Abstract
Inherited mutations of the breast cancer susceptibility gene BRCA1 confer a high risk for breast cancer development. The 300RXKK and 266KXK motifs have been identified previously as sites for acetylation of the estrogen receptor-α (ER-α), and 302K was also found to be a site for BRCA1-mediated mono-ubiquitination of ER-α in vitro. Here we show that ER-α proteins with single or double lysine mutations of these motifs (including K303R, a cancer-associated mutant) are resistant to inhibition by BRCA1, even though the mutant ER-α proteins retain the ability to bind to BRCA1. We also found that BRCA1 overexpression reduced and knockdown increased the level of acetylated wild-type ER-α, without changing the total ER-α protein level. Increased acetylation of ER-α due to BRCA1 small interfering RNA was dependent upon phosphatidylinositol 3-kinase/Akt signaling and on up-regulation of the coactivator p300. In addition, using an in vitro acetylation assay, we found that in vitro-translated wild-type BRCA1 but not a cancer-associated point mutant (C61G) inhibited p300-mediated acetylation of ER-α. Furthermore, BRCA1 overexpression increased the levels of mono-ubiquitinated ER-α protein, and a BRCA1 mutant that is defective for ubiquitin ligase activity but retains other BRCA1 functions (I26A) did not ubiquitinate ER-α or repress its activity in vivo. Finally, ER-α proteins with mutations of the 300RXKK or 266KXK motifs showed modest or no BRCA1-induced ubiquitination. We propose a model in which BRCA1 represses ER-α activity, in part, by regulating the relative degree of acetylation vs. ubiquitination of ER-α.
In this report, we show that BRCA1 inhibits estrogen receptor activity, in part, by inhibiting its acetylation and increasing its ubiquitination.
Recently, it has been recognized that in addition to phosphorylation and other posttranslational modifications, acetylation of the estrogen receptor-α (ER-α) is a potentially important physiological mechanism to control its activity. In this regard, it was reported that an ER-α mutation that is found in a subset of human mammary hyperplasias and cancer (A908G, which codes for the K303R point mutation) results in increased sensitivity of the receptor to low doses of 17β-estradiol (E2) (1). This mutation, which occurs within the hinge region of ER-α, affects a conserved acetylation motif of ER-α (300RXKK) that is acetylated by the transcriptional coactivator p300 both in vivo and in vitro (2). Mutations of the lysine residues of this motif conferred increased transcriptional activity to ER-α at low concentrations of E2. The incidence of the A908G mutation in breast cancer is unclear. Although several studies have failed to detect this mutation in invasive breast cancers (3,4,5); others have reported a low incidence of the mutation (6%) (6,7) and a high incidence (50%) (8). In the latter study, the mutation was found to be associated with biologically aggressive breast cancers in a univariate analysis (8).
Two additional conserved lysine residues of ER-α (K266 and K268) have also been identified as sites for in vivo acetylation by p300 (9). In that study, a lysine to glutamine substitution at these sites (K266/268Q) conferred increased DNA binding and increased transcriptional activity, again suggesting that acetylation can modulate ER-α function. ER-α has also been found to be a target for sumoylation within its hinge region, both in vitro and in vivo (10). The main sites of sumoylation appeared to be K266 and K268, and SUMO modification had the effect of increasing ER-α activity. Several previous studies indicate that ER-α can be the target of poly-ubiquitination and consequent degradation (11,12).
The breast and ovarian cancer susceptibility gene 1 (BRCA1) was identified based on its linkage to hereditary early-onset breast-ovarian cancer families (13). Subsequent studies have established that BRCA1 expression is absent or decreased in about 30–40% of sporadic breast cancers (14,15,16,17), suggesting that it may contribute to the pathogenesis of the much larger group of nonhereditary cancers. BRCA1 is a multifunctional protein, which can participate in a variety of molecular pathways, including those involved in DNA repair and recombination, cell cycle checkpoints, apoptosis, and transcriptional regulation (18,19,20). Of interest here are the findings that BRCA1 is a potent inhibitor of ER-α activity, in part, due to a direct interaction between the BRCA1 and ER-α proteins (21,22,23,24). In contrast, breast cancer-associated mutant BRCA1 proteins fail to inhibit ER-α activity (22,23). The absence of BRCA1 or knockdown of the endogenous BRCA1 protein leads to ligand-independent activation of ER-α and increased activity of the liganded receptor (25,26,27). In mouse models, a mammary-targeted deletion of the full-length Brca1 isoform conferred hypersensitivity to E2, and increased E2 signaling accelerated the development of mammary hyperplasias, preneoplastic mammary lesions, and adenocarcinomas (28,29). These findings suggest that the ability of BRCA1 to regulate ER-α activity is physiologically important.
Interestingly, the N terminus of BRCA1 in complex with another RING domain protein, BARD1 (BRCA1-associated RING domain 1), mediates an E3 ubiquitin ligase function (30,31), the physiological significance of which is unclear at present. In a recent study, it was found that ER-α is a potential substrate for the BRCA1/BARD1 ubiquitin ligase activity, based on in vitro ubiquitination assays (32). ER-α was found to be mono-ubiquitinated on K302, although the possibility of other ubiquitination sites was not ruled out. It is of importance that BRCA1/BARD1 caused only mono-ubiquitination of ER-α, which does not result in the targeting of ER-α for degradation. However, the physiological consequences of the mono-ubiquitination of ER-α due to BRCA1 were not described.
In this report, we studied the interaction of BRCA1 signaling and ER-α posttranslational modification. Our findings suggest that BRCA1 regulates acetylation vs. ubiquitination of ER-α in a reciprocal manner, and studies with mutant BRCA1 and ER-α proteins suggest that the ability of BRCA1 to cause posttranslational modification of ER-α activity determines its ability to repress ER-α activity.
Results
Mutations of the ER-α acetylation motif 300RXKK confer resistance to BRCA1
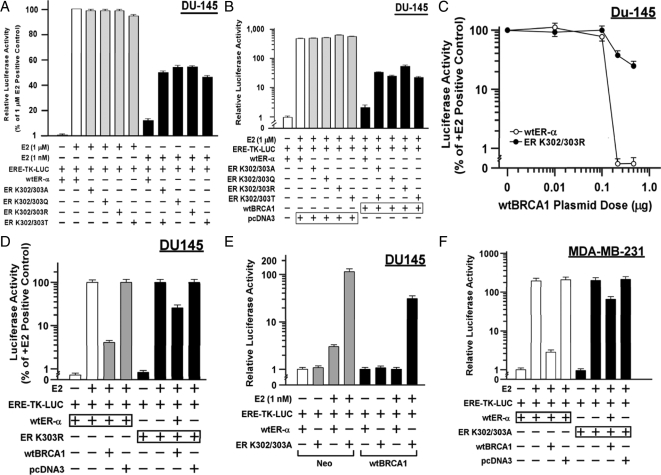
We studied four double lysine mutations of the ER-α acetylation motif: K302/303A, K302/303Q, K302/303R, and K302/303T. For these studies, we used DU-145 human prostate carcinoma cells, which are ER-α negative. The cells were transiently transfected with different ER-α expression vectors along with the estrogen-responsive reporter plasmid ERE-TK-Luc and then stimulated with very high (1 μm) and moderately low (1 nm) concentrations of E2 for 24 h before harvesting for luciferase assays. We found that at the very high E2 concentration, E2-stimulated ER-α activity levels were similar in each of the mutant receptors, as compared with the wild-type (wt) ER-α (Fig. 1A). However, at the low concentration, each mutant receptor was much more active than wt-ER-α (P < 0.001, two-tailed t tests).
Figure 1.
ER-α acetylation-site mutations confer resistance to repression by BRCA1. A, Subconfluent proliferating DU-145 cells were transfected overnight with the wt-ER-α or the indicated mutant vector and with the ERE-TK-Luc reporter (0.25 μg each vector per 2-cm2 well), washed, allowed to recover for several hours in fresh medium, and treated with either 1 μm or 1 nm E2 for 24 h. The cells were then harvested for luciferase assays. Luciferase activity values are expressed as a percentage of the 1 μm E2 positive control and are means ± sem of four replicate wells. B, Assays were performed as described for A, except that the cells were additionally cotransfected with 0.25 μg of either wtBRCA1 or empty pcDNA3 vector, as indicated. Here, luciferase values are expressed relative to the negative control (no E2, +wt-ER-α). C , wtBRCA1 plasmid dose-response curves for inhibition of activity of wt-ER-α vs. acetylation-site mutant ER-K302/303A. The E2 concentration was 1 μm, and the luciferase values are expressed as a percentage of the +E2 wt-ER-α value in the absence of wtBRCA1 vector. The total content of transfected DNA was equalized by the addition of empty pcDNA3 vector. D, Assays were performed as in B to test the ability of wtBRCA1 to inhibit wt-ER-α vs. acetylation-site mutant ER-K303R. The E2 concentration was 1 μm, and luciferase values are expressed as a percentage of the +E2 positive control. E, Effects of a low concentration of E2 (1 nm) on wt-ER-α or ER-K302/303A activity in DU-145 clones stably expressing wtBRCA1 or empty vector (Neo). Luciferase values are expressed relative to the negative control (0 E2, wt-ER-α). F, Ability of wtBRCA1 to inhibit the activity of wt-ER-α vs. ER-K302/303A in MDA-MB-231 cells. The E2 concentration was 1 μm. Values are expressed relative to the negative control (0 E2, wt-ER-α).
Next, we tested the mutant receptors for inhibition of ligand-stimulated activity (1 μm E2) by BRCA1 by cotransfecting a wtBRCA1 expression plasmid (or empty pcDNA3 vector). BRCA1 overexpression caused a reduction of wt-ER-α activity to less than 1% of that observed in the absence of wtBRCA1 (P < 0.001); whereas the mutant receptors retained 10- to 25-fold higher activity than did wt-ER-α (P < 0.001) (Fig. 1B). In these studies, BRCA1 overexpression was still able to remove most of the E2-stimulated ER-α activity, but the percentage of activity retained by the mutant receptors was greater than that retained by wt-ER-α. Figure 1C shows a wtBRCA1 plasmid dose response for E2-stimulated ER-α activity in which one of the mutant ERs (K302/303R) was compared with wt-ER-α. The wt-ER-α activity was reduced to near the baseline observed in the absence of E2 by a wtBRCA1 plasmid dose of 0.25 μg. At the same dose of wtBRCA1, the K302/303R mutant receptor still retained significant activity, which was only modestly diminished by 0.5 μg wtBRCA1.
Because the single mutant ER-α (K303R) has been observed in breast hyperplasias and cancers (1), we tested this mutant for sensitivity to BRCA1. As in the studies of the double mutants, K303R was significantly more resistant to repression by wtBRCA1 than was wt-ER-α (P < 0.001) (Fig. 1D). Next, we tested the sensitivity of a mutant receptor (K302/303A) vs. wt-ER-α to a low concentration of E2 (1 nm) in a DU-145 cell clone stably expressing wtBRCA1, as compared with a control (Neo) cell clone (33). For the Neo clone, E2 caused a 3-fold stimulation of wt-ER-α but a higher stimulation (122-fold) of K302/303A activity (P < 0.001) (Fig. 1E). For the wtBRCA1 clone, E2 caused no activation of wt-ER-α but still gave significant activation of the mutant receptor (66-fold). These findings suggest that stably overexpressed BRCA1 is also less able to repress the activity of acetylation mutant ER-α than wt-ER-α.
Finally, because none of these studies were carried out in breast cancer cells, we tested the effect of wtBRCA1 on the E2-stimulated K302/303A activity in a breast carcinoma cell line that lacks endogenous ER-α (MDA-MB-231). As in DU-145, wt-ER-α and ER-K302/303A gave roughly equal stimulation by a very high concentration of E2 (1 μm) in MDA-MB-231 cells, and K302/303A was much more resistant to repression by wtBRCA1 as compared with wt-ER-α in MDA-MB-231 cells (P < 0.001) (Fig. 1F). These data suggest that acetylation mutants are more active than wild-type ER-α at low concentrations of E2 but not at high concentrations of E2, and the mutant receptors are more resistant to repression by BRCA1 at high or low concentrations of E2. Although the acetylation-site mutant receptors are sensitive to low concentrations of E2, we found no evidence that they are active in the absence of E2.
The four double lysine mutant receptors were well expressed in DU-145 cells, and the expression levels were no higher than that of wt-ER-α (supplemental Fig. 1A, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). In addition, the expression of the exogenous receptors in DU-145 cells was comparable to that of endogenous ER-α in MCF-7 cells. Because repression of ER-α is mediated by physical association of BRCA1 and ER-α (22,23), we tested whether the resistance of the mutant receptors to BRCA1 is due to a loss of their ability to associate with BRCA1. DU-145 cells were transfected with mutant receptors, and immunoprecipitation-Western blotting was carried out. All of the mutant receptors coprecipitated with endogenous BRCA1, as did the wt-ER-α (supplemental Fig. 1B). As negative controls, no BRCA1 or ER-α was detected in immunoprecipitates using normal (nonimmune) IgG. These findings suggest that mutations of the 300RXKK motif do not prevent association of ER-α with BRCA1, although we cannot rule out subtle changes in the interaction.
BRCA1 knockdown further activates 300RXKK site mutant ER-α proteins
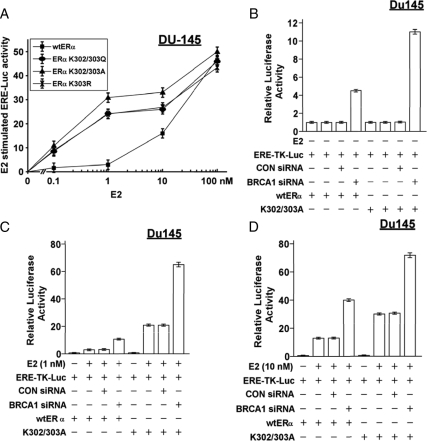
Previously, we showed that BRCA1 knockdown causes ligand-independent activation of ER-α (i.e. an increase in ERE-TK-Luc activity relative to the basal activity in the absence of E2) and further activates ER-α in the presence of E2 (25,26). Here, we tested whether BRCA1 small interfering RNA (siRNA) could enhance the activity of the mutant receptors, which are known to be hypersensitive to low concentrations of E2 (1,2,34). This would be true if the mechanisms by which BRCA1 siRNA and acetylation mutation enhance ER-α activity are not identical. Three different 300RXKK site mutants (K302/303A, K302/303Q, and K303R) were more active than wt-ER-α in DU-145 cells at lower concentrations of E2 (0.1–10 nm), whereas the receptors showed similar activity at 100 nm E2 (Fig. 2A). BRCA1 siRNA stimulated the activity of the unliganded K302/303A receptor by 11-fold, compared with 4.4-fold for the unliganded wt-ER-α (P < 0.001) (Fig. 2B).
Figure 2.
Effect of BRCA1 knockdown on activity of acetylation-site mutant ER-α proteins. A, DU-145 cells were transfected with the indicated ER-α expression vector and the ERE-TK-Luc reporter, exposed to the indicated concentration of E2 for 24 h, and harvested for luciferase assays. B–D, DU-145 cells were treated with BRCA1 siRNA (50 nm), control (CON) siRNA (50 nm), or no siRNA (vehicle only) for 48 h; transfected with the indicated ER-α vector and the ERE-TK-Luc reporter overnight; exposed to 0 E2 (B), 1 nm E2 (C), or 10 nm of E2 (D) for 24 h; and harvested for luciferase assays. In all panels, luciferase activity is expressed relative to control conditions (0 E2, wt-ER-α) as means ± sem of quadruplicate wells.
In Fig. 2C, as expected, at normal BRCA1 levels (i.e. in the presence of control siRNA or no siRNA), 1 nm E2 stimulated K302/303A activity to a greater extent (20-fold) than it stimulated wt-ER-α activity (3-fold, P < 0.001). BRCA1 siRNA further enhanced E2-stimulated activity of K302/303A (from 20- to 64-fold, P < 0.001) and wt-ER-α (from 3- to 10-fold, P < 0.001). Similarly, at 10 nm E2, BRCA1 siRNA enhanced the E2-stimulated activity of K302/303A (from 30- to 72-fold, P < 0.001) as well as wt-ER-α (from 12- to 40-fold, P < 0.001) (Fig. 2D). As a negative control, the control siRNA had little or no effect on ER-α activity. These findings suggest that 1) a 300RXKK mutant ER-α is hyperactivated by BRCA1 knockdown and that 2) the ligand-independent activity of the mutant ER-α due to loss of BRCA1 is greater than that for wt-ER-α.
BRCA1 regulates ER-α acetylation in MCF-7 cells
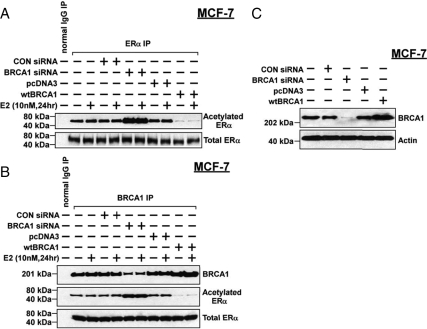
We next tested whether BRCA1 can regulate the acetylation state of ER-α in MCF-7 cells, which express endogenous wild-type ER-α. MCF-7 cells were pretreated with BRCA1 siRNA (vs. control siRNA, 50 nm for 48 h) or transiently transfected with wtBRCA1 (vs. empty pcDNA3 vector), treated with or without E2 (10 nm for 24 h), and harvested for immunoprecipitation of ER-α and Western blotting using antibodies against acetylated lysine or ER-α. E2 had little or no effect on ER-α acetylation under the different treatment conditions, nor did treatment with control siRNA or empty pcDNA3 vector (Fig. 3A). However, BRCA1 siRNA caused a significant increase in the levels of acetylated ER-α, whereas BRCA1 overexpression reduced the levels of acetylated ER-α. In contrast, none of the treatments had any obvious effect on total ER-α protein levels.
Figure 3.
BRCA1 regulates the levels of acetylated ER-α in MCF-7 cells. A, Subconfluent proliferating cells were treated with BRCA1 siRNA (50 nm), control (CON) siRNA (50 nm), or no siRNA (vehicle only). Alternatively, the cells were transfected overnight with wtBRCA1 or empty pcDNA3 vector, washed, and postincubated in fresh medium for 24 h. The cells were then treated with or without E2 (10 nm) for 24 h, after which they were immunoprecipitated using an anti-ER-α antibody and Western blotted using an antibody directed against acetyl-lysine and using an anti-ER-α antibody. A normal IgG immunoprecipitation (IP) was performed as a negative control. B, Assays were carried out as described for A, except that the immunoprecipitation was carried using an anti-BRCA1 antibody, and the precipitates were Western blotted using antibodies against BRCA1, acetyl-lysine, and ER-α. C, MCF-7 cells were treated with siRNA or transfected as described above and Western blotted to detect BRCA1 or actin (control for loading and transfer).
Figure 3B shows a similar experiment in which the immunoprecipitation was performed using an anti-BRCA1 antibody to test whether BRCA1 can associate with the acetylated ER-α. Although a number of different proteins can bind to BRCA1, we detected only one band at the expected relative molecular mass (Mr) of ER-α (66 kDa) when the BRCA1 immunoprecipitate was probed using anti-acetyl-lysine antibody, and presumably, this band represents acetylated ER-α. These results, which were validated in other experiments (see below), suggest that BRCA1 can bind to acetylated ER-α. Note that in cells treated with BRCA1 siRNA or wtBRCA1, the amount of acetylated ER-α bound to BRCA1 was increased or decreased, respectively, consistent with the effect of manipulating BRCA1 levels on total cellular levels of acetylated ER-α. Densitometric quantification of the effects of BRCA1 over- and underexpression on the levels of acetylated ER-α (corresponding to Fig. 3, A and B) is shown in supplemental Fig. 2. Figure 3C shows the effects of BRCA1 siRNA and wtBRCA1 transfection on the levels of BRCA1 protein in MCF-7 cells.
Increased ER-α acetylation due to BRCA1 siRNA requires phosphatidylinositol (PI) 3-kinase signaling
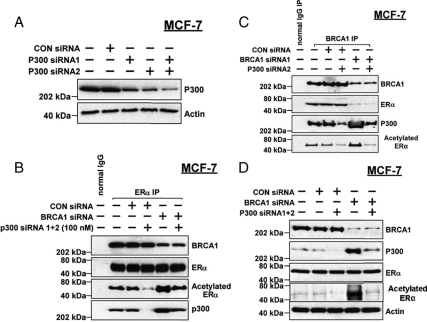
We investigated the signaling pathways for ER-α acetylation by the use of selective pharmacological inhibitors. The inhibitors were tested at concentrations reported to be active based on published literature, as described before (25). The inhibitors tested (pathways altered) were LY294002 (PI3-kinase), PD98059 (MAPK kinase-1/2), GF109203α (protein kinase C), SB202190 (p38), rapamycin (mammalian target of rapamycin), and PP1 (Src family kinases). As expected, BRCA1 siRNA (but not control siRNA) increased the levels of acetylated ER-α in MCF-7 cells as compared with vehicle-treated cells (Fig. 4A). Of the inhibitors tested, only LY294002 blocked the ability of BRCA1 siRNA to increase levels of acetylated ER-α. Neither LY294002 nor any other inhibitor appeared to alter total ER-α protein levels. Like LY294002, wortmannin, another selective inhibitor of PI3-kinase, blocked the increased ER-α acetylation due to BRCA1 siRNA (Fig. 4B), but neither inhibitor reduced ER-α acetylation to below the basal level in control-treated cells. Consistent with these findings, a dominant-negative (DN) p85 (regulatory subunit of PI3-kinase) expression vector also blocked the BRCA1 siRNA-stimulated acetylation of ER-α (data not shown).
Figure 4.
Effects of signaling inhibitors on ER-α acetylation due to BRCA1 knockdown. A, Subconfluent proliferating MCF-7 cells were treated with BRCA1 siRNA or control (CON) siRNA (50 nm for 48 h), incubated with the indicated inhibitor for 24 h, and harvested for immunoprecipitation-Western blotting to determine the levels of acetylated and total ER-α as in Fig. 3. B,Assays were performed as described for A, using the PI3-kinase inhibitor wortmannin. C, Assays were performed as described above, except that after treatment with siRNA, the cells were transfected overnight with DN-Akt or empty pcDNA3 vector before immunoprecipitation (IP)-Western blotting. D, Assays were performed as described for C, except that the cells were transfected with wtAkt rather than DN-Akt. E, Western blot showing the expression of the DN-Akt and wtAkt proteins after vector transfections. The inhibitors tested (concentrations) were as follows:LY294002 (100 μm), PD98059 (30 μm), GF109302X (30 μm), SB202190 (10 μm), rapamycin (10 ng/ml), PP1 (10 μm), and wortmannin (100 nm).
Because c-Akt is a major serine/threonine kinase in the PI3-kinase pathway that can phosphorylate ER-α and alter its activity (25,35), we tested whether Akt could modulate the ER-α acetylation state. Expression of a DN Akt protein that blocks signaling downstream of Akt also blocked the ability of BRCA1 siRNA to up-regulate levels of acetylated ER-α (Fig. 4C). On the other hand, overexpression of wtAkt did not further enhance the levels of acetylated ER-α beyond that due to BRCA1 siRNA (Fig. 4D). The expression of the wtAkt and DN-Akt proteins was validated by Western blotting (Fig. 4E). These findings suggest that the increased acetylation of ER-α caused by BRCA1 knockdown is mediated through a PI3-kinase/Akt-dependent signaling pathway.
Role of p300 in ER-α hyperacetylation due to BRCA1 knockdown
Because p300 was previously implicated in ER-α acetylation (2,34) and because we previously showed that BRCA1 overexpression inhibits p300 expression (33,36,37), we tested the role of p300 in BRCA1 regulation of ER-α acetylation. A combination of two different p300 siRNAs substantially reduced the protein levels of p300 in MCF-7 cells (Fig. 5A). To test the role of p300, MCF-7 cells were treated with control siRNA, BRCA1 siRNA, and/or p300 siRNA and harvested for immunoprecipitation of ER-α followed by Western blotting. p300 siRNA by itself had no effect on the content of BRCA1 or ER-α in the ER-α immunoprecipitate (Fig. 5B). However, p300 siRNA reduced the content of acetylated ER-α. BRCA1 siRNA alone increased the content of acetylated ER-α and of p300 associated with ER-α. p300 siRNA blocked the increase in acetylated ER-α due to BRCA1 siRNA. Similar results were obtained using immunoprecipitates of BRCA1 (Fig. 5C). Thus, p300 siRNA reduced the content of acetylated ER-α and of p300 associated with BRCA1 and blocked the increase in acetylated ER-α in BRCA1 siRNA-treated cells.
Figure 5.
Role of p300 in hyperacetylation of ER-α due to BRCA1 knockdown. A,MCF-7 cells were treated with the indicated siRNA (50 nm) for 48 h and then harvested to detect p300 and actin (loading control). B,Cells were pretreated with the indicated siRNA or combination of siRNAs for 48 h and harvested to assess ER-α acetylation by immunoprecipitation-Western blotting, as described in Fig. 3. C, Cells were treated as indicated with siRNAs and subjected to immunoprecipitation for BRCA1 and Western blotting for BRCA1, ER-α (total), p300, and acetyl-lysine. D, Cells were treated with siRNAs as indicated, and nonprecipitated cell lysates were Western blotted to detect BRCA1, p300, ER-α (total), acetyl-lysine, and actin. CON, Control.
A straight Western blot corresponding to the same five treatment conditions shows the efficacy of the BRCA1 and p300 knockdowns and a substantial increase in p300 protein levels due to BRCA1 siRNA (Fig. 5D). None of the treatments altered the total ER-α protein levels. As noted above, the anti-acetyl-lysine antibody detected a band of similar Mr to acetylated ER-α, but we cannot be certain that this represents acetylated ER-α, because ER-α may not be the only acetylated protein of that size in MCF-7 lysates. However, we note that BRCA1 siRNA caused a large increase in that band that was not seen in cells treated with BRCA1 siRNA plus p300 siRNA. These findings suggest that the increase in ER-α acetylation caused by BRCA1 knockdown is mediated, in part, via increased p300 levels.
BRCA1 inhibits in vitro acetylation of ER-α by p300
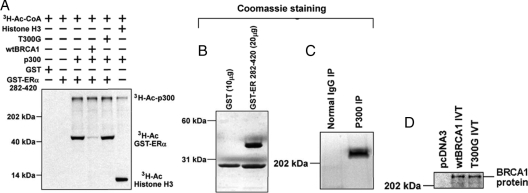
We used an in vitro acetylation assay (2) to test the effect of wtBRCA1 or a point mutant (T300G) on ER-α acetylation by p300. T300G is a breast cancer-associated mutation of the N-terminal RING domain of BRCA1 (C61G) that abolishes the ability of BRCA1 to repress ER-α activity (22). Here, we used a glutathione S-transferase (GST)-linked fragment of ER-α (amino acids 282-420), which contains the 300RXKK motif and the BRCA1-binding region (22,23). GST only was used as a negative control, and histone H3 was used as a positive control for the acetylation reaction. All four lanes containing p300 showed evidence of autoacetylation of p300, whereas the lane containing histone H3 also showed acetylation of that protein (Fig. 6A). Lanes with p300 and GST-ER-α showed acetylation of GST-ER-α that was blocked by addition of wtBRCA1 but not the T300G mutant. As a negative control, p300 failed to acetylate GST by itself. The expression of GST-ER 282-420, p300 (from a p300 immunoprecipitate), and in vitro-translated wtBRCA1 and T300G proteins is shown in Fig. 6, B–D. These findings suggest a direct mechanism for BRCA1 inhibition of ER-α acetylation by BRCA1.
Figure 6.
BRCA1 inhibits in vitro acetylation of ER-α by p300. A, In vitro acetylation assays were carried out as described in Materials and Methods. These assays test the ability of p300 (generated from a p300 immunoprecipitation) to acetylate a GST-ER-α (amino acids 282-420) chimeric protein using [3H]acetyl coenzyme A as the substrate. Assay reactions were carried out in the absence or presence of in vitro-translated (IVT) wtBRCA1 or mutant full-length BRCA1 (T300G). Histone H3 was used as a positive control substrate for acetylation by p300. Bands corresponding to autoacetylated p300 (3H-Ac-p300), acetylated GST-ER 282-420 (3H-Ac-GST-ERα), and acetylated histone (3H-Ac-Histone H3) are indicated. B–D, The GST-ER 282-420 (B) and immunoprecipitated (IP) p300 proteins (C) were analyzed by SDS-PAGE and detected by Coomassie staining; D Western blot showing the IVT wtBRCA1 and BRCA1-T300G proteins (0.5 μg). As a negative control, IVT was carried out using an empty pcDNA3 vector.
Effect of phosphorylation status on BRCA1 regulation of ER-α acetylation
To study the relationship of phosphorylation and acetylation, we tested expression vectors encoding ER-α mutant proteins with serine to alanine mutations of two key phosphorylation sites (S118 and S167) in the activation function-1 (AF-1) domain. In DU-145 cells transfected with wt-ER-α, wtBRCA1 reduced and BRCA1 siRNA increased the levels of acetylated wt-ER-α, as expected (supplemental Fig. 3A). Although the basal levels of acetylated ER-α were similar in cells transfected with ER-S118A, there was little or no increase in acetylation of ER-S118A due to BRCA1 siRNA. However, wtBRCA1 reduced the acetylation of ER-S118A. Similarly, BRCA1 siRNA had little or no effect on acetylation of ER-S167A, but wtBRCA1 reduced the acetylation of ER-S167A (supplemental Fig. 3B). Quantification of these experiments is provided in supplemental Fig. 3, C and D. These findings suggest that phosphorylation of ER-α may modulate the ability of endogenous BRCA1 to block ER-α acetylation (see Discussion).
Effect of acetylation-site mutations on BRCA1 regulation of ER-α acetylation
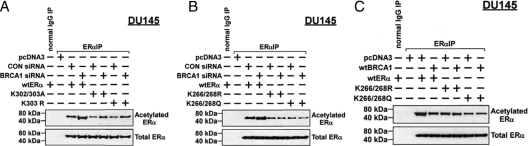
As noted above, two sites for acetylation have been identified within the hinge region of ER-α, 300RXKK, and K266/268. We tested the effect of mutations of one site on the ability of BRCA1 to regulate the residual ER-α acetylation (presumably that of the second site). As before, BRCA1 siRNA significantly stimulated acetylation of wt-ER-α. The basal levels of acetylation of ER-α mutants K302/303A and K303R were reduced relative to wt-ER-α (Fig. 7A). However, BRCA1 siRNA still caused an increase in acetylation of the two mutants. For K266/268R and K266/268Q, there was also a reduction in the basal levels of acetylation, compared with wt-ER-α (Fig. 7B). However, BRCA1 knockdown did not increase the acetylation of these mutant receptors. Although wtBRCA1 reduced the acetylation of wt-ER-α, it had little or no effect on acetylation of K266/268R or K266/268Q (Fig. 7C). Quantification of the experiments shown in Fig. 7, A–C, is provided in supplemental Fig. 4. These findings suggest that mutations of K266/268 but not K302/303 interfere with the ability of BRCA1 to regulate ER-α acetylation (see Discussion).
Figure 7.
Effect of acetylation-site mutations on BRCA1 regulation of ER-α acetylation. DU-145 cells were pretreated with the indicated siRNA (50 nm) for 48 h, transfected with wt-ER-α or mutant ER-α, and harvested for immunoprecipitation (IP)-Western blotting to determine the levels of acetylated ER-α and total ER-α. Alternatively, the cells were cotransfected overnight with wtBRCA1 or empty pcDNA3 vector and with wt-ER-α or mutant ER-α and then subjected to immunoprecipitation-Western blotting as above. The ER-α mutants tested were K302/303A and K303R (A) and K266/268R and K266/268Q (B and C). CON, Control.
Mutations of acetylation site K266/268 confer resistance to repression by BRCA1
DU-145 cells were cotransfected with the ERE-TK-Luc reporter and the indicated expression vectors, exposed to E2 (10 nm) for 24 h, and harvested for luciferase assays. For wt-ER-α, 10 nm E2 caused a 16-fold increase in reporter activity that was reduced to 3-fold (an 81% reduction) when the cells were cotransfected with wtBRCA1 (Fig. 8). The E2-stimulated ER-α activity was twice as high for the K266/268Q mutant (32-fold). However, wtBRCA1 reduced the E2-stimulated activity of K266/268Q to only 18-fold (a 44% reduction). Finally, for the K266/268R mutant, the E2-stimulated activity was similar to that of wt-ER-α, but wtBRCA1 reduced this activity by only 33%. These findings suggest that both K266/268Q and K266/268R are resistant to repression by wtBRCA1, as were the K302/303 mutants.
Figure 8.

Mutations of acetylation site K266/268 confer resistance to BRCA1-mediated repression. Subconfluent proliferating DU-145 cells were cotransfected overnight with the ERE-TK-Luc reporter and the indicated expression vectors, washed, allowed to recover for several hours, treated with or without E2 (10 nm) for 24 h, and harvested for luciferase assays. Luciferase values are expressed relative to control conditions (wt-ER-α, no E2) and are means ± sem of four replicate wells.
Regulation of ER-α ubiquitination by BRCA1
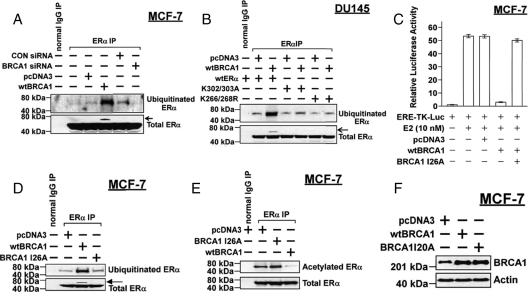
Because ubiquitination and acetylation are often competing processes and ER-α is an in vitro target for mono-ubiquitination by the BRCA1/BARD1 heterodimer (32), we tested the effect of BRCA1 on ubiquitination of ER-α using an antibody that detects mono-ubiquitin and poly-ubiquitin chains attached to proteins. MCF-7 cells were transfected with or without wtBRCA1 vector or treated with or without BRCA1 siRNA, subjected to immunoprecipitation using an anti-ER-α antibody, and Western blotted using anti-ubiquitin or anti-ER-α antibody. wtBRCA1 caused a large increase in the amount of ubiquitinated ER-α at a molecular weight consistent with mono-ubiquitination, and BRCA1 siRNA appeared to decrease levels of ubiquitinated ER-α, although the basal levels of ubiquitinated ER-α were already low (Fig. 9A). Based on our usual immunoprecipitation-Western blotting conditions, we did not see the slightly higher Mr ubiquitinated ER-α band corresponding to wtBRCA1-transfected cells in previously shown Western blots using anti-ER-α antibody, probably because the percentage of mono-ubiquitinated ER-α was small. However, when we immunoprecipitated 1000 μg cell protein rather than 500 μg, loaded all of the precipitated protein (rather than one half), and ran the gel longer to achieve greater separation of bands, a small band corresponding to ubiquitinated ER-α was visible (Fig. 9A, total ER-α Western blot, band indicated by arrow), indicating that the percentage of ER-α ubiquitination due to wtBRCA1 was small. Taken together with Fig. 3, these findings suggest that BRCA1 overexpression causes ubiquitination at the expense of acetylation, whereas BRCA1 underexpression does the opposite. We note here that treatment with E2 (10 nm for 24 h) had no effect on the levels of ubiquitinated ER-α without or with wtBRCA1 present (data not shown).
Figure 9.
BRCA1 regulates ubiquitination of ER-α in vivo. A, Subconfluent proliferating MCF-7 cells were transfected overnight with wtBRCA1 or empty pcDNA3 vector, washed, and postincubated for 24 h in fresh medium to allow gene expression. The cells were then harvested and subjected to immunoprecipitation (IP) for ER-α and Western blotting using antibodies directed against ubiquitin (monoubiquitin plus polyubiquitin chains) and against ER-α. B, DU-145 cells were transfected with the indicated vectors, harvested, and subjected to immunoprecipitation-Western blotting as in A. C, MCF-7 cells were transfected overnight with wtBRCA1, BRCA1 mutant I26A, or empty pcDNA3 vector plus the ERE-TK-Luc reporter; exposed to E2 (10 nm) for 24 h; and harvested for luciferase assays. Luciferase values are expressed relative to control conditions (0 E2, no transfection) as means ± sem of quadruplicate wells. D, MCF-7 cells were transfected with wtBRCA1, ubiquitination-defective point mutant BRCA1-I26A, or empty pcDNA3 vector and assayed for their effect on the levels of ubiquitinated ER-α as described for A. E, Assays were performed as in D, except that the cells were Western blotted with an anti-acetyl-lysine antibody rather and an anti-ubiquitin antibody. MCF-7 cells were transiently transfected as indicated and then assayed for ER-α acetylation by immunoprecipitation-Western blotting. F, Expression of wtBRCA1 and BRCA1-I26A in transfected MCF-7 cells, as demonstrated by Western blotting. Note that in A, B, and D, to detect the small mono-ubiquitinated ER-α band in the anti-ER-α Western blot (arrow), ER-α immunoprecipitations were carried out using twice the normal amount of cell protein (1000 μg instead of 500 μg), and all of the precipitated protein was subjected to Western blotting rather than half. In addition, the gel was run for a longer amount of time (3.5 h rather than 2 h) to further separate the bands. CON, Control.
We performed a similar study using DU-145 cells transfected with either wt-ER-α, K302/303A, or K266/268R. Here, the basal levels of ubiquitinated ER-α appear to be slightly reduced in the mutant receptors, and importantly, there was only a modest, if any, increase in ubiquitination due to BRCA1 overexpression (Fig. 9B). Densitometry corresponding to the experiments shown in Fig. 9, A and B, is provided in supplemental Fig. 5. The implications of these findings are considered in Discussion.
Previously, a point mutation of BRCA1 (I26A) was identified that disrupts the BRCA1/BARD1 ubiquitin ligase function but has little or no effect on several DNA damage-response-related functions of BRCA1, including its ability to mediate homology-directed DNA repair (38). We tested the ability of the BRCA1-I26A mutant protein to inhibit E2-stimulated ER-α activity in MCF-7 cells. As shown in Fig. 9C, wtBRCA1 strongly inhibited ER-α activity (from 53-fold to 3-fold, P < 0.001), but BRCA1-I26A caused only a modest reduction in activity (from 53-fold to 50-fold). In contrast to wtBRCA1, BRCA1-I26A had little or no effect on the levels of ubiquitinated ER-α in MCF-7 cells (Fig. 9D), and BRCA1-I26A did not reduce the levels of acetylated ER-α (Fig. 9E). In the experiment shown, there was an increase in ER-α acetylation in cells transfected with BRCA1-I26A as compared with pcDNA3 vector. Finally, we found that BRCA1 I26A was as well expressed as wtBRCA1 in MCF-7 cells (Fig. 9F). These findings suggest that the ubiquitination function of BRCA1 may be important for the repression of ER-α activity.
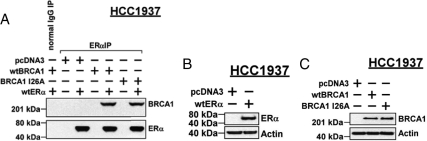
To test the ability of BRCA1-I26A to associate with ER-α, we used HCC1937 breast cancer cells, which are homozygous for a truncated mutant BRCA1 protein (5382insC). Because these cells are also ER-α negative, we transfected in a wt-ER-α expression vector. Immunoprecipitation-Western blotting revealed that in cells transfected with wtBRCA1 or BRCA1-I26A, immunoprecipitation of ER-α yielded about equal quantities of BRCA1 (Fig. 10A). Figure 10, B and C, provides Western blots showing expression of the ectopic proteins. Thus, the I26A mutation does not appear to impair BRCA1-ER-α association.
Figure 10.
BRCA1-I26A mutation does not abrogate BRCA1 association with ER-α in vivo. A, HCC1937 cells, which do not express wtBRCA1, were transfected as indicated and subjected to immunoprecipitation (IP) for ER-α and Western blotting for BRCA1 and ER-α. B and C, Western blots are provided to show the expression of exogenous ER-α (B) and BRCA1 (C) proteins.
Discussion
We showed that mutations in an acetylation motif (300RXKK) in the hinge region of ER-α (amino acids 263-315) render ER-α partially resistant to repression by BRCA1. The mutant ER-α proteins cannot be acetylated due to substitutions at one or both lysine residues. As in previous studies (1,2), the acetylation-site mutant proteins showed increased activity in the presence of low concentrations of E2 as compared with wild-type ER-α. The resistance of the mutant receptors to BRCA1 was not due to loss of BRCA1 binding, because BRCA1 associated with the mutant receptors in vivo. Point mutations of a second acetylation motif (K266/268) also rendered ER-α less sensitive to repression by BRCA1. Thus, ER-α mutants K266/268Q and K266/268R were significantly more resistant to BRCA1 than wild-type ER-α. As described earlier (9), the K266/268Q mutant (but not the K266/268R mutant) showed increased E2-stimulated ER-α activity as compared with wt-ER-α. These findings indicate that mutations on lysine residues of ER-α that are capable of being acetylated render ER-α resistant to repression by BRCA1.
In a previous study, ER-α proteins with mutations of several Akt-sensitive sites in the AF-1 domain (S118 or S167) showed reduced ligand-independent activation due to BRCA1 knockdown as compared with wild-type ER-α (25). In contrast, in the present study, we found that a mutation of the acetylation motif (K302/303A) conferred increased ligand-independent activation due to BRCA1 knockdown. Furthermore, the phosphorylation-site mutants showed less E2-stimulated activity in the setting of BRCA1 underexpression, whereas the acetylation-site mutants showed greater E2-stimulated activity as compared with wild-type ER-α. In the same previous study (25), we also showed that stimulation of ER-α activity due to BRCA1 siRNA was mediated, in part, by an Akt-dependent phosphorylation of serine-167 in the AF-1 domain of ER-α. Herein, our findings suggest that this mechanism remains intact in the acetylation-site mutant receptors.
We further showed that BRCA1 regulates the in vivo acetylation state of ER-α in MCF-7 cells. Thus, overexpression of BRCA1 caused deacetylation, whereas knockdown caused hyperacetylation of ER-α. Inhibition of PI3-kinase or Akt blocked the ability of BRCA1 siRNA to stimulate ER-α acetylation, suggesting that the increased acetylation of ER-α driven by the loss of BRCA1 is due, in part, to an Akt-dependent phosphorylation event. In this regard, it was reported that acetylation of ER-α was regulated, in part, by a protein kinase A-dependent phosphorylation site (S305), whereby phosphorylation at this site blocked acetylation at K303 of ER-α (34). Although S305 is adjacent to the acetylation motif, our findings suggest that Akt-dependent phosphorylation sites (S167 and S118) in the AF-1 domain may positively regulate ER-α acetylation. Consistent with this idea, the S118A and S167A mutant proteins showed a reduction or abrogation of BRCA1 siRNA-induced acetylation but no reduction in basal acetylation.
Our studies suggest that BRCA1 and p300 may play both direct and indirect roles in the acetylation and activity of ER-α. This study and previous data (2,9) show that p300 can directly acetylate ER-α. Our previous work revealed that BRCA1 overexpression causes down-regulation of p300 expression and that exogenous p300 rescues the BRCA1 repression of ER-α activity (36). In that study, we showed that the histone acetyl transferase (HAT) domain of p300 was not required for the rescue and that a portion of p300 missing the HAT domain disrupted the binding of BRCA1 to ER-α in GST capture assays and rescued the BRCA1 repression of ER-α activity. Here, BRCA1 may inhibit acetylation by preventing the binding of p300 to ER-α and/or by mono-ubiquitinating ER-α on the acetylation sites.
As noted above, Akt may regulate acetylation via phosphorylation of the AF-1 domain of ER-α. In addition, it was reported that p300 is phosphorylated by Akt on S1834 both in vitro and in vivo and that this phosphorylation is required for its HAT activity (39). Thus, enhanced activity of p300 as a transcriptional cofactor could, in part, explain the increased acetylation of ER-α due to BRCA1 siRNA, because BRCA1 knockdown stimulates Akt kinase activity (25). BRCA1 siRNA also causes an increase in p300 protein levels, and knockdown of p300 blocked the increase in ER-α acetylation due to BRCA1 siRNA. Thus, the increased acetylation caused by BRCA1 knockdown could be due to a combination of increased abundance of p300 in addition to an increased HAT related to phosphorylation of p300 by Akt.
T300G encodes a full-length BRCA1 protein with a point mutation in the N-terminal RING domain of BRCA1 (C61Gly). This mutation does not abrogate the binding of BRCA1 to ER-α in vitro (which may occur through several sites) (23) but does abrogate the BRCA1 repression of ER-α activity (22). The T300G mutation disrupts the interaction of BRCA1 with BARD1 and abrogates the BRCA1/BARD1 E3 ubiquitin ligase function (31,40). We do not know for certain why this mutant protein failed to inhibit p300-mediated acetylation of ER-α in the in vitro assay, but it is possible that even though the T300G mutant protein can still bind ER-α in GST capture assays (22), it is not able to compete with p300 for binding to ER-α as effectively as wtBRCA1. Alternatively, it may bind to ER-α but not be able to block access of the HAT domain of p300 to the acetylation sites on ER-α. Consistent with the idea of a direct mechanism for BRCA1 inhibition of ER-α acetylation, previous studies have established direct interactions of p300 and ER-α, BRCA1 and ER-α, and BRCA1 and p300 (36,41).
Interestingly, when we studied BRCA1 regulation of acetylation of ER-α in receptors with mutations of one of the two major acetylation sites (300RXKK or K266/268), we found that the K266/268 site mutations not only reduced the levels of acetylated ER-α but also abrogated the ability of BRCA1 to regulate ER-α acetylation. In contrast, although the 300RXKK mutants also showed reduced basal levels of ER-α, these mutants showed an increase in acetylation due to BRCA1 knockdown. These findings suggest that BRCA1 may regulate acetylation primarily at the K266/268 site rather than the 300RXKK site, even though mutation of lysine residues at either site renders ER-α resistant to BRCA1-mediated repression. Alternatively, the K266/268 site mutations cause a change in the configuration of ER-α that renders it less susceptible to regulation of acetylation on the 300RXKK site.
Our findings suggest that ER-α is hypoacetylated when BRCA1 levels are high and hyperacetylated when BRCA1 levels are low. The hypoacetylated ER-α protein observed when BRCA1 is overexpressed has little or no activity, as documented using ERE-TK-Luc reporter assays. Conversely, hyperacetylation of the ER-α protein and higher transcriptional activity are observed when BRCA1 is knocked down. Any model for BRCA1 regulation of ER-α activity must account for the findings that 300RXKK and K266/268 site mutations render ER-α resistant to BRCA1. We propose that the acetylation-site mutants are resistant to BRCA1 because the same sites are also potential ubiquitination sites for the BRCA1/BARD1 ubiquitin ligase activity (Fig. 11). As noted earlier, the BRCA1/BARD1 complex mono-ubiquitinates ER-α on residue K302 (32). This study used an ER-α construct consisting of amino acid residues 302-552, so it would not have detected ubiquitination on K266/268. Because ubiquitination and acetylation are often competing processes, it is quite possible that BRCA1/BARD1 also ubiquitinates ER-α on K266 and/or K268. This possibility is consistent with our finding that both the K302/303A and K266/268R mutants gave little or no increase in ubiquitination due to BRCA1 overexpression. However, the loss of BRCA1-mediated ubiquitination could also be due to conformational changes in the ER-α protein due to the mutations. In the model shown in Fig. 11, mono-ubiquitinated ER-α, although not a target for proteasomal degradation, is less active than either unmodified or acetylated ER-α.
Figure 11.

Model for BRCA1 regulation of ER-α acetylation and activity.
The model shown in Fig. 11 is oversimplified, because other mechanisms than acetylation and ubiquitination may contribute to BRCA1 inhibition of ER-α activity. Thus, we previously showed that a mutant p300 protein missing the HAT domain (which is required for acetylation) could still rescue the wtBRCA1-mediated repression of ER-α activity (36). We note that it is still possible that p300 could recruit other proteins with HAT activity to the ER-α complex, although we also found that steroid receptor coactivator 1 (SRC1) and the p300/CBP-associated factor (PCAF) failed to rescue the BRCA1 repression of ER-α activity. Recruitment of corepressors to the ER-α complex is another mechanism by which BRCA1 may inhibit ER-α activity (42,43). In addition, as noted earlier, phosphorylation of ER-α on serine residues within the AF-1 domain (S118 and S167) due to BRCA1 knockdown could contribute to hyperactivity of ER-α (25) independently of any effect on acetylation or ubiquitination.
Our results suggest that mono-ubiquitination is required for BRCA1 repression of ER-α activity, but they do not absolutely prove this point. On the one hand, we found that a mutant BRCA1 protein selectively deficient for the ubiquitin ligase function (I26A) failed to ubiquitinate ER-α or to repress its activity. The I26 residue of BRCA1 mediates the proper binding and positioning of the ubiquitin-conjugating enzyme UbcH5c (40). On the other hand, the I26A mutant is also defective for ubiquitination of other substrates than ER-α, and we did not rule out the possibility that it could be deficient for other functions of BRCA1. However, the finding that two other ubiquitin ligase-defective BRCA1 mutants (C61G and C64G) fail to inhibit ER-α activity (22) is consistent with an important role for ubiquitination in BRCA1 inhibition of ER-α activity. The I26A mutant protein did not block acetylation of ER-α, as did wtBRCA1. This observation, along with the findings that mutations of lysine residues of acetylation sites 300RXKK and K266/268 of ER-α also caused reduced ubiquitination of ER-α, suggests a reciprocal relationship between acetylation and ubiquitination of ER-α.
Our studies do not address the quantitative extent of acetylation or ubiquitination of ER-α. However, they do suggest that only a small percentage of the total ER-α is ubiquitinated in response to BRCA1 overexpression (Fig. 9, A and B). Similarly, it was found that BRCA1 caused the in vivo ubiquitination of only a small percentage of γ-tubulin, even though it was able to cause the ubiquitin-dependent inhibition of centrosome function (44). One possibility is that BRCA1-dependent ubiquitination of ER-α contributes to but is not sufficient for inhibition of ER-α activity, and other functions of BRCA1 contribute to the inhibition (e.g. down-regulation of p300 expression or recruitment of a corepressor complex). Another possibility is that not all of the ER-α in the cell is transcriptionally activatable by E2, so that it is not necessary to ubiquitinate all ER-α molecules to block E2-stimulated ER-α activity. Alternatively, the ubiquitinated ER-α might function as a dominant inhibitor of the function of the nonubiquitinated ER-α and thus may have a greater effect on overall ER-α activity than expected, especially because ER-α cycles on and off an estrogen-responsive enhancer site multiple times in response to exposure to E2 (45). Further studies will be required to clarify how mono-ubiquitination regulates ER-α activity.
Materials and Methods
Cell lines and culture
Human breast (MCF-7 and HCC1937) and prostate (DU-145) cancer cell lines were obtained from American Type Culture Collection (Manassas, VA) and cultured as described before (25,33). The DU-145 BRCA1-overexpressing (DU-145/wtBRCA1) and control (DU-145/Neo) cell clones were described earlier (33). Briefly, the cells were grown in DMEM plus 5% (vol/vol) fetal calf serum, l-glutamine (5 mm), nonessential amino acids (5 mm), penicillin (100 U/ml), and streptomycin (100 μg/ml) (all obtained from BioWhittaker, Walkersville, MD).
Reagents
E2 was obtained from Sigma Chemical Co. (St. Louis, MO). Histone H3 was obtained from Millipore (catalog item 14-494; Billerica, MA) [3H]Acetyl coenzyme A (200 mCi/mmol) was obtained from PerkinElmer Life Sciences (Boston, MA). The signal transduction inhibitors used in this study and their sources were LY294002 (Sigma), wortmannin (BioMol Research Laboratories, Plymouth Meeting, PA), PD98059 (Biomol), GF109302X (LC Laboratories, Woburn, MA), rapamycin (Biomol), 4-amino-5-(4-methylphenyl)-7-(t-butyl) pyrazolo[3,4-d]-pyrimidine (PP1) (Biomol), and SB202190 (CalBiochem, La Jolla, CA). These agents were dissolved in dimethylsulfoxide and diluted into culture medium at the time of experiments. The antibody reagents used in this study are described below.
Expression vectors, reporters, and transient transfections
The wtBRCA1 expression vector was created by cloning the BRCA1 cDNA into the pcDNA3 vector (Invitrogen, Carlsbad, CA) using artificially engineered 5′-HindIII and 3′-NotI sites (33). The BRCA1-T300G expression vector was described earlier (22). The ubiquitination-defective BRCA1 mutant expression vector (BRCA1-I26A) was created by site-directed mutagenesis of the wtBRCA1 cDNA within the pcDNA3 vector (44) and was generously provided to us by Dr. Jeffrey D. Parvin (Harvard Medical School, Boston, MA). The ER-α expression vector pCMV-ER-α was used to express wt-ER-α. The estrogen-responsive reporter ERE-TK-Luc is composed of the vitellogenin A2 estrogen-responsive enhancer (ERE) controlling a minimal thymidine kinase promoter (TK81) and luciferase in plasmid pGL2 (46). Assays of ER-α transcriptional activity using this reporter are described below. The wtAkt and DN kinase-dead mutant (K179A) Akt (DN-Akt) in the pCIS2 expression vector were provided by Dr. M. J. Quon (National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD) (47). The ER-α mutant expression vectors (K302/303A, K302/303Q, K302/303T, K302/303R, and K303R) have been described earlier (2). The ER-α mutant expression vectors pCMV-hERα K266/268R and pCMV-hERα K266/268Q were generously provided by Dr. W. Lee Kraus (Cornell University, Ithaca, NY) and have been described earlier (9).
For transient transfections, subconfluent proliferating cells were transfected overnight with the vectors of interest or the empty pcDNA3 vector (Invitrogen) (10 μg plasmid DNA per 100-mm dish) using Lipofectamine (Life Technologies, Gaithersburg, MD) and washed to remove the excess vector and Lipofectamine. To determine the transfection efficiency, cultures were cotransfected with plasmid pRSV-β-gal (Promega Corp., Madison, WI) to allow staining with X-gal reagent and visualization of transfected (blue-staining) cells.
siRNA treatments
Double-stranded siRNA to knock down BRCA1 and p300 protein levels (BRCA1 siRNA and p300 siRNA) and a control siRNA that is not homologous to human DNA sequences were chemically synthesized by Ambion (Austin, TX). The efficacy of the BRCA1 siRNAs and p300 siRNA was validated by Western blotting. In this study, we used a combination of four different BRCA1 siRNAs and two different p300 siRNAs at a total concentration of 100 nm. The sequences of the four BRCA1 siRNAs were 5′-CAGCTACCCTTCCATCATA-3′, 5′-GGGATACCATGCAACATAA-3′, 5′-GAAGGAGCTTTCATCATTC-3′, and 5′ CTAGAAATCTGTTGCTATG-3′. We used a combination of two different p300 siRNAs at a total concentration of 100 nm. The sequences of the two p300 siRNAs were 5′-GCACAAAUGUCUAGUUCUUTT-3′ and 5′-AAGAACUAGACAUUUGUGCTT-3′. For siRNA treatments, subconfluent proliferating cells were treated with gene-specific or control siRNAs (final concentration of 100 nm) using siPORT Amine transfection reagent (Ambion), according to the manufacturer’s instructions. Western blotting experiments showed that a minimum of 48 h exposure to siRNAs was required to obtain a large reduction of the BRCA1 and p300 protein levels.
Assay of ER-α activity
Subconfluent proliferating cells in 24-well dishes were transfected overnight with 0.25 μg of each indicated vector plus the ERE-TK-Luc reporter in serum-free DMEM containing Lipofectamine. The total transfected DNA was kept constant by addition of the appropriate control vector. The cells were washed, incubated in phenolphthalein-free DMEM containing 5% charcoal-stripped serum (obtained from the Tissue Culture Shared Resource of the Lombardi Comprehensive Cancer Center) (0.2 ml/well) with or without E2 (10 nm or as indicated) for 24 h, and harvested for luciferase assays. For each assay condition tested in each experiment performed, four replicate wells were tested.
Immunoprecipitation
After the indicated transfections and/or treatments, the cells were then harvested, and whole-cell extracts were prepared as described before in immunoprecipitation buffer [10 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 0.5% IGEPAL CA-630 (Sigma, catalog no. I8896), 10% glycerol, 1 mm sodium orthovanadate, and protease inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany)] (23,25). Each immunoprecipitation was carried out using 2 μg antibody and 500 μg extract protein, except where noted otherwise. The extracts were incubated anti-ER-α H184 (sc-7207, rabbit polyclonal IgG; Santa Cruz Biotechnology, Santa Cruz, CA) or with a combination of anti-BRCA1 mouse monoclonals (Ab-1+Ab-2+Ab-3; Calbiochem; San Diego, CA), and the precipitated proteins were collected using protein A/G agarose (Santa Cruz). After low-speed centrifugation to remove the supernatants, the resin or agarose was washed with PBS, collected in boiling Laemmli sample buffer, and subjected to SDS-PAGE and Western blotting (see below). Except where otherwise noted, one half of the immunoprecipitated protein was used for Western blotting. For each experiment, a control immunoprecipitation using an equal quantity of normal mouse or rabbit IgG (Santa Cruz) was carried out.
Western blotting
After the indicated treatment, the cells were harvested, and whole-cell lysates were prepared using RIPA buffer (Santa Cruz), as described earlier (23,25). Equal aliquots of whole-cell protein (either 100 μg unprecipitated whole-cell lysate or one half of the precipitated protein from 500 μg whole-cell lysate) were electrophoresed on 4–12% SDS-polyacrylamide gradient gels, transferred to nitrocellulose membranes (Millipore, Bedford, MA), and blotted using primary antibodies directed against BRCA1 (C-20, rabbit polyclonal, 1:200 dilution; Santa Cruz), ER-α (F10, mouse monoclonal, sc-8002, 1:500; Santa Cruz), total Akt (catalog item 9271, 1:500; Cell Signaling Technology, Beverly, MA), p300 (rabbit polyclonal, catalog item sc-585, 1:400; Santa Cruz), acetyl lysine (mouse monoclonal, catalog item 9681, 1:300; Cell Signaling Technology), ubiquitin (mouse monoclonal FK2, 1:400; Biomol, Plymouth Meeting, MA), and α-actin (goat polyclonal, catalog item sc-1615, 1:400; Santa Cruz). The membranes were then blotted with the appropriate secondary antibodies (1:1000; Santa Cruz), and the blotted proteins were visualized using the enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Piscataway, NJ), with colored markers (Bio-Rad Laboratories, Hercules, CA) as molecular size standards.
In vitro acetylation assay
The source of histone acetyl transferase was enzyme p300 immunoprecipitated from cultured MCF-7 cells. For immunoprecipitation, the protein concentration was adjusted to 2 μg/μl in 1000 μl whole-cell lysate (prepared using RIPA buffer). An anti-p300 antibody (rabbit polyclonal, catalog item sc-584; Santa Cruz) was added (2 μg/1000 μl extract), and the extract was incubated for 2 h at 4 C. The acetylation assay was performed as described before (48). The reaction mixture contained 5 μg substrate (GST-ER-α 282-420 or histone H3), 200 ng enzyme (immunoprecipitated p300), and 5 μm [3H]acetyl coenzyme A (0.5 mCi/ml). The reaction mixture (final volume of 50 μl) was incubated at 30 C for 1 h. Then, 5 μl reaction mixture was electrophoresed on a 4–20% SDS-polyacrylamide gradient gel, and the gel was subjected to fluorography. The GST and GST-ERα 282-420 proteins were generated from cDNAs cloned into the GST vector (p-GEX), expressed in Escherichia coli, and purified by affinity chromatography. The wtBRCA1 and mutant BRCA1 (T300) proteins were generated by in vitro translation and transcription carried out using 1.0 μg plasmid DNA, with the TNT-coupled rabbit reticulocyte lysate system (Promega), according to the manufacturer’s instructions.
Statistical methods
Where appropriate, statistical comparisons were made using the two-tailed Student’s t test.
Supplementary Material
Footnotes
This research was supported in part by United States Public Health Service Grants RO1-CA82599 and RO1-CA80000 to E.M.R.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 3, 2009
Abbreviations: AF-1, Activation function-1; BARD1, BRCA1-associated RING domain 1; BRCA1, breast and ovarian cancer susceptibility gene 1; DN, dominant negative; E2, 17β-estradiol; ER-α, estrogen receptor-α; GST, glutathione S-transferase; HAT, histone acetyl transferase; Mr, relative molecular mass; PI, phosphatidylinositol; siRNA, small interfering RNA; wt, wild type.
References
- Fuqua SA, Wiltschke C, Zhang QX, Borg A, Castles CG, Friedrichs WE, Hopp T, Hilsenbeck S, Mohsin S, O'Connell P, Allred DC 2000 A hypersensitive estrogen receptor-α mutation in premalignant breast lesions. Cancer Res 60:4026–4029 [PubMed] [Google Scholar]
- Wang C, Fu M, Angeletti RH, Siconolfi-Baez L, Reutens AT, Albanese C, Lisanti MP, Katzenellenbogen BS, Kato S, Hopp T, Fuqua SA, Lopez GN, Kushner PJ, Pestell RG 2001 Direct acetylation of the estrogen receptor α hinge region by p300 regulates transactivation and hormone sensitivity. J Biol Chem 276:18375–18383 [DOI] [PubMed] [Google Scholar]
- Davies MP, O'Neill PA, Innes H, Sibson DR 2005 Hypersensitive K303R oestrogen receptor-α variant not found in invasive carcinomas. Breast Cancer Res 7:R113–R118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Yamashita H, Toyama T, Omoto Y, Sugiura H, Hara Y, Haruki N, Kobayashi S, Iwase H 2003 Estrogen receptor α mutation (A-to-G transition at nucleotide 908) is not found in different types of breast lesions from Japanese women. Breast Cancer 10:70–73 [DOI] [PubMed] [Google Scholar]
- Tebbit CL, Bentley RC, Olson Jr JA, Marks JR 2004 Estrogen receptor α (ESR1) mutant A908G is not a common feature in benign and malignant proliferations of the breast. Genes Chromosomes Cancer 40:51–54 [DOI] [PubMed] [Google Scholar]
- Conway K, Parrish E, Edmiston SN, Tolbert D, Tse CK, Geradts J, Livasy CA, Singh H, Newman B, Millikan RC 2005 The estrogen receptor-α A908G (K303R) mutation occurs at a low frequency in invasive breast tumors: results from a population-based study. Breast Cancer Res 7:R871–R880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway K, Parrish E, Edmiston SN, Tolbert D, Tse CK, Moorman P, Newman B, Millikan RC 2007 Risk factors for breast cancer characterized by the estrogen receptor α A908G (K303R) mutation. Breast Cancer Res 9:R36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herynk MH, Parra I, Cui Y, Beyer A, Wu MF, Hilsenbeck SG, Fuqua SA 2007 Association between the estrogen receptor α A908G mutation and outcomes in invasive breast cancer. Clin Cancer Res 13:3235–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Woo EM, Chong YT, Homenko DR, Kraus WL 2006 Acetylation of estrogen receptor α by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Mol Endocrinol 20:1479–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentis S, Le Romancer M, Bianchin C, Rostan MC, Corbo L 2005 Sumoylation of the estrogen receptor α hinge region regulates its transcriptional activity. Mol Endocrinol 19:2671–2684 [DOI] [PubMed] [Google Scholar]
- Li L, Li Z, Howley PM, Sacks DB 2006 6AP and calmodulin reciprocally regulate estrogen receptor stability. J Biol Chem 281:1978–1985 [DOI] [PubMed] [Google Scholar]
- Luo M, Koh M, Feng J, Wu Q, Melamed P 2005 Cross talk in hormonally regulated gene transcription through induction of estrogen receptor ubiquitylation. Mol Cell Biol 25:7386–7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, Bell R, Rosenthal J, Hussey C, Tran T, McClure M, Frye C, Hattier T, Phelps R, Haugen-Strano A, Katcher H, Yakumo K, Gholami Z, Shaffer D, Stone S, Bayer S, Wray C, Bogden R, Dayananth P, Ward J, Tonin P, Narod S, Bristow PK, Norris FH, Helvering L, Morrison P, Rosteck P, Lai M, Barrett JC, Lewis C, Neuhausen S, Cannon-Albright L, Goldgar D, Wiseman R, Kamb A, Skolnick MH 1994 A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266:66–71 [DOI] [PubMed] [Google Scholar]
- Wilson CA, Ramos L, Villaseñor MR, Anders KH, Press MF, Clarke K, Karlan B, Chen JJ, Scully R, Livingston D, Zuch RH, Kanter MH, Cohen S, Calzone FJ, Slamon DJ 1999 Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat Genet 21:236–240 [DOI] [PubMed] [Google Scholar]
- Esteller M, Silva JM, Dominguez G, Bonilla F, Matias-Guiu X, Lerma E, Bussaglia E, Prat J, Harkes IC, Repasky EA, Gabrielson E, Schutte M, Baylin SB, Herman JG 2000 Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst 92:564–569 [DOI] [PubMed] [Google Scholar]
- Staff S, Isola J, Tanner M 2003 Haplo-insufficiency of BRCA1 in sporadic breast cancer. Cancer Res 63:4978–4983 [PubMed] [Google Scholar]
- Wei M, Grushko TA, Dignam J, Hagos F, Nanda R, Sveen L, Xu J, Fackenthal J, Tretiakova M, Das S, Olopade OI 2005 BRCA1 promoter methylation in sporadic breast cancer is associated with reduced BRCA1 copy number and chromosome 17 aneusomy. Cancer Res 65:10692–10699 [DOI] [PubMed] [Google Scholar]
- Rosen EM, Fan S, Pestell RG, Goldberg ID 2003 BRCA1 gene in breast cancer. J Cell Physiol 196:19–41 [DOI] [PubMed] [Google Scholar]
- Scully R, Xie A, Nagaraju G 2004 Molecular functions of BRCA1 in the DNA damage response. Cancer Biol Ther 3:521–527 [DOI] [PubMed] [Google Scholar]
- Polanowska J, Martin JS, Garcia-Muse T, Petalcorin MI, Boulton SJ 2006 A conserved pathway to activate BRCA1-dependent ubiquitylation at DNA damage sites. EMBO J 25:2178–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Wang J, Yuan R, Ma Y, Meng Q, Erdos MR, Pestell RG, Yuan F, Auborn KJ, Goldberg ID, Rosen EM 1999 BRCA1 inhibition of estrogen receptor signaling in transfected cells. Science 284:1354–1356 [DOI] [PubMed] [Google Scholar]
- Fan S, Ma YX, Wang C, Yuan RQ, Meng Q, Wang JA, Erdos M, Goldberg ID, Webb P, Kushner PJ, Pestell RG, Rosen EM 2001 Role of direct interaction in BRCA1 inhibition of estrogen receptor activity. Oncogene 20:77–87 [DOI] [PubMed] [Google Scholar]
- Ma YX, Tomita Y, Fan S, Wu K, Tong Y, Zhao Z, Song LN, Goldberg ID, Rosen EM 2005 Structural determinants of the BRCA1: estrogen receptor interaction. Oncogene 24:1831–1846 [DOI] [PubMed] [Google Scholar]
- Kawai H, Li H, Chun P, Avraham S, Avraham HK 2002 Direct interaction between BRCA1 and the estrogen receptor regulates vascular endothelial growth factor (VEGF) transcription and secretion in breast cancer cells. Oncogene 21:7730–7739 [DOI] [PubMed] [Google Scholar]
- Ma Y, Hu C, Riegel AT, Fan S, Rosen EM 2007 Growth factor signaling pathways modulate BRCA1 repression of estrogen receptor-α activity. Mol Endocrinol 21:1905–1923 [DOI] [PubMed] [Google Scholar]
- Jones LP, Li M, Halama ED, Ma Y, Lubet R, Grubbs CJ, Deng CX, Rosen EM, Furth PA 2005 Promotion of mammary cancer development by tamoxifen in a mouse model of Brca1-mutation-related breast cancer. Oncogene 24:3554–3562 [DOI] [PubMed] [Google Scholar]
- Zheng L, Annab LA, Afshari CA, Lee WH, Boyer TG 2001 BRCA1 mediates ligand-independent transcriptional repression of the estrogen receptor. Proc Natl Acad Sci USA 98:9587–9592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LP, Tilli MT, Assefnia S, Torre K, Halama ED, Parrish A, Rosen EM, Furth PA 2008 Activation of estrogen signaling pathways collaborates with loss of Brca1 to promote development of ERα-negative and ERα-positive mammary preneoplasia and cancer. Oncogene 27:794–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Katiyar P, Jones LP, Fan S, Zhang Y, Furth PA, Rosen EM 2006 The breast cancer susceptibility gene BRCA1 regulates progesterone receptor signaling in mammary epithelial cells. Mol Endocrinol 20:14–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume R, Fukuda M, Maeda I, Nishikawa H, Oyake D, Yabuki Y, Ogata H, Ohta T 2001 The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J Biol Chem 276:14537–14540 [DOI] [PubMed] [Google Scholar]
- Brzovic PS, Rajagopal P, Hoyt DW, King MC, Klevit RE 2001 Structure of a BRCA1-BARD1 heterodimeric RING-RING complex. Nat Struct Biol 8:833–837 [DOI] [PubMed] [Google Scholar]
- Eakin CM, Maccoss MJ, Finney GL, Klevit RE 2007 Estrogen receptor α is a putative substrate for the BRCA1 ubiquitin ligase. Proc Natl Acad Sci USA 104:5794–5799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Wang JA, Yuan RQ, Ma YX, Meng Q, Erdos MR, Brody LC, Goldberg ID, Rosen EM 1998 BRCA1 as a potential human prostate tumor suppressor: modulation of proliferation, damage responses and expression of cell regulatory proteins. Oncogene 16:3069–3082 [DOI] [PubMed] [Google Scholar]
- Cui Y, Zhang M, Pestell R, Curran EM, Welshons WV, Fuqua SA 2004 Phosphorylation of estrogen receptor α blocks its acetylation and regulates estrogen sensitivity. Cancer Res 64:9199–9208 [DOI] [PubMed] [Google Scholar]
- Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H 2001 Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor α: a new model for anti-estrogen resistance. J Biol Chem 276:9817–9824 [DOI] [PubMed] [Google Scholar]
- Fan S, Ma YX, Wang C, Yuan RQ, Meng Q, Wang JA, Erdos M, Goldberg ID, Webb P, Kushner PJ, Pestell RG, Rosen EM 2002 p300 modulates the BRCA1 inhibition of estrogen receptor activity. Cancer Res 62:141–151 [PubMed] [Google Scholar]
- Fan S, Yuan R, Ma YX, Xiong J, Meng Q, Erdos M, Zhao JN, Goldberg ID, Pestell RG, Rosen EM 2001 Disruption of BRCA1 LXCXE motif alters BRCA1 functional activity and regulation of RB family but not RB protein binding. Oncogene 20:4827–4841 [DOI] [PubMed] [Google Scholar]
- Reid LJ, Shakya R, Modi AP, Lokshin M, Cheng JT, Jasin M, Baer R, Ludwig T 2008 E3 ligase activity of BRCA1 is not essential for mammalian cell viability or homology-directed repair of double-strand DNA breaks. Proc Natl Acad Sci USA 105:20876–20881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Chen CC 2005 Akt phosphorylation of p300 at Ser-1834 is essential for its histone acetyltransferase and transcriptional activity. Mol Cell Biol 25:6592–6602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzovic PS, Keeffe JR, Nishikawa H, Miyamoto K, Fox 3rd D, Fukuda M, Ohta T, Klevit R 2003 Binding and recognition in the assembly of an active BRCA1/BARD1 ubiquitin-ligase complex. Proc Natl Acad Sci USA 100:5646–5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao GM, Janknecht R, Ruffner H, Hunter T, Verma IM 2000 CBP/p300 interact with and function as transcriptional coactivators of BRCA1. Proc Natl Acad Sci USA 97:1020–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden RI, Brody LC 1999 BRCA1 interacts with components of the histone deacetylase complex. Proc Natl Acad Sci USA 96:4983–4988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Li R, Lu Y, Shupnik MA 2009 Decreased BRCA1 confers tamoxifen resistance in breast cancer cells by altering estrogen receptor-coregulator interactions. Oncogene 28:575–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran S, Starita LM, Simons AM, Parvin JD 2006 Identification of domains of BRCA1 critical for the ubiquitin-dependent inhibition of centrosome function. Cancer Res 66:4100–4107 [DOI] [PubMed] [Google Scholar]
- Métivier R, Penot G, Hübner MR, Reid G, Brand H, Kos M, Gannon F 2003 Estrogen receptor directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751–763 [DOI] [PubMed] [Google Scholar]
- Henttu PM, Kalkhoven E, Parker MG 1997 AF-2 activity and recruitment of steroid receptor coactivator 1 to the estrogen receptor depend on a lysine residue conserved in nuclear receptors. Mol Cell Biol 17:1832–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong LN, Chen H, Li Y, Zhou L, McGibbon MA, Taylor SI, Quon MJ 1997 Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol Endocrinol 11:1881–1890 [DOI] [PubMed] [Google Scholar]
- Kraus WL, Manning ET, Kadonaga JT 1999 Biochemical analysis of distinct activation functions in p300 that enhance transcription initiation with chromatin templates. Mol Cell Biol 19:8123–8135 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.