Abstract
Selective estrogen receptor modulators (SERMs), such as tamoxifen (TAM), have been used extensively for the treatment and prevention of breast cancer and other pathologies associated with aberrant estrogen receptor (ER) signaling. These compounds exhibit cell-selective agonist/antagonist activities as a consequence of their ability to induce different conformational changes in ER, thereby enabling it to recruit functionally distinct transcriptional coregulators. However, the observation that SERMs can also regulate aspects of calcium signaling and apoptosis in an ER-independent manner in some systems suggests that some of the activity of drugs within this class may also arise as a consequence of their ability to interact with targets other than ER. In this study, we demonstrate that 4-hydroxy-TAM (4OHT), an active metabolite of TAM, directly binds to and modulates the transcriptional activity of the aryl hydrocarbon receptor (AHR). Of specific interest was the observation, that in the absence of ER, 4OHT can induce the expression of AHR target genes involved in estradiol metabolism, cellular proliferation, and metastasis in cellular models of breast cancer. The potential role for AHR in SERM pharmacology was further underscored by the ability of 4OHT to suppress osteoclast differentiation in vitro in part through AHR. Cumulatively, these findings provide evidence that it is necessary to reevaluate the relative roles of ER and AHR in manifesting the pharmacological actions and therapeutic efficacy of TAM and other SERMs.
4-hydroxy-Tamoxifen directly binds to and modulates the aryl hydrocarbon receptor in the absence of estrogen receptor, thereby modulating gene expression and suppressing in vivo osteoclastogenesis.
Breast cancer is the most common cancer diagnosed in women, leading to an estimated 40,000 deaths in 2007 in the United States alone (1). Notably, the majority of breast cancers express the estrogen receptor α (ERα), a member of the nuclear receptor (NR) superfamily of ligand-inducible transcription factors, and thus respond to the mitogenic actions of estrogen(s). The role of ERβ in breast cancer is unclear, but it appears to repress the actions of ERα when the two receptors are coexpressed (2). Regardless, the pharmacotherapy of ERα-positive breast cancer relies heavily on the ability to 1) block the synthesis of estradiol using aromatase inhibitors or GnRH agonists, or 2) competitively inhibit the activity of the receptor using selective ER modulators (SERMs) or selective ER down-regulators (3).
Although initially classified as competitive antagonists based on their ability to oppose estrogen action in the breast, it has become clear that SERMs, such as tamoxifen (TAM) and raloxifene (RAL), are pharmacologically more complex in that they manifest agonist or antagonist activity in a cell-selective manner. Both TAM and RAL function as antagonists in the breast and as agonists in the bone. These activities led to the development of TAM for the treatment and prevention of breast cancer and of RAL for the prevention of breast cancer and the treatment and prevention of osteoporosis. Highlighting the SERM activity even further is the observation that TAM, but not RAL, manifests agonist activity in the uterus, and the more recent finding that although TAM decreases the risk of both invasive and noninvasive breast cancer, RAL only decreases the risk for invasive breast cancer in women at elevated risk for this disease (4). The prevailing hypothesis used to describe the tissue-specific activities attributed to these compounds is that they induce different conformational changes in ER that engender the differential interaction of the receptor with functionally distinct coregulators. Thus, it is proposed that the biological activity of SERMs is determined, to a large extent, by the relative and absolute expression level of both coactivator and corepressor proteins in different cell types (5).
Although ER is the primary target of SERMs, there is increasing evidence that these molecules may exhibit off-target effects that contribute to their pharmacological activity. TAM, for instance, has been associated with two distinct types of off-target activity. The first refers to the ability of TAM to facilitate the ectopic interaction of ER with cofactor proteins with which the receptor would not normally interact in the presence of a physiologically relevant agonist (6). For instance, it has been shown that the corepressor proteins nuclear receptor copressor and silencing mediator of retinoid and thyroid hormone receptor interact with TAM-bound ER in MCF7 cells, resulting in repression of target gene expression (7). The second type of off-target activity relates to actions of a particular compound that occur in the absence of ER. Among the most studied pertain to TAM as a reversible or irreversible inhibitor of protein kinase C (8) and of calmodulin (9) and to its ability to regulate phospholipase D activity (10). Additionally, it has been shown that both TAM and its metabolite 4-hydroxy-TAM (4OHT) bind to and inhibit the transcriptional activity of estrogen-related receptor γ (11). It appears, therefore, that the pharmacological actions of SERMs are likely to reflect a composite of their actions in both ER-dependent and -independent pathways, although the relative contribution of these functionally distinct mechanisms to overall activity remains to be determined. Recognition of the potential contributions of ER-independent pathways in SERM pharmacology has heightened interest in identifying other targets of these drugs. Thus we have undertaken a genomic approach to identify genes that are modulated by TAM and other SERMs in an ER-independent manner. Using this approach, we have determined that the aryl hydrocarbon receptor (AHR) is a direct target of SERMs and that manipulation of AHR in cellular models of breast cancer and bone has a dramatic effect on the pharmacological activity of members of this class of compounds. These data highlight the need to understand the contribution of AHR modulation to the therapeutic efficacy of SERMs and the utility of targeting this receptor for the treatment of various endocrinopathies.
Results
Multiple AHR target genes are regulated by the SERM 4OHT
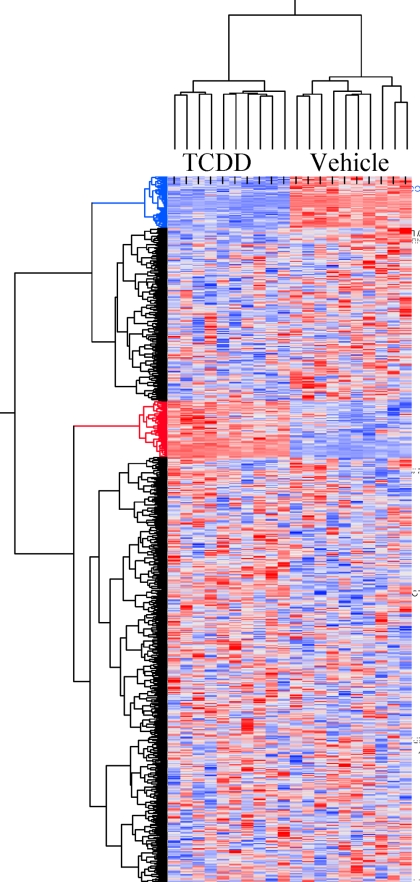
As an initial step in defining the ER-independent processes that are impacted by SERMs, we used a bioinformatics approach to identify genes that were differentially regulated by 4OHT in ERα-positive MCF7 breast cancer cells. To define the spectrum of 4OHT-regulated genes, we took advantage of the published dataset GSE848 (12). In addition, we performed an analysis of the genes induced in MCF7 cells treated with the canonical AHR agonist TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin). The overlap of gene expression profiles in the 4OHT and TCDD datasets was subsequently evaluated (Fig. 1). From this analysis, we determined that a subset of 4OHT target genes was also subject to regulation by TCDD, the identities of which are presented in Table 1. Notably, two distinct classes of genes were identified, those regulated coordinately by 4OHT and TCDD (e.g. CYP1A1 and achaete-scute complex homolog 1) and those regulated in opposite directions (such as selectin L and PDZ domain containing 1). We concluded from these studies that the SERM 4OHT has the ability to function as an AHR agonist on some genes. Interestingly, among the genes identified as 4OHT responsive were CYP1A1 and CYP1B1, two classic AHR targets. These findings prompted us to evaluate the potential role(s) of AHR in defining the pharmacology of tamoxifen and other SERMs.
Figure 1.
Regulation of TAM target genes by TCDD. Probe sets with a fold change between 4OHT and vehicle of more than 3 were cross-referenced to HG-133 plus 2.0 platform identifications through common Entrez identifiers, and corresponding probe sets were clustered for TCDD- and vehicle-treated samples using Ward algorithm. The diagram depicts 1016 probe sets, representing 450 unique genes. Clusters color coded blue and red encompass genes that are, respectively, down-regulated and up-regulated by TCDD.
Table 1.
The list of genes that are subject to dual regulation by 4OHT and TCDD
| Gene | Fold change TCDD vs. vehicle | Fold change 4OHT vs. vehicle in GSE848 | |
|---|---|---|---|
| Genes coordinatelyregulated | |||
| CYP1A1 | Cytochrome P450, family 1, subfamily A, polypeptide 1 | 118.6 | 57.2 |
| CYP1B1 | Cytochrome P450, family 1, subfamily B, polypeptide 1 | 12.2 | 20.2 |
| RAP2A | RAP2A, member of RAS oncogene family | 2.1 | 6.8 |
| TCF7L2 | Transcription factor 7-like 2 (T-cell specific, HMG-box) | 1.9 | 5.7 |
| PCDH8 | Protocadherin 8 | 2.4 | 5.7 |
| ME1 | Malic enzyme 1, NADP(+)-dependent, cytosolic | 2.1 | 4.4 |
| DRD1 | Dopamine receptor D1 | 2.8 | 4.2 |
| VIPR1 | Vasoactive intestinal peptide receptor 1 | 3.5 | 3.2 |
| SLC7A5 | Solute carrier family 7 (cationic amino acidtransporter, y+ system), member 5 | 2.2 | 3.0 |
| FLJ13305 | Hypothetical protein FLJ13305 | −1.9 | −3.0 |
| FN1 | Fibronectin 1 | −1.7 | −3.1 |
| CDH11 | Cadherin 11, type 2, OB-cadherin (osteoblast) | −2.4 | −3.1 |
| ASCL1 | Achaete-scute complex homolog 1 (Drosophila) | −3.4 | −3.3 |
| SVIL | Supervillin | −1.8 | −3.7 |
| COL4A6 | Collagen, type IV, α 6 | −1.6 | −3.9 |
| ADA | Adenosine deaminase | −1.6 | −4.2 |
| SLC1A1 | Solute carrier family 1 (neuronal/epithelial highaffinity glutamate transporter, system Xag), member 1 | −2.6 | −4.7 |
| HSPA2 | Heat shock 70 kDa protein 2 | −1.7 | −5.5 |
| Genes regulated inoppositedirections | |||
| SELL | Selectin L (lymphocyte adhesion molecule 1) | 3.0 | −3.2 |
| MALL | Mal, T-cell differentiation protein-like | 1.7 | −3.2 |
| B4GALT1 | UDP-Gal:βGlcNAc β 1,4-galactosyltransferase, polypeptide 1 | 1.7 | −3.3 |
| C20orf194 | Chromosome 20 open reading frame 194 | 1.6 | −3.3 |
| SLC9A7 | Solute carrier family 9 (sodium/hydrogenexchanger), member 7 | 1.6 | −3.4 |
| KCNN4 | Potassium intermediate/small conductancecalcium-activated channel, subfamily N, member 4 | 2.4 | −3.7 |
| TFAP2A | Transcription factor AP-2 α (activatingenhancer-binding protein 2 α) | 2.0 | −3.8 |
| SPTBN1 | Spectrin, β, nonerythrocytic 1 | 1.6 | −8.2 |
| CALCR | Calcitonin receptor | −2.7 | 6.4 |
| RASGRP1 | RAS guanyl-releasing protein 1 (calcium andDAG-regulated) | −5.5 | 5.3 |
| MYBL1 | v-myb Myeloblastosis viral oncogene homolog(avian)-like 1 | −11.9 | 5.0 |
| FRK | Fyn-related kinase | −1.6 | 4.4 |
| FLJ13236 | Hypothetical protein FLJ13236 | −1.5 | 3.9 |
| SEMA3C | Sema domain, Ig domain (Ig), short basic domain, secreted, (semaphorin) 3C | −1.5 | 3.8 |
| FOS | v-fos FBJ murine osteosarcoma viral oncogenehomolog | −2.4 | 3.8 |
| RHOBTB1 | ρ-related BTB domain containing 1 | −1.6 | 3.7 |
| CDC2 | Cell division cycle 2, G1 to S and G2 to M | −2.4 | 3.6 |
| IGFBP4 | IGF-binding protein 4 | −2.0 | 3.4 |
| PDZK1 | PDZ domain containing 1 | −6.4 | 3.4 |
These genes are regulated by 8-h treatment by 4OHT by more than ±3 fold in the GSE848 dataset and are significantly regulated by TCDD with a fold change of more than ±1.5 fold. The data from the probesets that passed the selection criteria are reported. Significance criteria are described in Materials and Methods. In cases when multiple probesets representing the same gene passed selection, the average fold change is provided.
4OHT-dependent up-regulation of CYP1A1 expression does not require ER
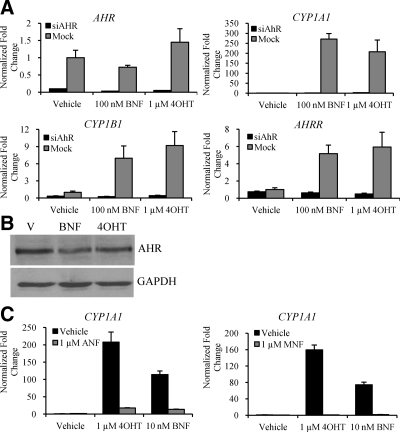
The ability of TAM, 4OHT, and other SERMs to regulate AHR target genes was evaluated in MCF7 cells, a well-characterized model of estrogen-dependent breast cancer. Because CYP1A1 was found to be robustly up-regulated by the SERMs TAM, 4OHT, and RAL by microarray analysis (12,13,14,15), and because the CYP1A1 promoter contains multiple consensus AHR response elements (AHREs) (16), we analyzed its expression in MCF7 cells and used this as a proxy for AHR activation. The specific goal was to determine the relative contribution of AHR and ER in 4OHT-dependent up-regulation of classical AHR target genes. It has been shown by others that AHR and ER physically interact (17,18,19,20), suggesting the possibility that 4OHT action on AHR target genes requires both receptors. To address this possibility, we used small interfering RNA (siRNA) to decrease the expression of ERα in MCF7 cells and then examined the induction of CYP1A1 after 4OHT treatment. We were unable to detect ERβ in MCF7 cells and thus assumed ERα to be the only ER subtype present. Using two different siRNA constructs targeting ERα [small interfering (si)ERα A and siERα C], ERα expression was reduced by greater than 90% (Fig. 2A). In this experiment it was determined that 4OHT treatment led to a significant increase in both CYP1A1 and CYP1B1 expression in control cells as well as in cells transfected with siERα (Fig. 2A). Induction of the ER target gene, the progesterone receptor (PR), by estradiol (E2) was completely blocked by siERα, but not by the control siRNA (supplemental Fig. 1A published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Interestingly, the promoter of CYP1B1 contains a functionally active estrogen response element in addition to AHREs (21); however, our data suggest that AHR is more critical in transducing the effects of 4OHT on this promoter in this particular context.
Figure 2.
4OHT activates AHR in an ER-independent manner. A, ERα-positive MCF7 cells were transfected with either of two unique siRNA duplexes to ERα (siERα A and siERα C) or siRNA control (Mock). After 48 h, the cells were treated for 4 h with either vehicle or 100 nm 4OHT. Activation of AHR was also examined in the ERα-negative breast cancer cell lines (B) MDA-MB-231 treated for 4 h with vehicle, 10 nm BNF, or 100 nm 4OHT or (C) SKBR3 treated for 4 h with vehicle, 100 nm BNF, or 100 nm 4OHT. D, SKBR3 cells were treated for 4 h with vehicle or increasing concentrations of 4OHT. For all experiments, mRNA was analyzed by quantitative RT-PCR for ERα (A), CYP1A1 (A, B, and C), or CYP1B1 (A, B, and C) expression. Data are the mean ± sem for triplicate amplification reactions from one representative experiment. Each experiment was repeated at least three independent times with very similar results.
To further confirm that 4OHT does not require ER to activate AHR, we examined the ability of 4OHT to induce CYP1A1 and CYP1B1 expression in two ER-negative breast cancer cell lines (MDA-MB-231 and SKBR3). In both cell lines, 4OHT treatment resulted in a significant increase in CYP1A1 and CYP1B1 expression (Fig. 2, B and C), similar to the classic AHR agonist β-naphthoflavone (BNF), used in our studies as a positive control. These data suggest that 4OHT-mediated regulation of AHR target genes does not require ER expression, and thus regulation of AHR is an ER-independent off-target effect of 4OHT.
It has been determined that the circulating levels of 4OHT in patients treated with 20 mg TAM ranges from 3–200 nm (22,23). Furthermore, concentrations of drug from 100 nm to 1 μm are generally used in in vitro studies of ER action (22,23). Considering these reference data, it is important to note that significant induction of CYP1A1 was observed at concentrations of 4OHT as low as 100 nm (Fig. 2D). The maximal activity elicited by 4OHT did not reach that of BNF, most likely due to differing affinities for AHR, although the time course of activation was similar (supplemental Fig. 1B). The activation of AHR leads to a transient up-regulation of CYP1A1, perhaps explaining why these gene targets are not noticed in previous microarray studies where treatment times of 24–48 h were generally employed. Further, significant variability in the absolute magnitude of response to AHR stimulation when analyzing CYP1A1 expression was observed, most likely a consequence of the steep dose-response curve in this system. However, other confounders such as cell density, exposure to UV light, and disruption of cell-cell contact have also been shown to impact the magnitude of CYP1A1 induction in in vitro cell models (24,25,26). Not withstanding these complexities, we noted robust and consistent regulation of AHR at doses between 100 nm and 1 μm 4OHT (see Fig. 2) and decided therefore to use these pharmacologically relevant doses in our subsequent studies.
4OHT regulates AHR transcriptional activity andits stability
Although increased expression of CYP1A1 is a well-characterized marker of AHR activation, it was important to show that AHR was required for 4OHT-induced up-regulation of the selected AHR-responsive genes. Targeting siRNA to AHR [small interfering (si)AHR] blocked the induction of CYP1A1 and CYP1B1 by both 4OHT and BNF in ER-negative SKBR3 cells (Fig. 3A) and ER-positive MCF7 cells (supplemental Fig. 1C).
Figure 3.
Regulation of AHR target genes by 4OHT requires expression of AHR. A, SKBR3 cells were transfected with siRNA to AHR (siAHR) or siRNA control (Mock). After 48 h, the cells were treated for 4 h with either vehicle, 1 μm 4OHT, or 100 nm BNF. mRNA expression of AHR, CYP1A1, CYP1B1, and AHRR was analyzed by quantitative RT-PCR. B, AHR protein degradation was analyzed in SKBR3 cells treated for 6 h with either vehicle, 100 nm BNF, or 1 μm 4OHT. Cells were harvested, and 60 μg whole-cell extract was resolved by SDS-PAGE, transferred to nitrocellulose, and subjected to immunoblotting for AHR or glyceraldehyde-3-phosphate dehydrogenase as a loading control. A representative blot is shown. C, CYP1A1 mRNA expression was examined in SKBR3 cells after treatment for 4 h with vehicle, 1 μm 4OHT, or 10 nm BNF in the absence or presence of either 1 μm ANF or 1 μm MNF. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; V, vehicle.
To assess whether 4OHT functions as a classical AHR agonist, we also examined the ability of 4OHT to modulate the expression of another AHR target gene, the AHR repressor (AHRR). AHRR plays an important role within a negative feedback loop that regulates AHR activity. In Fig. 3A, it is clear that AHRR was also regulated by 4OHT in an AHR-dependent and ER-independent manner. Although not an exhaustive analysis, our data strongly suggest that 4OHT can recapitulate many of the activities of classic AHR agonists in terms of target gene regulation.
In a process linked to DNA binding and transcriptional activation, AHR undergoes ubiquitin-dependent degradation through the 26S proteosome pathway in the presence of agonists such as BNF and TCDD (27). In ER-negative SKBR3 breast cancer cells, we showed that treatment with BNF or 4OHT for 6 h led to an equivalent decrease in AHR protein expression (Fig. 3B). Therefore, 4OHT modulated both AHR activation and degradation in the manner expected of a classical AHR agonist.
We next sought to determine whether AHR target gene regulation by 4OHT was inhibited by AHR antagonists. When treated in combination with the antagonists α-naphthoflavone (ANF) or 3-methoxy-4-nitroflavone (MNF) (28,29,30), 4OHT and BNF were unable to elicit maximal expression of CYP1A1 (Fig. 3C). Further, increasing concentrations of MNF competed in a dose-dependent manner with 4OHT to inhibit AHR (data not shown), suggesting that 4OHT may be a direct modulator of AHR.
4OHT directly increases AHRtranscriptional activity
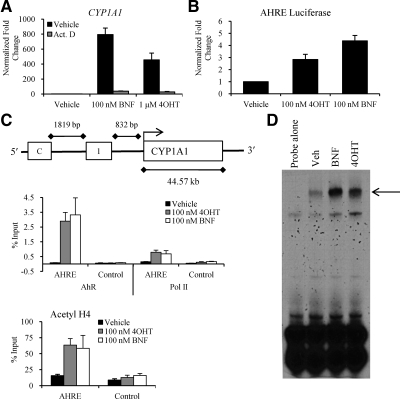
The question remained whether 4OHT-mediated up-regulation of CYP1A1 mRNA expression was a direct effect on the transcriptional activity of AHR. To address this issue, we cotreated cells with 4OHT or BNF and the transcriptional inhibitor actinomycin D (ActD). This treatment blocked the induction of CYP1A1 in SKBR3 cells (Fig. 4A), proving that 4OHT-mediated up-regulation of CYP1A1 required active transcription.
Figure 4.
4OHT directly binds to and increases AHR transcriptional activity. Panel A, CYP1A1 mRNA expression in SKBR3 cells was analyzed by quantitative RT-PCR after cells were pretreated for 1 h with either vehicle or 100 ng/ml ActD followed by treatment for 4 h with vehicle, 100 nm BNF, or 1 μm 4OHT. Panel B, Luciferase reporter activity was assessed in SKBR3 cells transfected overnight with pAHRE-TK-luc3 and then treated overnight with either vehicle, 100 nm 4OHT, or 100 nm BNF. The responses to 4OHT and BNF were significantly different from vehicle (P < 0.05 by ANOVA with Tukey’s multiple comparison test). Panel C, A schematic of the CYP1A1 promoter region, where 1 indicates the AHRE and C indicates the distal control region. SKBR3 cells were treated for 75 min with vehicle, 100 nm 4OHT, or 100 nm BNF. Cells were harvested after cross-linking and subjected to immunoprecipitation with either control IgG or antibodies to AHR, RNA polymerase II (Pol II), or acetylated histone H4 (Acetyl H4). After reversal of the cross-link, DNA was isolated and subjected to qPCR analysis. There was no significant recruitment of AHR to a distal region of the CYP1A1 promoter. Panel D, AHR/ARNT binding to DNA was examined by EMSA. AHR and ARNT were translated in vitro from pCDNA3.1nv5-AHR and pCDNA3.1nv5-ARNT. Proteins were allowed to heterodimerize in the presence of ligand before addition of radiolabeled oligo. Protein-DNA complexes resolved on a 5% nondenaturing acrylamide gel were dried and subjected to autoradiography. Act.D, Actinomycin D; Veh, vehicle.
To definitively demonstrate whether 4OHT activated transcription through an AHRE, we used a reporter construct containing a consensus AHRE fused to luciferase and transfected this into ER-negative breast cancer cells. Both 4OHT and BNF increased AHR transcriptional activity as assessed by increased luciferase expression (Fig. 4B). From this, we concluded that 4OHT increases expression of AHR target genes by increasing AHR transcriptional activity directly.
The most direct way a ligand affects target gene expression is by modulating the recruitment of the transcription factor to DNA elements. Using chromatin immunoprecipitation in SKBR3 cells, we found that treatment with 4OHT for 75 min increased AHR binding to the CYP1A1 promoter region, and that this increase in binding was concomitant with an increase in the recruitment of both RNA polymerase II and acetylated histone H4 (Fig. 4C). This effect was recapitulated in ERα-positive MCF7 cells (supplemental Fig. 1D). Therefore, it appeared that 4OHT directly influenced the transcriptional activity of AHR in an ER-independent manner by increasing occupancy of AHR at DNA-response elements within target genes.
Direct activation of AHR by 4OHT
EMSAs have been used as a surrogate to examine ligand binding to AHR because they assess the essential hallmarks of AHR activation, i.e. dimerization with AHR nuclear translocator (ARNT) and heterodimeric binding to DNA (31). For these assays we prepared recombinant human AHR and ARNT protein separately in vitro and subsequently combined the recombinant proteins in the presence of vehicle, BNF, or 4OHT. The protein-ligand mixture was then incubated with a 32P-labeled oligonucleotide containing a single copy of a consensus AHRE and resolved on a nondenaturing acrylamide gel. Both BNF and 4OHT increased the binding of the AHR-ARNT complex to the AHRE as evidenced by retardation in the mobility of the radiolabeled probe (Fig. 4D). We confirmed that neither AHR nor ARNT bound to DNA alone under any treatment condition (data not shown). The fact that 4OHT increased the DNA-binding ability of the AHR/ARNT complex in a defined system makes it likely that 4OHT directly binds to AHR and influences its biological activity in a direct manner. Because there is precedent for ligand binding to AHR outside the ligand-binding pocket, it will be necessary to use more detailed binding studies to determine whether 4OHT binds within the ligand-binding pocket or to a second site on the receptor (32,33,34).
Many SERMs modulate AHR activity
4OHT is a primary, but not the only, metabolite of TAM that is found in the serum and/or mammary tissue of breast cancer patients treated with TAM. Indeed, metabolites such as N-desmethyl-TAM and endoxifen have also been identified and their pharmacological properties have been partially characterized (35,36,37,38,39). Because the circulating levels of both TAM and 4OHT are high enough to activate ER, it is unclear what portion of the in vivo biological activity is attributable to TAM, 4OHT, or endoxifen. As a result, we examined whether the parent compound TAM or the secondary metabolite endoxifen possessed the ability to regulate AHR signaling. In SKBR3 cells, it was determined that 4OHT was the most active component over the specified dose range, although both TAM and endoxifen significantly induced CYP1A1 expression (Fig. 5A). Interestingly, given the results of this analysis, both TAM and 4OHT would be predicted to activate AHR at pharmaceutically relevant concentrations. This was not the case with endoxifen, which only exhibited AHR-modulatory activity at exceedingly high concentrations relative to what has been measured in patients (40). Thus, both TAM and 4OHT exhibit a significant ER-independent ability to modulate the AHR signaling axis, a result of potential clinical significance.
Figure 5.
TAM metabolites and many SERMs activate AHR. A, Dose-dependent regulation of CYP1A1 mRNA by TAM, 4OHT, or endoxifen (Endo) in SKBR3 cells. Cells were treated for 4 h and mRNA was analyzed by quantitative RT-PCR. Data were fit to a sigmoidal dose-response curve with variable slope and a bottom value set at 1 using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). B, MCF7 cells were treated for 4 h with the vehicle or 100 nm ligand, unless otherwise indicated. cDNA from RNA extracted from treated cells was analyzed by quantitative RT-PCR. Veh, Vehicle; coum, coumestrol; PPT, propyl pyrazole triol.
We extended our studies of AHR pharmacology to other NR modulators to determine whether the ability to regulate AHR was shared among this class of drugs. Interestingly, many other synthetic ER ligands, including RAL, ICI 182,780 (ICI), diarylproprionitrile (DPN), and RU58668, possessed the ability to activate AHR in breast cancer cells, as assessed by up-regulation of CYP1A1 expression (Fig. 5B). However, none of the ligands with an estradiol steroidal core structure were able to activate AHR, i.e. 17α- and 17β-ethinyl-E2 did not induce CYP1A1 expression (Fig. 5B and data not shown). The ability of DPN to robustly induce CYP1A1, and thus potentially activate AHR, is intriguing given its widespread use as an ERβ-selective ligand (41,42,43,44). It is possible that the observed phenotypic responses to DPN in systems such as the brain, prostate, and immune system may be a composite of separate activation of the ERβ- and AHR-signaling pathways or a consequence of cross talk between these two receptors, illustrating the need for caution when interpreting results from experiments utilizing DPN and other NR ligands. Of course, further studies are warranted to determine the extent to which the biological activities of these ligands are mediated through AHR.
4OHT blocks osteoclast (OC) differentiation through AHR
TAM and RAL preserve bone mineral density in postmenopausal women, and this is thought to be a reflection of the ability of these compounds to manifest partial ER-agonist activity in the bone (45). More specifically, it has been shown that estrogens and 4OHT inhibit OC differentiation, an activity that may explain the antiresorptive activity of these compounds (46,47). AHR also plays a role in both osteoblast (OB) and OC biology, but the physiological role of AHR modulation is not yet clear. In this regard, AHR agonists have been shown to decrease OC differentiation and activity (48). Additionally, some studies suggest that AHR is expressed and active in OBs, and further that the AHR agonist 3-methylcholanthrene inhibits both OB proliferation and calcification of bones in vitro and in vivo (49,50). We therefore wanted to test the possibility that a portion of the positive effects of 4OHT in the bone is mediated by AHR. To accomplish this, we used an in vitro OC differentiation assay in which the mouse monocyte cell line, RAW264.7, was induced to differentiate by treatment with macrophage-colony-stimulating factor (M-CSF) and receptor activator for nuclear factor κB (RANKL) for 8 d. During this process, cells were also treated with vehicle or increasing concentrations of E2, BNF, RAL, 4OHT, or ICI and subsequently evaluated for OC differentiation by quantitating the presence of multinucleated, tartrate-resistant acid phosphatase (TRAP)-positive cells. The combination of surface expression of TRAP and the presence of multinucleation is a hallmark of a mature OCs and thus can be used to monitor the differentiation process. As expected, E2, 4OHT, and RAL suppress OC differentiation. In addition, it was observed that BNF also suppresses OC differentiation to a comparable degree as the ER ligands (Fig. 6A). Neither vehicle [ethanol or dimethylsulfoxide (DMSO)] nor ICI had a significant effect on OC differentiation. Next, we treated the cells during the differentiation process with E2, BNF, RAL, or 4OHT in the presence of either an ER antagonist (ICI) or an AHR antagonist (ANF). Although ICI and RAL robustly induced CYP1A1 expression in breast cancer cells, neither ligand significantly activated AHR in OCs (Fig. 6C), another example of context-selective differences between 4OHT/TAM and RAL. Surprisingly, we found that the ability of 4OHT to suppress OC differentiation was significantly inhibited by cotreatment with ANF (Fig. 6B), indicating that 4OHT may require AHR for maximal activity in inhibiting OC differentiation, and may not solely work through ER. In contrast, the ability of RAL to inhibit OC differentiation required only ER expression and did not depend on AHR functionality. A similar reliance on AHR for TAM activity in this model system was confirmed using siRNA-mediated knockdown of AHR expression (data not shown). These provocative OC differentiation data were independently verified in a second laboratory (supplemental Fig. 1E). Thus, it appears that 4OHT and RAL may differ in their relative requirements for ER vs. AHR in different tissue contexts, thereby extending the SERM context beyond ER to AHR. Further in vitro and in vivo studies are necessary to determine the extent of AHR involvement in mediating the biological effects of 4OHT, and perhaps other NR modulators.
Figure 6.
4OHT requires AHR to efficiently inhibit OC differentiation. A, Differentiating RAW264.7 cells were treated with vehicle or increasing concentrations of E2, BNF, 4OHT, RAL, or ICI. B, Differentiating RAW264.7 cells were treated with vehicle, 100 nm E2, 100 nm BNF, 100 nm 4OHT, or 100 nm RAL in the presence of either 1 μm ICI or 1 μm ANF. For both A and B, total TRAP-positive multinucleated cells were counted after 8 d. C, CYP1A1 expression was analyzed after treatment of differentiated RAW264.7 cells for 6 h with vehicle or increasing concentrations of E2, BNF, 4OHT, RAL, or ICI. cDNA from RNA extracted from treated cells was analyzed by quantitative RT-PCR.
Discussion
TAM has been used clinically for more than 30 yr to treat breast cancer and for more than 10 yr as a prevention strategy for women at high risk of developing this disease. The activities of TAM, 4OHT, and endoxifen in breast cancer have primarily been attributed to their ability to inhibit ER signaling. Although clearly an ER antagonist in the breast, the ability of TAM to exhibit context-selective agonist properties enables it to function as an antiresorptive agent in the bone. The mechanisms underlying the increased risk for endometriosis and endometrial cancer, blood clots, and stroke in TAM-treated patients are not well understood. Furthermore, it is not entirely clear how TAM, RAL, or other SERMs exhibit tissue-specific activities through a single signaling pathway. Differential cofactor expression and tissue-selective kinase signaling pathways could certainly play a role in this activity; however, it is also quite likely that there exist mechanisms of action of SERMs that are unrelated to their ability to bind ER. We identified one such ER-independent mechanism of action of TAM and its metabolites.
In this study we show that 4OHT regulates AHR transcriptional activity in an ER-independent manner in cellular models of estrogen action and bone remodeling. In these models, 4OHT induced the expression of many AHR target genes, including CYP1A1, CYP1B1, and AHRR in an AHR-dependent and ER-independent manner. 4OHT directly affected the transcriptional activity of AHR, as shown by sensitivity to the transcriptional inhibitor ActD. Further, 4OHT increased AHR binding to the CYP1A1 promoter region in a manner analogous to the known AHR agonist BNF. Importantly, we found that 4OHT bound directly to AHR as assessed by EMSA, an assay that recapitulates ligand binding, dimerization with ARNT, and heterodimeric binding to an AHRE.
Although all SERMs lead to preservation of bone mineral density in postmenopausal women, they do not share a common mechanism of action in the bone. We therefore wished to determine whether the ability of 4OHT to regulate AHR was involved in its actions in the skeleton. Because 4OHT and RAL inhibit osteoclastogenesis, we examined the requirement for ER and/or AHR in mediating the effects of these two ligands, using E2 and BNF as controls for ER and AHR, respectively. Using this approach, we determined that a significant portion of the ability of 4OHT to block osteoclastogenesis required AHR, whereas RAL depended entirely on ER expression for its function in this model system. The extent to which other SERMs with bone-protective activity act through AHR remains to be determined, but this presents a potential explanation for differing mechanisms of action among SERMs in the bone.
4OHT acting through AHR highlights a previously unappreciated activity of SERMs
It is known that the AHR and ER pathways engage in cross talk through direct interaction, and that they also impact the other’s activity by competing for transcriptional regulators such as Sp1. Further, studies show that agonist-activated AHR induces proteosome-dependent degradation of ER protein (18). Together, these data suggest that AHR activation may be antiestrogenic and could thus have antitumorigenic activity in the breast. AHR is required for proper development of the mammary gland and its differentiation during pregnancy, thereby placing AHR as an important regulator of both cell proliferation and differentiation (51). Further, a microarray analysis comparing gene expression in normal breast to breast carcinoma showed that CYP1A1 is down-regulated in the cancerous tissue, suggesting that AHR activity may be decreased during tumorigenesis (data not shown).
Despite the established cross talk and independent roles of ER and AHR in mammary gland physiology, it is interesting to speculate that AHR activation may impact ER action by inducing the enzymes responsible for E2 metabolism. The metabolism of E2 is controlled in a large part by the cytochrome P450 enzymes (for minireview, see Ref. 52). In particular, actions initiated by CYP1A1 and CYP1B1 lead to hydroxy-, methoxy-, and quinone- derivatives of E2. CYP1A1 converts E2 to 2-hydroxy-estradiol, which is transformed by catechol-O-methyl transferase to form 2-methoxy-estradiol, an antitumorigenic metabolite (53). On the other hand, 4-hydroxylation of E2 by CYP1B1 leads to estradiol-3,4-quinone, which is associated with DNA adduct formation that can result in DNA damage and genotoxic stress and thereby potentially promote or accelerate tumorigenesis (54). AHR up-regulation of CYP1A1 vs. CYP1B1 varies in a cell-specific manner, therefore influencing the pathway by which E2 is metabolized in a particular cell (55). The observation that 4OHT may directly affect the local metabolism of E2 through activation of AHR offers another potential mechanism of action for this compound. On the other hand, the possibility that 4OHT increases the metabolism of E2 to the protumorigenic estradiol-3,4-quinone through AHR offers a mechanism by which resistance to 4OHT could arise. Here, the 4OHT- and AHR-dependent increase in DNA-damaging agents would increase genomic instability, leading to accumulation of mutations within the tumor that allow growth signaling to occur in the face of ER inhibition.
Despite efficacy in in vitro and in vivo models of breast cancer, many SERMs and antiestrogens have shown disappointing activity in the clinic. For example, fulvestrant (ICI 182,780), a high-affinity antagonist with no agonist activity on ER in both in vitro and in vivo models of breast cancer, is not superior to TAM as a first line of therapy in breast cancer (56). The lack of a robust response in patients perhaps points to potential pharmacokinetic limitations, such as compound inactivation by the UGT family of glucuronidation enzymes. The most highly expressed UDP-glucuronosyltransferase (UGT) family member in the breast is UGT1A4, which is capable of producing significant amounts of fulvestrant-glucuronide (57) and is largely regulated directly by AHR (58). Recent studies have shown that TAM, 4OHT, and endoxifen can also be glucuronidated as a pathway of metabolism and excretion (59). It is therefore possible that by activating AHR, SERMs and antiestrogens are concomitantly inducing their own metabolism and excretion, thereby reducing their efficacy. The ability of each therapeutic to induce AHR, and thus up-regulate metabolic enzymes, could contribute to disappointing in vivo efficacy that is not well modeled in rodents with vastly different metabolic systems and AHR-signaling pathways (60,61). Thus, the tissue-specific expression levels of AHR and UGT family members could greatly influence the efficacy of antihormonal therapy in inhibiting breast cancer growth or progression.
Potential impacts of AHR on otherestrogen-responsive tissues
In addition to breast cancer cell lines, we have found that 4OHT activates AHR in cell lines derived from tissues such as the liver, endometrium, and bone (data not shown). It is interesting to speculate on the role(s) that 4OHT-activated AHR may play in the bone and the endometrium, two tissues in which AHR plays important but unspecified role(s). Although many SERMs display ER agonist activity in the bone, leading to preservation of bone mineral density under estrogen-deprived conditions, differences in the efficacy among this class of drugs have suggested that their mechanism of action may not be identical. In addition to the well-established tissue-selective actions of SERMs, there are also site-selective activities within a tissue, and this is quite apparent in bone where SERM action leads to estrogen-equivalent activity at some sites but not so robustly at others (trabeculae vs. periosteum; vertebral vs. femoral, etc.). It is possible that some differences in SERM activity in the bone microenvironment stem from tissue-specific and site-specific modulation of ER signaling as well as from regulation of AHR. Despite potentially conflicting data, it is obvious that AHR plays a role in bone development and homeostasis; thus it will be important to define what portion, if any, of the actions of 4OHT, and perhaps other SERMs, in bone are mediated by AHR, and to determine whether this ER-independent effect is therapeutically desirable.
In the Breast Cancer Prevention Trial, premenopausal women taking TAM had an increased risk for leiomyomas, endometriosis, and ovarian cysts (62). TAM is an ER agonist in the endometrium and results in increased steroidogenesis in the ovary, thereby increasing local E2 production and leading to abnormal proliferation (63,64). Similar to the bone, the precise role of AHR in the endometrium is unclear, with some studies pointing to an inhibitory (65,66) and others to a promoting (67,68,69) role in endometriosis. Whereas clearly AHR is expressed in the endometrium and is ligand responsive, the role it plays in the pathology of endometriosis is not yet fully understood. Because TAM therapy is associated with increased risk for endometriosis, studies are warranted to define the potential role of AHR in mediating the actions of TAM and 4OHT in this disease.
The availability of an Ahr−/− mouse has made possible studies designed to further understand the role of AHR in development, homeostatic processes, and disease progression. Although viable, these mice exhibit fertility and mammary gland development defects (51,70), poor development of the immune system and the liver (71), and various pathologies of the skin, heart, uterus, and lung (72). Given this range of phenotypes, it appears certainly possible that cross talk between ER and AHR is a normal physiological process and that abnormalities in this regulatory axis may have pathological consequences. Regardless, it now seems prudent to consider AHR a bona fide target of TAM and 4OHT, although the role of this receptor in mediating the positive and negative effects of SERMs remains to be determined.
Materials and Methods
Biochemicals and plasmids
4OHT, α-naphthoflavone, β-naphthoflavone, DMSO, and real-time quantitative PCR (qPCR) primers were purchased from Sigma Chemical Co. (St. Louis, MO). Actinomycin D was purchased from Calbiochem (San Diego, CA). 3-Methoxy-4-nitroflavone was a gift from Dr. Stephen Safe (Texas A&M University, College Station, TX). pAHRE-TK-luc3 was a gift from Dr. Alvaro Puga (University of Cincinnati, Cincinnati, OH). pCDNA3.1nv5-AHR containing full-length human AHR was subcloned from pSPORT-AHR (a gift from Dr. Chris Bradfield, University of Wisconsin-Madison, Madison, WI) with KpnI and NotI. pCDNA3.1nv5-ARNT containing full-length human ARNT was subcloned from pSPORT-ARNT (a gift from Dr. Chris Bradfield, University of Wisconsin-Madison) with SalI and EcoRV (details available upon request).
Mammalian cell culture and transienttransfection assays
All cell lines were obtained from American Type Culture Collection (Manassa, VA). MCF7 cells were maintained in DMEM/F12 (Invitrogen, Carlsbad, CA) supplemented with 8% fetal bovine serum (FBS), 1 mm sodium pyruvate, and 0.1 mm nonessential amino acids. MDA-MB-231 cells were maintained in DMEM (Sigma) supplemented with 8% FBS, 1 mm sodium pyruvate, and 0.1 mm nonessential amino acids. SKBR3 cells were maintained in RPMI 1640 (Sigma) supplemented with 8% FBS, 1 mm sodium pyruvate, and 0.1 mm nonessential amino acids. RAW264.7 cells were maintained in DMEM containing 8% FBS. All cell lines were propagated in a 37 C incubator with 5% CO2.
For transient transfections, SKBR3 cells were plated in phenol red-free media containing 8% charcoal-stripped serum (Hyclone Laboratories, Inc., Logan, UT), 1 mm sodium pyruvate, and 0.1 mm nonessential amino acids 24 h before transfection with pCMV-βgal and AHRE-TK-luc3 by Lipofectin (Invitrogen)-mediated transfection as described previously in detail (73). Ligands were added 24 h after transfection and cells were assayed after overnight treatment. Luminescence and β-galactosidase activity were measured on a Fusion luminometer (PerkinElmer, Waltham, MA). Results are expressed as average luciferase activity (normalized to β-galactosidase for transfection efficiency) ± sem relative to vehicle-treated samples for three independent experiments performed in triplicate.
Western blotting
SKBR3 or MCF7 cells were plated in phenol red-free media containing 8% charcoal-stripped serum, 1 mm sodium pyruvate, and 0.1 mm nonessential amino acids. For siRNA experiments, cells were plated in the presence of 40 nm siAHR, siERα, or siRNA control (Stealth siRNA, Invitrogen) using DharmaFECT-1 (Dharmacon, Lafayette, CO) as a transfection reagent. For all experiments, cells were treated 48 h after plating with the indicated ligand for 4 h, followed by preparation of whole-cell extracts. Proteins were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and then detected using the following antibodies from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA): ERα (D12), AHR, Cytokeratin 18 (DC-10), and glyceraldehyde-3-phosphate dehydrogenase.
RNA isolation and quantitative PCR
For studies with MCF7, SKBR3, and MDA-MB-231, cells were seeded in phenol red-free media containing 8% charcoal-stripped serum, 1 mm sodium pyruvate, and 0.1 mm nonessential amino acids. After 48 h, cells were treated for the indicated time with the appropriate ligand(s). The media were not changed at the time of ligand treatment to avoid activation of AHR by tryptophan derivatives in culture media (74). For studies with RAW264.7, cells were seeded and grown in differentiating media [αMEM with 8% FBS and supplemented with 30 ng/ml RANKL (R&D Systems, Minneapolis, MN) and 20 ng/ml M-CSF (R&D Systems)] for 8 d, and then treated for 6 h with the indicated ligand. For all studies, cells were harvested and total RNA was isolated using the Aurum Total RNA Mini Kit (Bio-Rad Laboratories, Inc., Hercules, CA). RNA (1 μg) was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad). The Bio-Rad iCycler Realtime PCR System was used to amplify and quantitate levels of target gene cDNA. qPCR reactions were performed with 1 μl cDNA, 10 μm specific primers, and iQ SYBRGreen supermix (Bio-Rad). Data are normalized to the 36B4 (human) or mActinB (mouse) housekeeping gene and presented as fold induction over vehicle. Data are the mean ± sem for triplicate amplification reactions from one representative experiment. Each experiment was repeated at least three independent times with very similar results. Primers used are as follows (all human except as indicated). CYP1A1: forward (F), TGCAGAAGATGGTCAAGGAG; reverse (R), AGCTCCAAAGAGGTCCAAGA; CYP1A1 (mouse): F, ATTGTGCCTGCCTCCTACTT; R, AGGATCTGAGGTTCCTGTGG; CYP1B1: F, CTGGATTTGGAGAACGTACCG; R, TGATCCAATTCTGCCTGCAC; AHRR: F, AGGCTGCTGTTGGAGTCTCT; R, TTCTGGTGCATTACATCCGT; AHR: F, TCCACCTCAGTTGGCTTTGTTTGC; R, TCGTGCACAGCTCTGCTTCAGTAT; ERα: F, GAAAGGTGGGATACGAAAAGACC; R, GTTGGCAGCTCTCATGTCTCC; 36B4: F, GGACATGTTGCTGGCCAATAA; R, GGGCCCGAGACCAGTGTT; mActinB (mouse): F, CCTTCCTTCTTGGGTATGGA; R, TGGTACCACCAGACAGCACT.
For siRNA experiments, cells were plated in the presence of 40 nm siAHR, siERα, or siRNA control (Stealth siRNA, Invitrogen) using DharmaFECT-1 (Dharmacon) as a transfection reagent. After 48 h, cells were treated with the indicated ligand for 4 h, then harvested and assayed for RNA expression as detailed above.
Chromatin immunoprecipitation
Cells were grown to 90% confluence in 15-cm dishes in phenol red-free media supplemented with 8% charcoal-stripped serum, 1 mm sodium pyruvate, and 0.1 mm nonessential amino acids. Cells were then treated with vehicle, 100 nm BNF, or 100 nm 4OHT for 75 min. The remainder of the procedure has been described previously (75). The antibodies used are as follows: AHR SA-210 (BIOMOL Research Laboratories, Plymouth Meeting, PA), anti-acetyl histone H4 06-866 (Millipore, Billerica, MA), RNA polymerase II sc-899 (Santa Cruz Biotechnology). Primers are as follows. AHRE: F, CCTGGGCCCGAGTCTTTC; R, AGTGCTTTGATTGGCAGAGC; and Control: F, CAAGGTGGTGATGGAATGAG; R, TTAGCCTCCACCTAGGTTCC.
EMSA
In vitro transcription and translation was performed using the Promega TNT Quick Coupled Transcription/Translation T7 System per the manufacturer’s directions. Briefly, 40 μl TNT Quick Master Mix was combined with 3 μl PCR Enhancer solution, 5 μl plasmid DNA (25 ng pCDNA3.1nv5-AHR or 50 ng pCDNA3.1nv5-ARNT), and 2 μl 1 mm methionine. After 90 min at 30 C, AHR and ARNT were mixed 1:1, diluted 1:2 with 2× HEGD (1×: 25 mm HEPES, 1.5 mm EDTA, 10% glycerol, and 1 mm dithiothreitol), and allowed to incubate for 15 min at room temperature (RT) with vehicle, BNF, or 4OHT (∼33 μm each ligand). Protein-ligand mixtures were then incubated with binding buffer (0.2 μg/μl BSA, 2 mm dithiothreitol, 100 mm NaCl, and 0.02 μg/μl sheared salmon sperm DNA in HEG) for 15 min at RT. 32P-labeled oligo (for sequence, see Ref. 76) was then added to the mixture and allowed to incubate for 20 min at RT. Samples were resolved on a 5% nondenaturing acrylamide gel, dried, and analyzed by autoradiography.
OC differentiation assay
RAW264.7 cells were seeded in differentiating media [αMEM with 8% FBS and supplemented with 30 ng/ml RANKL (R&D Systems) and 20 ng/ml M-CSF (R&D Systems)] in a 48-well plate at 2000 cells per well along with ligands at the indicated concentrations. Media were changed and new ligands added every 2 d. After 8 d, cells were stained for TRAP (Sigma) according to the manufacturer’s instructions. TRAP-positive cells with at least three nuclei were counted. Data represent the average percent suppression from a representative experiment. For the independent data confirmation shown in supplemental Fig. 1E, the differentiation assay was performed as above with the following changes: RAW264.7 cells were cultured in 10% FBS supplemented with only RANKL and analyzed after 5 d.
Analysis of global gene expression
The list of 4OHT-regulated genes was generated by analyzing the gene expression data from MCF7 cells treated with 4OHT or vehicle for 8 h (12). The dataset (GSE848) was obtained from National Center for Biotechnology Information Gene Expression Omnibus. Change in gene expression was assessed as the difference of means of log2-transformed intensities for 4OHT- and vehicle-treated samples; raw values less than 1 were truncated to 1. Due to the small sample size (two replicates per condition), selection was performed based solely on fold change, with the cutoff set at ±3. Cross-referencing between HG-U95 and HG-133plus2.0 platforms was done through common Entrez identifiers. Expression data from MCF7 cells treated with TCDD or vehicle (DMSO) were log2-transformed and normalized by robust multichip average (RMA). Significantly regulated genes were identified by one-way ANOVA; family-wise error rate was controlled by Holm step-down procedure with α = 0.05. Hierarchical clustering of probe sets corresponding to 4OHT-regulated genes in TCDD/DMSO-treated samples was performed using Ward algorithm.
Supplementary Material
Acknowledgments
We thank Suzanne Wardell, Dave Gooden, and Eric Toone for their assistance. We also thank the members of the McDonnell laboratory for critical review of the manuscript.
Footnotes
This work was supported by the Department of Defense Breast Cancer Program Predoctoral Traineeship Award BC050609 (to C.D.D.) and National Institutes of Health Grants 5R37DK048807 (to D.P.M.) and DK-056059 (to J.W.P.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 9, 2009
Abbreviations: ActD, Actinomycin D; AHR, aryl hydrocarbon receptor; AHRE, AHR response element; AHRR, AHR repressor; ANF, α-naphthoflavone; ARNT, AHR nuclear translocator; BNF, β-naphthoflavone; DPN, diarylproprionitrile; E2, estradiol; ER, estrogen receptor; ICI, ICI 182,780; M-CSF, macrophage-colony-stimulating factor; MNF, 3-methoxy-4-nitroflavone; NR, nuclear receptor; OB, osteoblast; OC, osteoclast; 4OHT, 4-hydroxy-tamoxifen; qPCR, quantitative PCR; RAL, raloxifene; RANKL, receptor activator of nuclear factor κB ligand; SERM, selective ER modulator; siAHR, small interfering AHR; siER, small interfering ER; siRNA, small interfering RNA; TAM, tamoxifen; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TRAP, tartrate-resistant acid phosphatase; UGT, UDP-glucuronosyltransferase.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ 2008 Cancer statistics, 2008. CA Cancer J Clin 58:71–96 [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP 1999 The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140:5566–5578 [DOI] [PubMed] [Google Scholar]
- Howell A, Howell SJ, Clarke R, Anderson E 2001 Where do selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) now fit into breast cancer treatment algorithms? J Steroid Biochem Mol Biol 79:227–237 [DOI] [PubMed] [Google Scholar]
- Vogel VG 2009 The NSABP study of tamoxifen and raloxifene (STAR) trial. Expert Rev Anticancer Ther 9:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, O'Malley BW 2004 Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev 25:45–71 [DOI] [PubMed] [Google Scholar]
- Norris JD, Paige LA, Christensen DJ, Chang CY, Huacani MR, Fan D, Hamilton PT, Fowlkes DM, McDonnell DP 1999 Peptide antagonists of the human estrogen receptor. Science 285:744–746 [DOI] [PubMed] [Google Scholar]
- Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M 2000 Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843–852 [DOI] [PubMed] [Google Scholar]
- Gundimeda U, Chen ZH, Gopalakrishna R 1996 Tamoxifen modulates protein kinase C via oxidative stress in estrogen receptor-negative breast cancer cells. J Biol Chem 271:13504–13514 [DOI] [PubMed] [Google Scholar]
- Lopes MC, Vale MG, Carvalho AP 1990 Ca2(+)-dependent binding of tamoxifen to calmodulin isolated from bovine brain. Cancer Res 50:2753–2758 [PubMed] [Google Scholar]
- Eisen SF, Brown HA 2002 Selective estrogen receptor (ER) modulators differentially regulate phospholipase D catalytic activity in ER-negative breast cancer cells. Mol Pharmacol 62:911–920 [DOI] [PubMed] [Google Scholar]
- Coward P, Lee D, Hull MV, Lehmann JM 2001 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor γ. Proc Natl Acad Sci USA 98:8880–8884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasor J, Stossi F, Danes JM, Komm B, Lyttle CR, Katzenellenbogen BS 2004 Selective estrogen receptor modulators: discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res 64:1522–1533 [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Weiss J, Lee EJ, Pillai S, Ishikawa T, Ariazi EA, Jameson JL 2005 ERE-independent ERα target genes differentially expressed in human breast tumors. Mol Cell Endocrinol 245:53–59 [DOI] [PubMed] [Google Scholar]
- Itoh T, Karlsberg K, Kijima I, Yuan YC, Smith D, Ye J, Chen S 2005 Letrozole-, anastrozole-, and tamoxifen-responsive genes in MCF-7aro cells: a microarray approach. Mol Cancer Res 3:203–218 [DOI] [PubMed] [Google Scholar]
- Montano MM, Katzenellenbogen BS 1997 The quinone reductase gene: a unique estrogen receptor-regulated gene that is activated by antiestrogens. Proc Natl Acad Sci USA 94:2581–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison MS, Fisher JM, Whitlock Jr JP 1988 Inducible, receptor-dependent protein-DNA interactions at a dioxin-responsive transcriptional enhancer. Proc Natl Acad Sci USA 85:2528–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge CM, Kaur K, Swanson HI 2000 The aryl hydrocarbon receptor interacts with estrogen receptor α and orphan receptors COUP-TFI and ERRα1. Arch Biochem Biophys 373:163–174 [DOI] [PubMed] [Google Scholar]
- Wormke M, Stoner M, Saville B, Walker K, Abdelrahim M, Burghardt R, Safe S 2003 The aryl hydrocarbon receptor mediates degradation of estrogen receptor α through activation of proteasomes. Mol Cell Biol 23:1843–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockings JK, Thorne PA, Kemp MQ, Morgan SS, Selmin O, Romagnolo DF 2006 The ligand status of the aromatic hydrocarbon receptor modulates transcriptional activation of BRCA-1 promoter by estrogen. Cancer Res 66:2224–2232 [DOI] [PubMed] [Google Scholar]
- Khan S, Barhoumi R, Burghardt R, Liu S, Kim K, Safe S 2006 Molecular mechanism of inhibitory aryl hydrocarbon receptor-estrogen receptor/Sp1 cross talk in breast cancer cells. Mol Endocrinol 20:2199–2214 [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y, Nakajima M, Kyo S, Kanaya T, Inoue M, Yokoi T 2004 Human CYP1B1 is regulated by estradiol via estrogen receptor. Cancer Res 64:3119–3125 [DOI] [PubMed] [Google Scholar]
- MacCallum J, Cummings J, Dixon JM, Miller WR 2000 Concentrations of tamoxifen and its major metabolites in hormone responsive and resistant breast tumours. Br J Cancer 82:1629–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA 2003 Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95:1758–1764 [DOI] [PubMed] [Google Scholar]
- Cho YC, Zheng W, Jefcoate CR 2004 Disruption of cell-cell contact maximally but transiently activates AhR-mediated transcription in 10T1/2 fibroblasts. Toxicol Appl Pharmacol 199:220–238 [DOI] [PubMed] [Google Scholar]
- Ikuta T, Kobayashi Y, Kawajiri K 2004 Cell density regulates intracellular localization of aryl hydrocarbon receptor. J Biol Chem 279:19209–19216 [DOI] [PubMed] [Google Scholar]
- Sindhu RK, Reisz-Porszasz S, Hankinson O, Kikkawa Y 1996 Induction of cytochrome P4501A1 by photooxidized tryptophan in Hepa lclc7 cells. Biochem Pharmacol 52:1883–1893 [DOI] [PubMed] [Google Scholar]
- Ma Q, Baldwin KT 2000 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced degradation of aryl hydrocarbon receptor (AhR) by the ubiquitin-proteasome pathway. Role of the transcription activaton and DNA binding of AhR. J Biol Chem 275:8432–8438 [DOI] [PubMed] [Google Scholar]
- Lu YF, Santostefano M, Cunningham BD, Threadgill MD, Safe S 1995 Identification of 3′-methoxy-4′-nitroflavone as a pure aryl hydrocarbon (Ah) receptor antagonist and evidence for more than one form of the nuclear Ah receptor in MCF-7 human breast cancer cells. Arch Biochem Biophys 316:470–477 [DOI] [PubMed] [Google Scholar]
- Blank JA, Tucker AN, Sweatlock J, Gasiewicz TA, Luster MI 1987 α-Naphthoflavone antagonism of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced murine lymphocyte ethoxyresorufin-O-deethylase activity and immunosuppression. Mol Pharmacol 32:169–172 [PubMed] [Google Scholar]
- Merchant M, Arellano L, Safe S 1990 The mechanism of action of α-naphthoflavone as an inhibitor of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced CYP1A1 gene expression. Arch Biochem Biophys 281:84–89 [DOI] [PubMed] [Google Scholar]
- Swanson HI 2002 DNA binding and protein interactions of the AHR/ARNT heterodimer that facilitate gene activation. Chem Biol Interact 141:63–76 [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR 2003 Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 43:309–334 [DOI] [PubMed] [Google Scholar]
- Fukuda I, Mukai R, Kawase M, Yoshida K, Ashida H 2007 Interaction between the aryl hydrocarbon receptor and its antagonists, flavonoids. Biochem Biophys Res Commun 359:822–827 [DOI] [PubMed] [Google Scholar]
- Gradelet S, Astorg P, Pineau T, Canivenc MC, Siess MH, Leclerc J, Lesca P 1997 Ah receptor-dependent CYP1A induction by two carotenoids, canthaxanthin and β-apo-8′-carotenal, with no affinity for the TCDD binding site. Biochem Pharmacol 54:307–315 [DOI] [PubMed] [Google Scholar]
- Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, Desta Z, Flockhart DA, Skaar TC 2004 Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 85:151–159 [DOI] [PubMed] [Google Scholar]
- Lien EA, Solheim E, Kvinnsland S, Ueland PM 1988 Identification of 4-hydroxy-N-desmethyltamoxifen as a metabolite of tamoxifen in human bile. Cancer Res 48:2304–2308 [PubMed] [Google Scholar]
- Lim YC, Desta Z, Flockhart DA, Skaar TC 2005 Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol 55:471–478 [DOI] [PubMed] [Google Scholar]
- Furr BJ, Jordan VC 1984 The pharmacology and clinical uses of tamoxifen. Pharmacol Ther 25:127–205 [DOI] [PubMed] [Google Scholar]
- Wu X, Hawse JR, Subramaniam M, Goetz MP, Ingle JN, Spelsberg TC 2009 The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor α for degradation in breast cancer cells. Cancer Res 69:1722–1727 [DOI] [PubMed] [Google Scholar]
- Borges S, Desta Z, Li L, Skaar TC, Ward BA, Nguyen A, Jin Y, Storniolo AM, Nikoloff DM, Wu L, Hillman G, Hayes DF, Stearns V, Flockhart DA 2006 Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther 80:61–74 [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA 2001 Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 44:4230–4251 [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ 2005 Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology 146:797–807 [DOI] [PubMed] [Google Scholar]
- Yu HP, Shimizu T, Choudhry MA, Hsieh YC, Suzuki T, Bland KI, Chaudry IH 2006 Mechanism of cardioprotection following trauma-hemorrhagic shock by a selective estrogen receptor-β agonist: up-regulation of cardiac heat shock factor-1 and heat shock proteins. J Mol Cell Cardiol 40:185–194 [DOI] [PubMed] [Google Scholar]
- Lewis DK, Johnson AB, Stohlgren S, Harms A, Sohrabji F 2008 Effects of estrogen receptor agonists on regulation of the inflammatory response in astrocytes from young adult and middle-aged female rats. J Neuroimmunol 195:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RL, Morgan G, Dalley D, Kelly PJ 1993 Tamoxifen reduces bone turnover and prevents lumbar spine and proximal femoral bone loss in early postmenopausal women. Bone Miner 22:87–94 [DOI] [PubMed] [Google Scholar]
- Shevde NK, Bendixen AC, Dienger KM, Pike JW 2000 Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci USA 97:7829–7834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL 2004 Postmenopausal osteoporosis, T cells, and immune dysfunction. Proc Natl Acad Sci USA 101:16711–16712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronov I, Heersche JN, Casper RF, Tenenbaum HC, Manolson MF 2005 Inhibition of osteoclast differentiation by polycyclic aryl hydrocarbons is dependent on cell density and RANKL concentration. Biochem Pharmacol 70:300–307 [DOI] [PubMed] [Google Scholar]
- Naruse M, Ishihara Y, Miyagawa-Tomita S, Koyama A, Hagiwara H 2002 3-Methylcholanthrene, which binds to the arylhydrocarbon receptor, inhibits proliferation and differentiation of osteoblasts in vitro and ossification in vivo. Endocrinology 143:3575–3581 [DOI] [PubMed] [Google Scholar]
- Ryan EP, Holz JD, Mulcahey M, Sheu TJ, Gasiewicz TA, Puzas JE 2007 Environmental toxicants may modulate osteoblast differentiation by a mechanism involving the aryl hydrocarbon receptor. J Bone Miner Res 22:1571–1580 [DOI] [PubMed] [Google Scholar]
- Hushka LJ, Williams JS, Greenlee WF 1998 Characterization of 2,3,7,8-tetrachlorodibenzofuran-dependent suppression and AH receptor pathway gene expression in the developing mouse mammary gland. Toxicol Appl Pharmacol 152:200–210 [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y, Nakajima M, Yokoi T 2005 Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett 227:115–124 [DOI] [PubMed] [Google Scholar]
- Mooberry SL 2003 New insights into 2-methoxyestradiol, a promising antiangiogenic and antitumor agent. Curr Opin Oncol 15:425–430 [DOI] [PubMed] [Google Scholar]
- Zahid M, Kohli E, Saeed M, Rogan E, Cavalieri E 2006 The greater reactivity of estradiol-3,4-quinone vs estradiol-2,3-quinone with DNA in the formation of depurinating adducts: implications for tumor-initiating activity. Chem Res Toxicol 19:164–172 [DOI] [PubMed] [Google Scholar]
- Spink DC, Spink BC, Cao JQ, DePasquale JA, Pentecost BT, Fasco MJ, Li Y, Sutter TR 1998 Differential expression of CYP1A1 and CYP1B1 in human breast epithelial cells and breast tumor cells. Carcinogenesis 19:291–298 [DOI] [PubMed] [Google Scholar]
- Robertson JF, Come SE, Jones SE, Beex L, Kaufmann M, Makris A, Nortier JW, Possinger K, Rutqvist LE 2005 Endocrine treatment options for advanced breast cancer–the role of fulvestrant. Eur J Cancer 41:346–356 [DOI] [PubMed] [Google Scholar]
- Chouinard S, Tessier M, Vernouillet G, Gauthier S, Labrie F, Barbier O, Bélanger A 2006 Inactivation of the pure antiestrogen fulvestrant and other synthetic estrogen molecules by UDP-glucuronosyltransferase 1A enzymes expressed in breast tissue. Mol Pharmacol 69:908–920 [DOI] [PubMed] [Google Scholar]
- Erichsen TJ, Ehmer U, Kalthoff S, Lankisch TO, Müller TM, Munzel PA, Manns MP, Strassburg CP 2008 Genetic variability of aryl hydrocarbon receptor (AhR)-mediated regulation of the human UDP glucuronosyltransferase (UGT) 1A4 gene. Toxicol Appl Pharmacol 230:252–260 [DOI] [PubMed] [Google Scholar]
- Sun D, Sharma AK, Dellinger RW, Blevins-Primeau AS, Balliet RM, Chen G, Boyiri T, Amin S, Lazarus P 2007 Glucuronidation of active tamoxifen metabolites by the human UDP glucuronosyltransferases. Drug Metab Dispos 35:2006–2014 [DOI] [PubMed] [Google Scholar]
- Cheung C, Gonzalez FJ 2008 Humanized mouse lines and their application for prediction of human drug metabolism and toxicological risk assessment. J Pharmacol Exp Ther 327:288–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KT, Aylward LL 2006 Human response to dioxin: aryl hydrocarbon receptor (AhR) molecular structure, function, and dose-response data for enzyme induction indicate an impaired human AhR. J Toxicol Environ Health B Crit Rev 9:147–171 [DOI] [PubMed] [Google Scholar]
- Chalas E, Costantino JP, Wickerham DL, Wolmark N, Lewis GC, Bergman C, Runowicz CD 2005 Benign gynecologic conditions among participants in the Breast Cancer Prevention Trial. Am J Obstet Gynecol 192:1230–1237; discussion 1237–1239 [DOI] [PubMed] [Google Scholar]
- Cohen I, Figer A, Tepper R, Shapira J, Altaras MM, Yigael D, Beyth Y 1999 Ovarian overstimulation and cystic formation in premenopausal tamoxifen exposure: comparison between tamoxifen-treated and nontreated breast cancer patients. Gynecol Oncol 72:202–207 [DOI] [PubMed] [Google Scholar]
- Gielen SC, Santegoets LA, Hanifi-Moghaddam P, Burger CW, Blok LJ 2008 Signaling by estrogens and tamoxifen in the human endometrium. J Steroid Biochem Mol Biol 109:219–223 [DOI] [PubMed] [Google Scholar]
- Collins NH, Lessey EC, Dusell CD, McDonnell DP, Fowler L, Palomino WA, Illera MJ, Yu X, Mo B, Houwing A, Lessey BA 2009 Characterization of antiestrogenic activity of the Chinese Herb, Prunella vulgaris, using in vitro and in vivo (mouse xenograft) models. Biol Reprod [Erratum (2009) 80:1306] 80:375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M, Khan KN, Fujishita A, Masuzaki H, Ishimaru T 2004 Histomorphometric alteration and cell-type specific modulation of arylhydrocarbon receptor and estrogen receptor expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin and 17β-estradiol in mouse experimental model of endometriosis. Reprod Toxicol 18:793–801 [DOI] [PubMed] [Google Scholar]
- Heilier JF, Nackers F, Verougstraete V, Tonglet R, Lison D, Donnez J 2005 Increased dioxin-like compounds in the serum of women with peritoneal endometriosis and deep endometriotic (adenomyotic) nodules. Fertil Steril 84:305–312 [DOI] [PubMed] [Google Scholar]
- Nayyar T, Bruner-Tran KL, Piestrzeniewicz-Ulanska D, Osteen KG 2007 Developmental exposure of mice to TCDD elicits a similar uterine phenotype in adult animals as observed in women with endometriosis. Reprod Toxicol 23:326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niskar AS, Needham LL, Rubin C, Turner WE, Martin CA, Patterson Jr DG, Hasty L, Wong LY, Marcus M 2009 Serum dioxins, polychlorinated biphenyls, and endometriosis: a case-control study in Atlanta. Chemosphere 74:944–949 [DOI] [PubMed] [Google Scholar]
- Abbott BD, Schmid JE, Pitt JA, Buckalew AR, Wood CR, Held GA, Diliberto JJ 1999 Adverse reproductive outcomes in the transgenic Ah receptor-deficient mouse. Toxicol Appl Pharmacol 155:62–70 [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ 1995 Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 268:722–726 [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Fernandez-Salguero P 1998 The aryl hydrocarbon receptor: studies using the AHR-null mice. Drug Metab Dispos 26:1194–1198 [PubMed] [Google Scholar]
- Hall JM, Chang CY, McDonnell DP 2000 Development of peptide antagonists that target estrogen receptor β-coactivator interactions. Mol Endocrinol 14:2010–2023 [DOI] [PubMed] [Google Scholar]
- Oberg M, Bergander L, Håkansson H, Rannug U, Rannug A 2005 Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol Sci 85:935–943 [DOI] [PubMed] [Google Scholar]
- DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP 2008 27-Hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol 22:65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WK, Chu R, Jain S, Reddy JK, Bradfield CA 1994 Baculovirus expression of the Ah receptor and Ah receptor nuclear translocater. Evidence for additional dioxin responsive element-binding species and factors required for signaling. J Biol Chem 269:26464–26471 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.