Clarke AR, McBride PA, McConnell I, Hope J. PrP gene dosage determines the timing but not the final intensity or distribution of lesions in scrapie pathology. Neurodegeneration. 1994;3:331–340. [PubMed] [Google Scholar]
- 5.Mallucci GR, Ratté S, Asante EA, Linehan J, Gowland I, Jefferys JG, Collinge J. Post-natal knockout of prion protein alters hippocampal CA1 properties, but does not result in neurodegeneration. EMBO J. 2002;21:202–210. doi: 10.1093/emboj/21.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llewelyn C, Hewitt P, Knight R, Amar K, Cousens S, Mackenzie J, Will R. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417–421. doi: 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- 7.Peden A, Head M, Diane L, Jeanne E, James W. Preclinical vCJD after blood transfusion in a codon 129 heterozygous patient. Lancet. 2004;364:527–529. doi: 10.1016/S0140-6736(04)16811-6. [DOI] [PubMed] [Google Scholar]
- 8.Wroe SJ, Pal S, Siddique D, Hyare H, Macfarlane R, Joiner S, et al. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: a case report. Lancet. 2006;368:2061–2067. doi: 10.1016/S0140-6736(06)69835-8. [DOI] [PubMed] [Google Scholar]
- 9.Mead S, Joiner S, Desbruslais M, Beck JA, O'Donoghue M, Lantos P, et al. Creutzfeldt-Jakob disease, prion protein gene codon 129VV, and a novel PrPSc type in a young British woman. Arch Neurol. 2007;64:1780–1784. doi: 10.1001/archneur.64.12.1780. [DOI] [PubMed] [Google Scholar]
- 10.Comoy EE, Casalone C, Lescoutra-Etchegaray N, Zanusso G, Freire S, Marcé D, et al. Atypical BSE (BASE) transmitted from asymptomatic aging cattle to a primate. PLoS ONE. 2008;3:3017. doi: 10.1371/journal.pone.0003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Büeler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 12.Mallucci G, Dickinson A, Linehan J, Klöhn PC, Brandner S, Collinge J. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science. 2003;302:871–874. doi: 10.1126/science.1090187. [DOI] [PubMed] [Google Scholar]
- 13.Collinge J, Owen F, Poulter M, Leach M, Crow TJ, Rossor MN, et al. Prion dementia without characteristic pathology. Lancet. 1990;336:7–9. doi: 10.1016/0140-6736(90)91518-f. [DOI] [PubMed] [Google Scholar]
- 14.Hill AF, Joiner S, Linehan J, Desbruslais M, Lantos PL, Collinge J. Species-barrier-independent prion replication in apparently resistant species. Proc Natl Acad Sci USA. 2000;97:10248–10253. doi: 10.1073/pnas.97.18.10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill AF, Collinge J. Subclinical prion infection. Trends in Microbiology. 2003;11:578–584. doi: 10.1016/j.tim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Collinge J, Palmer MS, Sidle KC, Gowland I, Medori R, Ironside J, Lantos P. Transmission of fatal familial insomnia to laboratory animals. Lancet. 1995;346:569–570. doi: 10.1016/s0140-6736(95)91405-6. [DOI] [PubMed] [Google Scholar]
- 17.Medori R, Montagna P, Tritschler HJ, LeBlanc A, Cortelli P, Tinuper P, et al. Fatal familial insomnia: a second kindred with mutation of prion protein gene at codon 178. Neurology. 1992;42:669–670. doi: 10.1212/wnl.42.3.669. [DOI] [PubMed] [Google Scholar]
- 18.Medori R, Tritschler HJ, LeBlanc AC. In: Chapter 18. Prusiner SB, Collinge J, Powell J, Anderton B, editors. London: Ellis Horwood; 1992. [Google Scholar]
- 19.Büeler H, Raeber A, Sailer A, Fischer M, Aguzzi A, Weissmann C. High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP gene. Mol Med. 1994;1:19–30. [PMC free article] [PubMed] [Google Scholar]
- 20.Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, et al. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 21.Colling SB, Collinge J, Jefferys JG. Hippocampal slices from prion protein null mice: disrupted Ca(2+)-activated K+ currents. Neurosci Lett. 1996;209:49–52. doi: 10.1016/0304-3940(96)12596-9. [DOI] [PubMed] [Google Scholar]
- 22.Tobler I, Gaus SE, Deboer T, Achermann P, Fischer M, Rülicke T, et al. Altered circadian activity rhythms and sleep in mice devoid of prion protein. Nature. 1996;380:639–642. doi: 10.1038/380639a0. [DOI] [PubMed] [Google Scholar]
- 23.Jeffrey M, Halliday WG, Bell J, Johnston AR, MacLeod NK, Ingham C, et al. Synapse loss associated with abnormal PrP precedes neuronal degeneration in the scrapie-infected murine hippocampus. Neuropathol Appl Neurobiol. 2000;26:41–54. doi: 10.1046/j.1365-2990.2000.00216.x. [DOI] [PubMed] [Google Scholar]
- 24.Betmouni S, Clements J, Perry VH. Vacuolation in murine prion disease: an informative artifact. Curr Biol. 1999;9:677–679. doi: 10.1016/s0960-9822(99)80437-0. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham C, Deacon RM, Chan K, Boche D, Rawlins JN, Perry VH. Neuropathologically distinct prion strains give rise to similar temporal profiles of behavioral deficits. Neurobiology of Disease. 2005;18:258–269. doi: 10.1016/j.nbd.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Deacon RM, Raley JM, Perry VH, Rawlins JN. Burrowing into prion disease. Neuroreport. 2001;12:2053–2057. doi: 10.1097/00001756-200107030-00052. [DOI] [PubMed] [Google Scholar]
- 27.Guenther K, Deacon RM, Perry VH, Rawlins JN. Early behavioural changes in scrapie-affected mice and the influence of dapsone. Eur J Neurosci. 2001;14:401–409. doi: 10.1046/j.0953-816x.2001.01645.x. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham C, Deacon R, Wells H, Boche D, Waters S, Diniz CP, et al. Synaptic changes characterize early behavioural signs in the ME7 model of murine prion disease. Eur J Neurosci. 2003;17:2147–2155. doi: 10.1046/j.1460-9568.2003.02662.x. [DOI] [PubMed] [Google Scholar]
- 29.Mallucci GR, White MD, Farmer M, Dickinson A, Khatun H, Powell AD, et al. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron. 2007;53:325–335. doi: 10.1016/j.neuron.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 31.Martinez J, Patkaniowska A, Urlaub H, Lührmann R, Tuschl T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002;110:563–574. doi: 10.1016/s0092-8674(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 32.Hutvágner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- 33.Doench JG, Petersen CP, Sharp PA. siRNAs can function as miRNAs. Genes Dev. 2003;17:438–442. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng Y, Yi R, Cullen BR. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc Natl Acad Sci USA. 2003;100:9779–9784. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorn G, Patel S, Wotherspoon G, Hemmings-Mieszczak M, Barclay J, Natt FJ, et al. siRNA relieves chronic neuropathic pain. Nucleic Acids Research. 2004;32:49. doi: 10.1093/nar/gnh044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thakker DR, Natt F, Hüsken D, Maier R, Müller M, van der Putten H, et al. Neurochemical and behavioral consequences of widespread gene knockdown in the adultmouse brain by using nonviral RNA interference. Proc Natl Acad Sci USA. 2004;101:17270–17275. doi: 10.1073/pnas.0406214101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hassani Z, Lemkine GF, Erbacher P, Palmier K, Alfama G, Giovannangeli C, et al. Lipid-mediated siRNA delivery downregulates exogenous gene expression in the mouse brain at picomolar levels. Gene Med. 2005;7:198–207. doi: 10.1002/jgm.659. [DOI] [PubMed] [Google Scholar]
- 39.Kumar P, Lee SK, Shankar P, Manjunath N. A single siRNA suppresses fatal encephalitis induced by two different flaviviruses. PLoS Medicine. 2006;3:96. doi: 10.1371/journal.pmed.0030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo MC, Zhang DQ, Ma SW, Huang YY, Shuster SJ, Porreca F, Lai J. An efficient intrathecal delivery of small interfering RNA to the spinal cord and peripheral neurons. Molecular Pain. 2005;1:29. doi: 10.1186/1744-8069-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan PH, Yang LC, Shih HC, Lan KC, Cheng JT. Gene knockdown with intrathecal siRNA of NMDA receptor NR2B subunit reduces formalin-induced nociception in the rat. Gene Ther. 2005;12:59–66. doi: 10.1038/sj.gt.3302376. [DOI] [PubMed] [Google Scholar]
- 42.Wang YL, Liu W, Wada E, Murata M, Wada K, Kanazawa I. Clinico-pathological rescue of a model mouse of Huntington's disease by siRNA. Neuroscience Research. 2005;53:241–249. doi: 10.1016/j.neures.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 43.Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 44.Paul CP, Good PD, Winer I, Engelke DR. Effective expression of small interfering RNA in human cells. Nat Biotechnol. 2002;20:505–508. doi: 10.1038/nbt0502-505. [DOI] [PubMed] [Google Scholar]
- 45.Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester WC, Shi Y. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA. 2002;99:5515–5520. doi: 10.1073/pnas.082117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blomer U, Naldini L, Kafri T, Trono D, Verma IM. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10:816–820. doi: 10.1038/nm1076. [DOI] [PubMed] [Google Scholar]
- 49.Harper SQ, Staber PD, He X, Eliason SL, Martins IH, Mao Q, et al. RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model. Proc Natl Acad Sci USA. 2005;102:5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Lebron E, Denovan-Wright EM, Nash K, Lewin AS, Mandel RJ. Intrastriatal rAAV-mediated delivery of anti-huntingtin shRNAs induces partial reversal of disease progression in R6/1 Huntington's disease transgenic mice. Mol Ther. 2005;12:618–633. doi: 10.1016/j.ymthe.2005.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Machida Y, Okada T, Kurosawa M, Oyama F, Ozawa K, Nukina N. rAAV-mediated shRNA ameliorated neuropathology in Huntington disease model mouse. Biochem Biophys Res Commun. 2006;343:190–197. doi: 10.1016/j.bbrc.2006.02.141. [DOI] [PubMed] [Google Scholar]
- 52.Raoul C, Abbas-Terki T, Bensadoun JC, Guillot S, Haase G, Szulc J, et al. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11:423–428. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- 53.Ralph GS, Radcliffe PA, Day DM, Carthy JM, Leroux MA, Lee DC, et al. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005;11:429–433. doi: 10.1038/nm1205. [DOI] [PubMed] [Google Scholar]
- 54.Daude N, Marella M, Chabry J. Specific inhibition of pathological prion protein accumulation by small interfering RNAs. J Cell Science. 2003;116:2775–2779. doi: 10.1242/jcs.00494. [DOI] [PubMed] [Google Scholar]
- 55.Tilly G, Chapuis J, Vilette D, Laude H, Vilotte JL. Efficient and specific downregulation of prion protein expression by RNAi. Biochem Biophys Res Commun. 2003;305:548–551. doi: 10.1016/s0006-291x(03)00805-2. [DOI] [PubMed] [Google Scholar]
- 56.Golding MC, Long CR, Carmell MA, Hannon GJ, Westhusin ME. Suppression of prion protein in livestock by RNA interference. Proc Natl Acad Sci USA. 2006;103:5285–5290. doi: 10.1073/pnas.0600813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pfeifer A, Eigenbrod S, Al-Khadra S, Hofmann A, Mitteregger G, Moser M, et al. Lentivector-mediated RNAi efficiently suppresses prion protein and prolongs survival of scrapie-infected mice. J Clin Invest. 2006;116:3204–3210. doi: 10.1172/JCI29236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruce ME. TSE strain variation. Br Med Bull. 2003;66:99–108. doi: 10.1093/bmb/66.1.99. [DOI] [PubMed] [Google Scholar]
- 59.Bruce ME, McBride PA, Jeffrey M, Scott JR. PrP in pathology and pathogenesis in scrapie-infected mice. Mol Neurobiol. 1994;8:105–112. doi: 10.1007/BF02780660. [DOI] [PubMed] [Google Scholar]
- 60.Kato S, Inoue K, Kobayashi K, Yasoshima Y, Miyachi S, Inoue S, et al. Efficient gene transfer via retrograde transport in rodent and primate brains using a human immunodeficiency virus type 1-based vector pseudotyped with rabies virus glycoprotein. Hum Gen Ther. 2007;18:1141–1151. doi: 10.1089/hum.2007.082. [DOI] [PubMed] [Google Scholar]
- 61.Mazarakis ND, Azzouz M, Rohll JB, Ellard FM, Wilkes FJ, Olsen AL, et al. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum Mol Genet. 2001;10:2109–2121. doi: 10.1093/hmg/10.19.2109. [DOI] [PubMed] [Google Scholar]
- 62.Hadaczek P, Kohutnicka M, Krauze MT, Bringas J, Pivirotto P, Cunningham J, Bankiewicz K. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum Gene Ther. 2006;17:291–302. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- 63.Oh S, Odland R, Wilson SR, Kroeger KM, Liu C, Lowenstein PR, et al. Improved distribution of small molecules and viral vectors in the murine brain using a hollow fiber catheter. J Neurosurgery. 2007;107:568–577. doi: 10.3171/JNS-07/09/0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanftner LM, Sommer JM, Suzuki BM, Smith PH, Vijay S, Vargas JA, et al. AAV2-mediated gene delivery to monkey putamen: evaluation of an infusion device and delivery parameters. Exp Neurol. 2005;194:476–483. doi: 10.1016/j.expneurol.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009 doi: 10.1038/ncb1901. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez-Alegre P, Bode N, Davidson BL, Paulson HL. Silencing primary dystonia: lentiviral-mediated RNA interference therapy for DYT1 dystonia. J Neurosci. 2005;25:10502–10509. doi: 10.1523/JNEUROSCI.3016-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sapru MK, Yates JW, Hogan S, Jiang L, Halter J, Bohn MC. Silencing of human alpha-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Exp Neurol. 2006;198:382–390. doi: 10.1016/j.expneurol.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 68.Aguzzi A, Heikenwalder M. Pathogenesis of prion diseases: current status and future outlook. Nat Rev Micro. 2006;4:765–775. doi: 10.1038/nrmicro1492. [DOI] [PubMed] [Google Scholar]
- 69.Croyle MA, Callahan SM, Auricchio A, Schumer G, Linse KD, Wilson JM, et al. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J Virol. 2004;78:912–921. doi: 10.1128/JVI.78.2.912-921.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan D, Gunther R, Duan W, Wendell S, Kaemmerer W, Kafri T, et al. Biodistribution and toxicity studies of VSVG-pseudotyped lentiviral vector after intravenous administration in mice with the observation of in vivo transduction of bone marrow. Mol Ther. 2002;6:19–29. doi: 10.1006/mthe.2002.0630. [DOI] [PubMed] [Google Scholar]
- 71.Spencer BJ, Verma IM. Targeted delivery of proteins across the blood-brain barrier. Proc Natl Acad Sci USA. 2007;104:7594–7599. doi: 10.1073/pnas.0702170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2008;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Birmingham A, Anderson E, Sullivan K, Reynolds A, Boese Q, Leake D, et al. A protocol for designing siRNAs with high functionality and specificity. Nat Protoc. 2007;2:2068–2078. doi: 10.1038/nprot.2007.278. [DOI] [PubMed] [Google Scholar]
- 74.Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]