Abstract
Background and Aims
l-Ascorbate (vitamin C) has well-documented roles in many aspects of redox control and anti-oxidant activity in plant cells. This Botanical Briefing highlights recent developments in another aspect of l-ascorbate metabolism: its function as a precursor for specific processes in the biosynthesis of organic acids.
Scope
The Briefing provides a summary of recent advances in our understanding of l-ascorbate metabolism, covering biosynthesis, translocation and functional aspects. The role of l-ascorbate as a biosynthetic precursor in the formation of oxalic acid, l-threonic acid and l-tartaric acid is described, and progress in elaborating the mechanisms of the formation of these acids is reviewed. The potential conflict between the two roles of l-ascorbate in plant cells, functional and biosynthetic, is highlighted.
Conclusions
Recent advances in the understanding of l-ascorbate catabolism and the formation of oxalic and l-tartaric acids provide compelling evidence for a major role of l-ascorbate in plant metabolism. Combined experimental approaches, using classic biochemical and emerging ‘omics’ technologies, have provided recent insight to previously under-investigated areas.
Key words: Ascorbate, tartrate, oxalate, grapes, Vitis, metabolism
INTRODUCTION
It is an exciting time in plant biology; in the past 10 years an eruption of molecular, metabolic and technological know-how has provided data and tools to explore further both model and non-model species, thereby vastly increasing our understanding of the workings of plants. The plant scientist or student in search of information on the topic of l-ascorbate (ASC) metabolism is met with abundant reviews focussing on the many functional roles of this uniquely important metabolite [l-ascorbic acid (Vitamin C) occurs principally in vivo as the monoanion; it is referred to as ASC in this Botanical Briefing]. The biosynthesis of ASC, and its roles as cellular anti-oxidant, stress response factor and enzyme co-factor have been the subject of many reviews over the past 5–10 years (inter alia Loewus, 1999; Arrigoni and De Tullio, 2000; Davey et al., 2000; Smirnoff, 2000; Smirnoff and Wheeler, 2000; Conklin, 2001; Smirnoff et al., 2001, 2004). Advances in our knowledge of ASC biosynthesis via different pathways continue to be made (reviewed in Valpuesta and Botella, 2004; Hancock and Viola, 2005; Ishakawa et al., 2006). Additionally, in recent years, ASC has been recognized to play pivotal roles in specific functions including redox signalling (reviewed in Noctor, 2006), response to pathogen insult (Conklin and Barth, 2004) and recently also as a component in the determination of flowering time (Barth et al., 2006). In many of these reviews, mention is made of an additional function of ASC, as the biosynthetic precursor of oxalic and l-tartaric acids (OA and TA, respectively) in certain plants. Until recently, progress in unravelling this aspect of ASC metabolism has lagged behind that in other areas. While the accumulation of TA is limited to a handful of plant genera (Stafford, 1959), OA, which in planta occurs predominantly as crystals of calcium oxalate (CaOx), is widely distributed. The accumulation of OA crystals in plant tissues is suggested to be involved in regulation of cellular calcium levels and sequestration of toxic metals, and to confer resistance to herbivory (Nakata, 2003; Franceschi and Nakata, 2005).
The in planta function of TA accumulation remains unclear. Most notably, TA is produced as the dominant organic acid in grape (Vitis vinifera) berries in contrast to the majority of cases where fruit acidity is conferred by malic, citric or ascorbic acids. TA biosynthesis during grape berry development is restricted to the initial 4 weeks post-anthesis; berry TA levels subsequently remain unchanged until maturation (Iland and Coombe, 1988). Economically, TA plays a critical role in determining the suitability of grapes for use in winemaking – berry TA is largely responsible for controlling juice pH and through TA addition during vinification, the winemaker can minimize oxidative and microbial spoilage, thereby promoting both organoleptic and ageing potentials of the finished wine. Recent investigations of ASC catabolism in grape berries showed that synthesis of OA and TA occurs within these organs, suggesting two distinct catabolic fates for ASC (DeBolt et al., 2004).
An important corollary to the role of ASC as a precursor compound for OA and TA arises from the impact of ASC diversion into biosynthetic functions and thus away from being available for the vital functional roles in which it participates (Ishakawa et al., 2006).
l-ASCORBATE: BIOSYNTHESIS, TRANSLOCATION AND MAINTENANCE IN PLANT CELLS
It is now generally accepted that three pathways exist in plants for ASC formation, arising from GDP-d-mannose, myo-inositol and methyl galacturonate, this latter arising from the decomposition of pectin during later stages of ripening in strawberry fruit (reviewed in Hancock and Viola, 2005). The pathway(s) arising from GDP-d-mannose are likely to be of critical importance because of its role as a key metabolic intermediate. Evidence from the cyt1 mutant in Arabidopsis, which was mapped to a gene encoding an enzyme catalysing GDP-mannose synthesis, showed lethality in seedlings (Lukowitz et al., 2001) (mutations at this locus have variously been termed cyt, soz and vtc – those used by the original authors are retained here). As well as causing reduced ASC levels; this mutation had many downstream consequences such as defects in cell-wall synthesis and reduction in N-linked glycosylation (Lukowitz et al., 2001).
The final step of each pathway of ASC biosynthesis involves the activity of either l-galactono-1,4-lactone dehydrogenase or l-gulono-1,4-lactone dehydrogenase. In contrast to the cytoplasmic location of all other enzymes in the pathways, l-galactono- and l-gulono-1,4-lactone dehydrogenases are located within the mitochondrion. The nature of the trans-membrane processes involved in ASC biosynthesis remains to be determined. Data suggesting that carrier-mediated uptake of ASC and dehydroascorbate (DHA) across the plasma membrane of barley (Hordeum vulgare) protoplasts is driven by a proton electrochemical gradient were presented by Rautenkranz et al. (1994). These authors proposed that vacuolar uptake did not involve a carrier. ASC accumulation in plant organs including fruit and storage tubers is likely to follow localized biosynthesis, but the mode of ASC translocation from other tissues was in part answered by Franceschi and Tarlyn (2002), who showed that transport of ASC from source to sink tissues can occur via the phloem. Similar results with potato (Solanum tuberosum) tubers were reported by Tedone et al. (2004). Furthermore, Hancock et al. (2003) demonstrated the presence not merely of ASC but moreover of several of its biosynthetic enzymes in phloem exudates from courgette (Cucurbita pepo). The appearance of stable and correctly folded ASC biosynthetic enzymes in phloem exudate suggests a complex secretory system; further developments in this area are keenly awaited.
Cellular ASC levels are subject to control not only by the rate of biosynthesis and factors associated with translocation and unloading into sink tissues, but also by the extent to which oxidized forms of ASC are regenerated into the fully reduced form (May and Asard, 2004). The functional properties of ASC derive from the ene-diol group at carbon atoms 2 and 3, which provides acidic and reducing (antioxidant) properties via ionization (pKa=4.17 and 11.17) and electron donation, respectively (Smirnoff, 1996). Well-characterized enzymatic and chemical processes exist whereby ASC is regenerated in vivo, thus providing for a maintenance of cellular antioxidant and redox capacity that contributes to the overall metabolic plasticity of the plant (May and Asard, 2004). An important component of ASC recycling via reduction of the monodehydroascorbate radical is provided in animals by cytochrome b561. Recent work indicates similar functions for plant cytochrome b561 (Griesen et al., 2004). In Arabidopsis thaliana, tonoplast membranes are highly enriched in one isoform of this cytochrome, suggesting a potential ASC-mediated link between vacuole and cytoplasm.
l-ASCORBATE CATABOLISM: PATHWAYS AND OUTCOMES
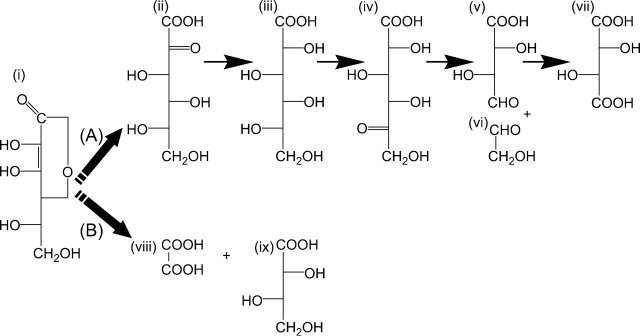
Early work elucidated the pathways by which ASC is catabolized to form variously OA, l-threonic acid and TA (reviewed in Bánhegyi and Loewus, 2004). In brief, intermediate compounds derived from the six-carbon ASC molecule undergo cleavage between carbon atoms 2 and 3 or 4 and 5. The cleavage reactions may follow a number of steps involving delactonization, oxidation and reduction of the intermediates. The identity of the intermediate compounds formed, and the order of reactions within the pathways was determined with radiotracer experiments and whole-plant studies. It became apparent that the alternative ASC cleavage pathways (2–3 or 4–5) showed species differences that remain unresolved to this day. In geraneaceous plants, Wagner and Loewus (1973) in a seminal paper showed that cleavage of ASC between carbon atoms 2 and 3 results in the formation of OA from carbon atoms 1 and 2, and l-threonic acid (which may be further oxidized to form TA) from carbon atoms 3 to 6. In grape berries, cleavage of the intermediate 5-keto-d-gluconic acid between carbon atoms 4 and 5 leads to TA formation, with the 2-carbon fragment of atoms 5 and 6 putatively recycled into central metabolic pathways (Fig. 1).
Fig. 1.
Proposed synthetic pathways of tartaric acid (A) and oxalic acid (B) in plants (adapted from Loewus, 1999). Intermediates and final products are (i) l-ascorbic acid (ASC); (ii) 2-keto-l-idonic acid; (iii) l-idonic acid; (iv) 5-keto-d-gluconic acid; (v) l-threo-tetruronic acid; (vi) glycoaldehyde; (vii) l-tartaric acid (TA); (viii) oxalic acid (OA); and (ix) l-threonic acid. The precise nature of the reaction steps indicated by the striped arrows at (A) and (B) is unknown.
2,3-CLEAVAGE OF ASC: FUNCTIONAL OR BIOSYNTHETIC OUTCOMES?
OA and TA biosynthesis represent catabolic fates for ASC. It is only within the last 8 or so years that substantial data concerning the biosynthesis of ASC have emerged. Therefore it is perhaps not surprising that nothing was known until recently of the molecular and biochemical processes associated with OA and TA biosynthesis.
An excellent review of the synthesis and roles of CaOx in plants appeared recently (Franceschi and Nakata, 2005). In plants that accumulate significant amounts of CaOx, the crystals conform to a limited number of morphologies. Arguably the most striking of these are raphides, needle-shaped crystals often clustered into bundles. Individual crystals may be longitudinally grooved; this has been proposed to allow toxins to be secreted into the wounds produced upon contact with the crystal tips (reviewed in Franceschi and Nakata, 2005).
Raphides occur mainly within the vacuoles of specialized cells called idioblasts. The high concentration of oxalate within these cells led to speculation concerning the source of the ASC from which the OA would be derived. This was solved by Keates et al. (2000) and further by Kostman et al. (2001), with the demonstration that idioblast cells of Pistia stratiotes were capable of both ASC and OA biosynthesis. Using a range of radiolabelled compounds, which were fed to idioblast protoplast preparations to minimize the possibilities of translocation from neighbouring cells, the existence of the Smirnoff–Wheeler–Running ASC biosynthetic pathway and of cleavage between carbon atoms 2 and 3 of ASC to yield OA was shown. An exciting development in this area is the suggestion that crystal formation within idioblasts is a directed process. In addition to specialized membrane structures, a calcium-binding protein uniquely localized to idioblast cells has been identified, which is suggested to play a role in controlling crystal growth and form (Li et al., 2003).
Evidence confirming a long-suggested anti-feeding role for OA was elegantly provided in a recent report, in which abrasion of the mouthparts of larval Spodoptera exigua following grazing on oxalate-containing Medicago truncatula, but not mutant lines lacking CaOx crystals, was observed by SEM (Korth et al., 2006). Interestingly, the effective crystals were prismatic in morphology, suggesting spontaneous formation rather than a directed mechanism. This finding highlights the value of mutants in the study of ASC catabolism; a number of lines of M. truncatula in which CaOx accumulates to lower levels than in wild-type have been characterized. Identification of the genetic lesions underlying these phenotypes is eagerly anticipated (Nakata, 2003).
An important paper (Green and Fry, 2005b) provided evidence for the apoplastic conversion of ASC to OA via the formation of DHA. This pathway in plants was proposed initially by the research of Loewus and co-workers (Yang and Loewus, 1975), who showed in-planta data supporting the role of DHA, but not of its delactonized hydrolysis product 2,3-diketo-l-gulonate, in the synthesis of OA and l-threonic acid from ASC. In cultured cells of Rosa sp., Green and Fry identified by feeding experiments using ASC radiolabelled with 14C on position 1, the hitherto uncharacterized intermediate 4-O-oxalyl-l-threonate, which was formed from the oxidation of ASC via DHA. The recovery also of 14C-labelled oxalate and unlabelled l-threonic acid from the culture medium provided evidence for the final steps in the pathway. Importantly, evidence was obtained for the enzymatic hydrolysis of 4-O-oxalyl-l-threonate by a soluble esterase enzyme presumably present within the apoplastic fluid of the cultured cells (Green and Fry, 2005a). In addition to identification of this intermediate and its subsequent fate, quantitative data for the extent of ASC oxidation to DHA via enzymatic and non-enzymatic reactions were determined. Approximately 50 % of the rate of ASC loss was determined to arise from non-enzymatic oxidation (Green and Fry, 2005b). This has potential importance for the proposed pro-oxidant role of ASC during cell expansion, since non-enzymatic oxidation of ASC is accompanied by production of hydrogen peroxide, a strong oxidant and potential precursor of hydroxyl radicals. Four steps of the pathway ASC to OA described by Green and Fry were proposed to potentially generate hydrogen peroxide. The authors suggest that apoplastic ascorbate may be a partial regulator of cell wall loosening, which is considered the primary determinant of rates of cell expansion, and also has implications accompanying physiological processes such as fruit ripening and softening.
GRAPE BERRIES: TISSUES DEMONSTRATING 2,3- AND 4,5-CLEAVAGE OF ASC
Recent work has unearthed significant features of the catabolism of ASC during grape berry development. Grapes do not accumulate ASC to high levels, and for table grapes it would be potentially of value to elevate natural ASC concentrations to enhance the nutritional benefits of their consumption. It is, however, in the area of TA metabolism that the greatest interest lies – grapes used for winemaking in many of the warmer climates of the world are harvested at much greater states of ripeness than is the case for the majority of old-world sites. This is manifest not only in higher sugar levels (and thereby potential alcohol levels in the finished wine) but also in lower juice acidity, increasing the risks of oxidative and microbial spoilage and moreover producing negative impacts on the organoleptic properties of the wine. Winemakers in these regions commonly add TA to control pH: TA has the added benefit of being essentially inert to microbial metabolism and, unlike malic acid, is not oxidized further during fermentations. Surprisingly perhaps, research aimed at determining the mechanism and control of TA metabolism during grape berry development has been largely unaddressed since the pioneering work of the Saito and Loewus groups in the 1970s and 1980s (reviewed in Bánhegyi and Loewus, 2004).
During preliminary experiments aimed at isolating enzyme extracts containing potential TA biosynthetic activities from grape berry protoplasts, large numbers of needle-shaped crystals were observed. Cursory examination of grapevine and winemaking literature suggested several possibilities for the identity of the crystals, including calcium tartrate, potassium hydrogen tartrate and CaOx. Further investigations indicated the strong possibility that the crystals were raphides of CaOx (Webb, 1999). Grapevines are not generally considered to be accumulators of oxalate and the intriguing possibility therefore suggested itself to us that two alternative pathways of ASC catabolism were occurring within the tissue of developing grape berries. Earlier examination of developing berries by TEM indicated the presence of smaller, star-shaped CaOx crystals (druses; Hardie et al., 1996); these were localized to the tissue surrounding the developing seed and were suggested to play a role in calcium regulation. It is tempting to speculate a potential seed-protectant anti-feeding function analogous to that proposed for herbivore-deterrent activity of the non-raphide CaOx crystals observed in M. truncatula (see above). In a survey of berries from 25 species of the family Vitaceae, a range of levels of berry oxalate concentration, including some species with almost zero, was observed (supporting information is given in fig. 5 of DeBolt et al., 2006). It will be of interest to examine the distribution and form of CaOx crystals within berries of the low-oxalate species, and to determine any consequences for berry metabolism associated with this phenotype.
Having confirmed the identity of crystal raphides isolated and purified from grape berry tissue using X-ray dispersion analysis (DeBolt et al., 2004), further evidence was shown in planta for the formation of OA and TA in attached immature (green) berries of V. vinifera ‘Cabernet Sauvignon’. ASC radiolabelled with 14C at position 1 was fed via a cotton thread into berries attached to a potted vine. Uptake and subsequent metabolism of the radiolabel was followed by HPLC analysis of acidified berry extracts and detection by combined absorbance and scintillation analyses. Radiolabel was recovered in both OA and TA fractions, indicating that ASC was cleaved between carbon atoms 2 and 3 to produce OA, and alternatively between carbon atoms 4 and 5 to produce TA. In accordance with earlier data (Saito and Kasai, 1982), over 70 % of the applied radiolabelled ASC was recovered as either OA (as CaOx crystals) or TA. The berries used for this study were approx. 4 weeks post-anthesis. Measurement of the relative flux through each pathway in berries sampled at different developmental stages will reveal the variation in ASC metabolism during berry ripening.
BIOCHEMICAL AND MOLECULAR CHARACTERIZATION OF TA SYNTHESIS IN PLANTS
The absence of homologous sequences isolated from more amenable experimental systems, and a significant degree of uncertainty regarding the enzymes likely to be involved in the biosynthetic pathway, necessitated an alternative approach to isolate and characterize the components of the TA biosynthesis pathway in grapevines (DeBolt et al., 2006). Large numbers of cDNA libraries prepared from particular tissues and at particular developmental stages of V. vinifera have been fully sequenced. The compilation of these datasets into (partially) annotated databases provides not only qualitative (viz. what genes are transcribed in that tissue at that developmental stage) but also quantitative (viz. how many cDNA clones representing each unique sequence were present) information. A hypothesis was proposed based on the known physiology of TA accumulation, namely that TA biosynthesis was restricted to immature leaves and berries, and was absent from other tissues and from berries at later stages of development. Further refinement was achieved by consideration of the likely reactions involved in the steps linking ASC to TA via the 4,5-cleavage pathway. At least three of the steps in the proposed pathway [Fig. 1; (ii)→(iii), (iii)→(iv) and (v)→(vii)] involve oxidation/reduction reactions for which dehydrogenase enzymes are potentially implicated. Each candidate cDNA identified on the basis of transcription pattern was conceptually translated and the resulting predicted amino acid sequences were used to scan a range of databases for motifs (short, characterized stretches of amino acids) known to be associated with enzymes possessing dehydrogenase activity. In this way, the list of potential candidates was reduced from several hundred to 10.
Analysis of the organic acid content and profiles of 25 species of vitaceous plants growing in the Winters vineyard at UC Davis resulted in the identification of one species, Ampelopsis aconitifolia, in which no TA accumulation occurred. When cDNA and genomic DNA from this species was used as a template for the amplification of each of the ten candidate sequences, one candidate was not amplified. Further characterization of this sequence revealed homology to several plant sorbitol dehydrogenases and, intriguingly, low level homology to a sequence encoding l-idonate dehydrogenase from Escherichia coli. l-Idonate is an intermediate in the conversion of ASC to TA; its oxidation to 5-keto-d-gluconate immediately precedes the cleavage event between carbon atoms 4 and 5 (Fig. 1). Recombinant protein expressed from this cDNA was tested in vitro for catalytic activity and was shown to be specific for the interconversion of l-idonate and 5-keto-d-gluconate using the co-factors NAD+ and NADH, respectively. Preliminary experimental data (C. M. Ford, unpubl. res.), suggest that the native enzyme is multimeric, and may exhibit co-operative kinetics indicative of a potential regulatory role within the pathway (DeBolt et al., 2006). This finding is in accordance with the results of Malipiero et al. (1987) and Saito et al. (1984), who showed, using a range of 14C-labelled intermediates, that the conversion of l-idonate to 5-keto-d-gluconate was rate-limiting for TA biosynthesis in leaves of Vitis sp.
Berries of the non-TA accumulating species A. aconitifolia were shown to contain approximately four times the levels of ASC commonly found in berries of V. vinifera (DeBolt et al., 2006), suggesting that the lack of a functional TA biosynthetic pathway resulted in unusual accumulation of its precursor. Interestingly, when berries of A. aconitifolia were fed with the TA precursors l-idonate or 5-keto-d-gluconate, TA biosynthetic capacity was not restored, suggesting that the biochemical competence for TA biosynthesis may exist over only a short period of berry development, or that additional as yet uncharacterized factors may be involved also in TA biosynthesis. TA is not present in any of the model plant species; therefore ASC deficient mutants like soz1 cannot be used to assess if differences in transcript or protein abundance correlate with TA levels. Viticultural trials were used to test the effect of different light exposure treatments on the levels of TA and ASC during grape development. HPLC and quantitative PCR analyses using samples extracted from high- and low-light treatments showed that ASC and TA levels increase with higher light exposure, as does the transcript level for idonate dehydrogenase (supporting information in fig. 9 of DeBolt et al., 2006). These data suggest a degree of transcriptional regulation of the TA pathway in grapes. From a cell biology standpoint, the control of ASC breakdown to specific end-point metabolites is an interesting question: ASC conversion to OA is limited to CaOx crystal formation in specialized cell types in the grape (Webb, 1999; Storey et al., 2003; DeBolt et al., 2004), while the conversion of ASC to TA occurred throughout the mesocarp (DeBolt et al., 2004). Further experiments are underway to determine more clearly the developmental interval of TA biosynthetic competence.
CONSEQUENCES OF ASC CATABOLISM: BENEFITS AND COSTS
By far the most common roles ascribed to ASC in cellular metabolism are those associated with its redox, enzyme cofactor and anti-oxidant activities. The use of ASC as a precursor for the biosynthesis of OA or TA must in some way be integrated with these functional roles, but clearly will impact on cellular ASC pool size and composition (Ishakawa et al., 2006). ASC diverted into biosynthetic functions is no longer available for other uses, suggesting that further ASC biosynthesis or a greater rate of recycling between oxidized and reduced forms of ASC is required in parallel to catabolism to maintain levels required for redox-associated roles. The control of this ‘uneasy alliance’ of metabolic outcomes remains unknown. Experiments in which the expression of specific ASC biosynthetic genes was up-regulated have not revealed unambiguous data on the regulation of the Smirnoff–Wheeler–Running pathway, neither did overexpression of ASC recycling-associated genes result in clear outcomes (Ishakawa et al., 2006). The recent discovery of a TA-biosynthetic gene (DeBolt et al., 2006) provides an important first step in addressing the control of ASC metabolism from the catabolic perspective. Further work is extending this knowledge by examining additional TA biosynthetic candidates.
TA biosynthesis is uncommon in fruits, and clearly represents an unusual fate for ASC. In an early paper (Bradford and Palmer, 1954), reference was made to TA accumulation in fruits of santol (Sandoricum koetjape). Intriguingly, two forms of santol fruit are known, yellow and red. The yellow santol may contain approx. 85 mg ASC per 100 g fruit pulp; in contrast the red santol contains approximately one-hundredth this level (Morton, 1987). Analysis of TA levels in the two forms of santol will test whether the pattern of ASC metabolism represented in V. vinifera and A. aconitifolia has wider botanic distribution.
CONCLUSIONS
ASC metabolism in plants fulfils a number of potentially conflicting roles: on the one hand, a continuous supply of ‘fully reduced’ ASC is vital for functions associated with redox maintenance and mediation of cellular processes, while, on the other hand, in specific tissues at particular stages in development, ASC is used as the precursor to the formation of OA and TA. Levels of accumulation of both acids (principally as oxalate and bitartrate salts) are frequently high, suggesting that a significant rate of ASC diversion into catabolic outcomes occurs when needed. Whether the use of ASC for biosynthetic purposes is regulated by developmental, environmental or tissue-specific cues remains unknown.
Elucidation of the mechanism of apoplastic oxalate formation from ASC via 4-O-oxalyl-l-threonate (Green and Fry, 2005a, b) provided details of the 2,3-cleavage reaction, including a proposal that an oxidase is associated with formation of the cyclic intermediate. At present there is no comparable model for the early stages of the ASC to TA pathway. Elucidation of these steps and, critically, the mechanism by which the fate of ASC is determined will provide the answer to the interesting and important biochemical question, ‘why do grape berries make TA?’ It is fortunate that the combination of both emergent ‘omics’ technologies and classical biochemical studies can to be used to begin unravelling this and other questions.
ACKNOWLEDGEMENTS
The authors thank Frank Loewus and Han Asard for their critical reviews of the manuscript.
LITERATURE CITED
- Arrigoni O, De Tullio MC. The role of ascorbic acid in cell metabolism: between gene-directed functions and unpredictable chemical reactions. Journal of Plant Physiology. 2000;157:481–488. [Google Scholar]
- Bánhegyi G, Loewus FL. Ascorbic acid catabolism: breakdown pathways in animals and plants. In: Asard H, May JM, Smirnoff N, editors. Vitamin C: functions and biochemistry in animals and plants. London: BIOS Scientific Publishers; 2004. pp. 31–48. [Google Scholar]
- Barth C, De Tullio M, Conklin PL. The role of ascorbic acid in the control of flowering time and the onset of senescence. Journal of Experimental Botany. 2006;57:1657–1665. doi: 10.1093/jxb/erj198. [DOI] [PubMed] [Google Scholar]
- Bradford VH, Palmer JK. The metabolism of the organic acids of tobacco leaves. VII. Effect of culture of excised leaves in solutions of (+) tartrate. Journal of Biological Chemistry. 1954;207:275–285. [PubMed] [Google Scholar]
- Conklin PL. Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant, Cell and Environment. 2001;24:383–394. [Google Scholar]
- Conklin PL, Barth C. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant, Cell and Environment. 2004;27:959–970. [Google Scholar]
- Davey MW, Van Montagu M, Inzé D, Sanmartin M, Kanellis A, Smirnoff N, et al. Plant l-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. Journal of the Science of Food and Agriculture. 2000;80:825–860. [Google Scholar]
- DeBolt S, Hardie WJ, Tyerman S, Ford CM. Composition and synthesis of raphide crystals and druse crystals in berries of Vitis vinifera L. cv. Cabernet Sauvignon: ascorbic acid as precursor for both oxalic and tartaric acids as revealed by radiolabelling studies. Australian Journal of Grape and Wine Research. 2004;10:134–142. [Google Scholar]
- DeBolt S, Cook DR, Ford CM. l-Tartaric acid synthesis from vitamin C in higher plants. Proceedings of the National Academy of Sciences of the USA. 2006;103:5608–5613. doi: 10.1073/pnas.0510864103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi VR, Nakata PA. Calcium oxalate in plants: formation and function. Annual Review of Plant Biology. 2005;56:41–71. doi: 10.1146/annurev.arplant.56.032604.144106. [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Tarlyn NM. l-Ascorbic acid is accumulated in source leaf phloem and transported to sink tissues in plants. Plant Physiology. 2002;130:649–656. doi: 10.1104/pp.007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MA, Fry SC. Apoplastic degradation of ascorbate: novel enzymes and metabolites permeating the plant cell wall. Plant Biosystems. 2005a;139:2–7. [Google Scholar]
- Green MA, Fry SC. Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-l-threonate. Nature. 2005b;433:83–87. doi: 10.1038/nature03172. [DOI] [PubMed] [Google Scholar]
- Griesen D, Su D, Berczi A, Asard H. Localization of an ascorbate-reducible cytochrome b561 in the plant tonoplast. Plant Physiology. 2004;134:726–734. doi: 10.1104/pp.103.032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RD, Viola R. Biosynthesis and catabolism of l-ascorbic acid in plants. Critical Reviews in Plant Sciences. 2005;24:167–188. [Google Scholar]
- Hancock RD, McRae D, Haupt S, Viola R. Synthesis of l-ascorbic acid in the phloem. BMC Plant Biology. 2003;3:7. doi: 10.1186/1471-2229-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie WJ, O'Brien TP, Jaudzems VG. Morphology, anatomy and development of the pericarp after anthesis in grape, Vitis vinifera L. Australian Journal of Grape and Wine Research. 1996;2:97–142. [Google Scholar]
- Iland PG, Coombe BG. Malate, tartrate, potassium, and sodium in flesh and skin of Shiraz grapes during ripening: concentration and compartmentation. American Journal of Enology and Viticulture. 1988;39:71–76. [Google Scholar]
- Ishakawa T, Dowdle J, Smirnoff N. Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiologia Plantarum. 2006;126:343–355. [Google Scholar]
- Keates SE, Tarlyn NM, Loewus FA, Franceschi VR. l-Ascorbic acid and l-galactose are sources for oxalic acid and calcium oxalate in Pistia stratiotes. Phytochemistry. 2000;53:433–440. doi: 10.1016/s0031-9422(99)00448-3. [DOI] [PubMed] [Google Scholar]
- Korth KL, Doege SJ, Park SH, Goggin FL, Wang Q, Gomez SK, et al. Medicago truncatula mutants demonstrate the role of plant calcium oxalate crystals as an effective defense against chewing insects. Plant Physiology. 2006;141:188–195. doi: 10.1104/pp.106.076737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostman TA, Tarlyn NM, Loewus FA, Franceschi VR. Biosynthesis of l-ascorbic acid and conversion of carbons 1 and 2 of l-ascorbic acid to oxalic acid occurs within individual calcium oxalate crystal idioblasts. Plant Physiology. 2001;125:634–640. doi: 10.1104/pp.125.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang D, Lynch-Holm VJ, Okita TW, Franceschi VR. Isolation of a crystal matrix protein associated with calcium oxalate precipitation in vacuoles of specialized cells. Plant Physiology. 2003;133:549–559. doi: 10.1104/pp.103.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewus FA. Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry. 1999;52:193–210. [Google Scholar]
- Lukowitz W, Nickle TC, Meinke DW, Last RL, Conklin PL, Somerville CR. Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proceedings of the National Academy of Sciences of the USA. 2001;98:2262–2267. doi: 10.1073/pnas.051625798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malipiero U, Ruffner HP, Rast DM. Ascorbic to tartaric acid conversion in grapevines. Journal of Plant Physiology. 1987;129:33–40. [Google Scholar]
- May J, Asard H. Ascorbate recycling. In: Asard H, May JM, Smirnoff N, editors. Vitamin C: functions and biochemistry in animals and plants. London: BIOS Scientific Publishers; 2004. pp. 139–157. [Google Scholar]
- Morton J. Santol. 1987. [18 March 2006]. http://newcorp.hort.purdue.edu/newcrop/morton/santol.html .
- Nakata PA. Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Science. 2003;164:901–909. [Google Scholar]
- Noctor G. Metabolic signalling in defence and stress: the central roles of soluble redox couples. Plant, Cell and Environment. 2006;29:409–425. doi: 10.1111/j.1365-3040.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Rautenkranz AAF, Li LJ, Mächler F, Märtinoia E, Oertli JJ. Transport of ascorbic and dehydroascorbic acids across protoplast and vacuole membranes isolated from barley (Hordeum vulgare L. cv. Gerbel) leaves. Plant Physiology. 1994;106:187–193. doi: 10.1104/pp.106.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Kasai Z. Conversion of l-ascorbic acid to l-idonic acid, l-idono gamma-lactone and 2-keto-l-idonic acid in slices of immature grapes (Vitis labrusca cultivar Delaware) Plant and Cell Physiology. 1982;23:499–508. [Google Scholar]
- Saito K, Morita SI, Kasai Z. Synthesis of l-dextro-tartaric acid from 5-keto-d-gluconic acid in Pelargonium. Plant and Cell Physiology. 1984;25:1223–1232. doi: 10.1104/pp.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. The function and metabolism of ascorbic acid in plants. Annals of Botany. 1996;78:661–669. [Google Scholar]
- Smirnoff N. Ascorbic acid: metabolism and functions of a multi-facetted molecule. Current Opinion in Plant Biology. 2000;3:229–235. [PubMed] [Google Scholar]
- Smirnoff N, Wheeler GL. Ascorbic acid in plants: biosynthesis and function. Critical Reviews in Plant Sciences. 2000;19:267–290. doi: 10.1080/10409230008984166. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Conklin PL, Loewus FA. Biosynthesis of ascorbic acid in plants: a renaissance. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:437–467. doi: 10.1146/annurev.arplant.52.1.437. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Running JA, Gaztek S. Ascorbate biosynthesis: a diversity of pathways. In: Asard H, May JM, Smirnoff N, editors. Vitamin C: functions and biochemistry in animals and plants. London: BIOS Scientific Publishers; 2004. pp. 7–29. [Google Scholar]
- Stafford HA. Distribution of tartaric acid in the leaves of certain angiosperms. American Journal of Botany. 1959;46:347–352. [Google Scholar]
- Storey R, Wyn Jones RG, Schachtman D, Treeby MT. Calcium-accumulating cells in the meristematic region of grapevine root apices. Functional Plant Biology. 2003;30:719–727. doi: 10.1071/FP02212. [DOI] [PubMed] [Google Scholar]
- Tedone L, Hancock RD, Alberino S, Haupt S, Viola R. Long-distance transport of l-ascorbic acid in potato. BMC Plant Biology. 2004;4:16. doi: 10.1186/1471-2229-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valpuesta V, Botella MA. Biosynthesis of l-ascorbic acid in plants: new pathways for an old antioxidant. Trends in Plant Science. 2004;9:573–577. doi: 10.1016/j.tplants.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Wagner G, Loewus FA. The biosynthesis of (+)-tartaric acid in Pelargonium crispum. Plant Physiology. 1973;67:591–593. doi: 10.1104/pp.52.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb MA. Cell-mediated crystallization of calcium oxalate in plants. The Plant Cell. 1999;11:751–761. doi: 10.1105/tpc.11.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JC, Loewus FA. Metabolic conversion of l-ascorbic acid to oxalic acid in oxalate-accumulating plants. Plant Physiology. 1975;56:283–285. doi: 10.1104/pp.56.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]