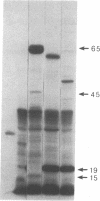
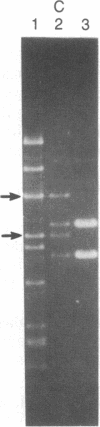
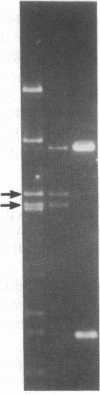
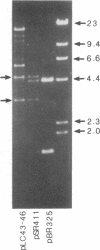
Abstract
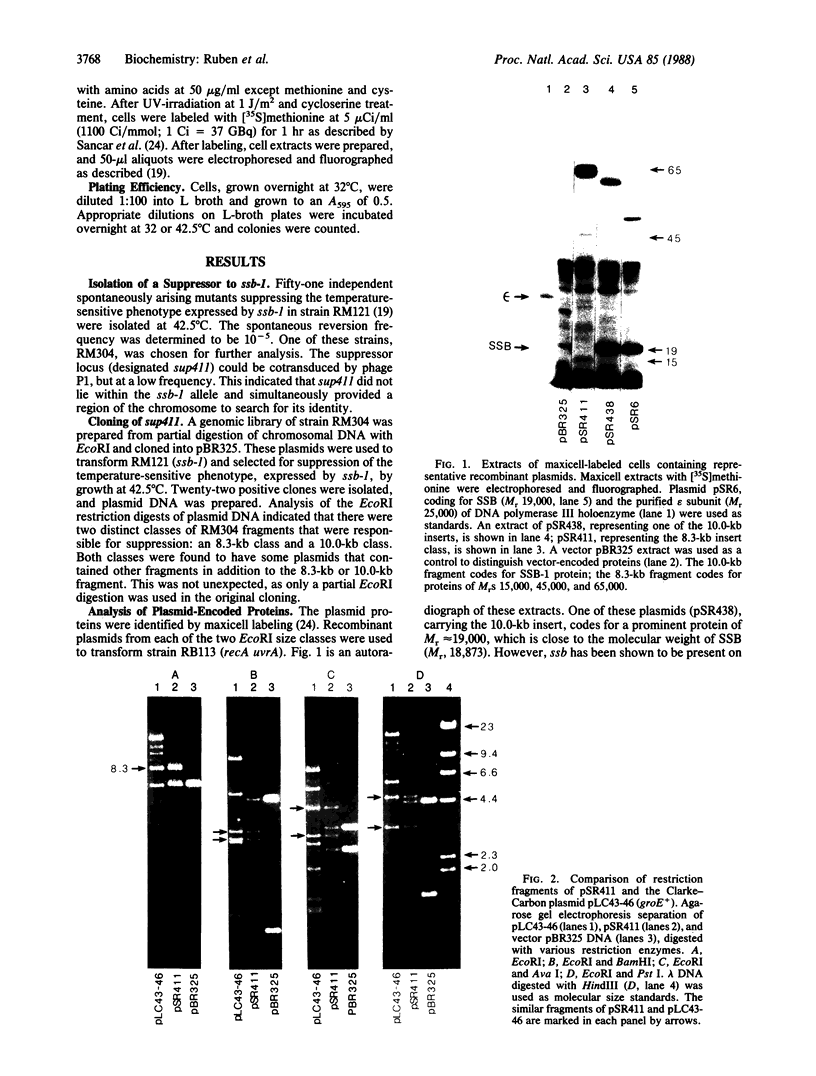
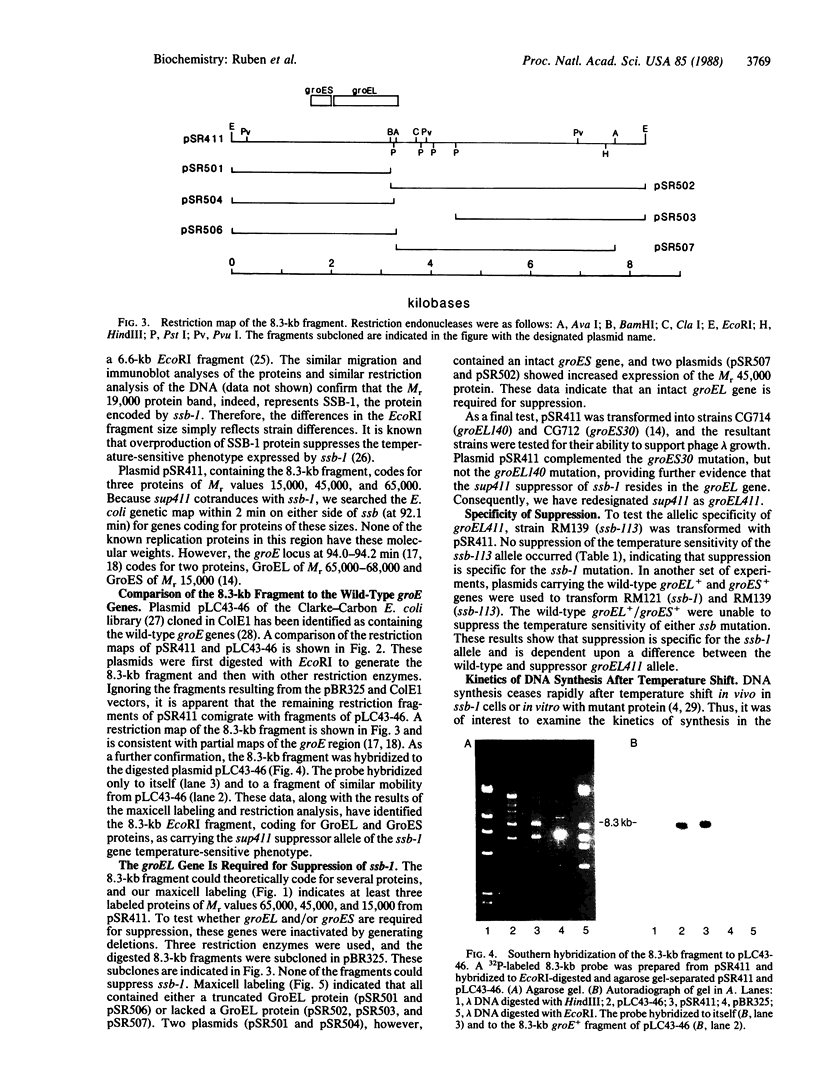
A series of spontaneous suppressors to the temperature-sensitive phenotype of the single-stranded DNA-binding protein mutation ssb-1 were isolated. A genomic library of EcoRI fragments from one of these suppressor strains was prepared by using pBR325 as the cloning vector. A 10.0-kilobase class of inserts was identified as carrying the ssb-1 gene itself. A second class of 8.3-kilobase inserts was shown to contain the groE region by (i) restriction analysis, (ii) Southern hybridization of the 8.3-kilobase insert to groE+ DNA, and (iii) identification of the gene products by similar migration on polyacrylamide gels. Subcloning demonstrated that an intact mutant groEL gene was necessary for suppression and that plasmids carrying the 8.3-kilobase insert could suppress mutants carrying groES- but not groEL- genes for phage lambda growth. The suppressor, designated as groEL411, was specific for the ssb-1 allele. In ssb-1 groEL411 cells, DNA synthesis stopped after a shift to 42.5 degrees C but rapidly recovered within minutes. The data suggest a direct interaction between the single-stranded DNA-binding protein and GroEL proteins in DNA replication.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. The lexA gene product represses its own promoter. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1932–1936. doi: 10.1073/pnas.77.4.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Murphy J. B., Whittier R. F., Lorensen E., Sninsky J. J. Amplification of ssb-1 mutant single-stranded DNA-binding protein in Escherichia coli. J Mol Biol. 1983 Feb 25;164(2):193–211. doi: 10.1016/0022-2836(83)90075-x. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Williams K. R. Single-stranded DNA binding proteins required for DNA replication. Annu Rev Biochem. 1986;55:103–136. doi: 10.1146/annurev.bi.55.070186.000535. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Fassler J. S., Tessman I., Tessman E. S. Lethality of the double mutations rho rep and rho ssb in Escherichia coli. J Bacteriol. 1985 Feb;161(2):609–614. doi: 10.1128/jb.161.2.609-614.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayet O., Louarn J. M., Georgopoulos C. Suppression of the Escherichia coli dnaA46 mutation by amplification of the groES and groEL genes. Mol Gen Genet. 1986 Mar;202(3):435–445. doi: 10.1007/BF00333274. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Kornberg A. Purified dnaA protein in initiation of replication at the Escherichia coli chromosomal origin of replication. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5817–5821. doi: 10.1073/pnas.80.19.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell B. E., Baker T. A., Kornberg A. In vitro assembly of a prepriming complex at the origin of the Escherichia coli chromosome. J Biol Chem. 1987 Jul 25;262(21):10327–10334. [PubMed] [Google Scholar]
- Geider K., Hoffmann-Berling H. Proteins controlling the helical structure of DNA. Annu Rev Biochem. 1981;50:233–260. doi: 10.1146/annurev.bi.50.070181.001313. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Casjens S. R., Kaiser A. D. Host participation in bacteriophage lambda head assembly. J Mol Biol. 1973 May 5;76(1):45–60. doi: 10.1016/0022-2836(73)90080-6. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Kaiser A. D., Wood W. B. Role of the host cell in bacteriophage morphogenesis: effects of a bacterial mutation on T4 head assembly. Nat New Biol. 1972 Sep 13;239(89):38–41. doi: 10.1038/newbio239038a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Jenkins A. J., March J. B., Oliver I. R., Masters M. A DNA fragment containing the groE genes can suppress mutations in the Escherichia coli dnaA gene. Mol Gen Genet. 1986 Mar;202(3):446–454. doi: 10.1007/BF00333275. [DOI] [PubMed] [Google Scholar]
- Low R. L., Shlomai J., Kornberg A. Protein n, a primosomal DNA replication protein of Escherichia coli. Purification and characterization. J Biol Chem. 1982 Jun 10;257(11):6242–6250. [PubMed] [Google Scholar]
- Meyer R. R., Glassberg J., Kornberg A. An Escherichia coli mutant defective in single-strand binding protein is defective in DNA replication. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1702–1705. doi: 10.1073/pnas.76.4.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. R., Glassberg J., Scott J. V., Kornberg A. A temperature-sensitive single-stranded DNA-binding protein from Escherichia coli. J Biol Chem. 1980 Apr 10;255(7):2897–2901. [PubMed] [Google Scholar]
- Meyer R. R., Rein D. C., Glassberg J. The product of the lexC gene of Escherichia coli is single-stranded DNA-binding protein. J Bacteriol. 1982 Apr;150(1):433–435. doi: 10.1128/jb.150.1.433-435.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. R., Voegele D. W., Ruben S. M., Rein D. C., Trela J. M. Influence of single-stranded DNA-binding protein on recA induction in Escherichia coli. Mutat Res. 1982 Jun;94(2):299–313. doi: 10.1016/0027-5107(82)90293-7. [DOI] [PubMed] [Google Scholar]
- Molineux I. J., Gefter M. L. Properties of the Escherichia coli DNA-binding (unwinding) protein interaction with nucleolytic enzymes and DNA. J Mol Biol. 1975 Nov 15;98(4):811–825. doi: 10.1016/s0022-2836(75)80012-x. [DOI] [PubMed] [Google Scholar]
- Molineux I. J., Gefter M. L. Properties of the Escherichia coli in DNA binding (unwinding) protein: interaction with DNA polymerase and DNA. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3858–3862. doi: 10.1073/pnas.71.10.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrical S. W., Lee J., Cox M. M. Continuous association of Escherichia coli single-stranded DNA binding protein with stable complexes of recA protein and single-stranded DNA. Biochemistry. 1986 Apr 8;25(7):1482–1494. doi: 10.1021/bi00355a003. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Phillips T. A., VanBogelen R. A., Smith M. W., Georgalis Y., Subramanian A. R. Identity of the B56.5 protein, the A-protein, and the groE gene product of Escherichia coli. J Bacteriol. 1981 Jan;145(1):513–520. doi: 10.1128/jb.145.1.513-520.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. Cloning of uvrA, lexC and ssb genes of Escherichia coli. Biochem Biophys Res Commun. 1979 Sep 12;90(1):123–129. doi: 10.1016/0006-291x(79)91598-5. [DOI] [PubMed] [Google Scholar]
- Sancar A., Wharton R. P., Seltzer S., Kacinski B. M., Clarke N. D., Rupp W. D. Identification of the uvrA gene product. J Mol Biol. 1981 May 5;148(1):45–62. doi: 10.1016/0022-2836(81)90234-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tessman E. S., Peterson P. K. Suppression of the ssb-1 and ssb-113 mutations of Escherichia coli by a wild-type rep gene, NaCl, and glucose. J Bacteriol. 1982 Nov;152(2):572–583. doi: 10.1128/jb.152.2.572-583.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., Murialdo H., Georgopoulos C. Identification of a second Escherichia coli groE gene whose product is necessary for bacteriophage morphogenesis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1629–1633. doi: 10.1073/pnas.78.3.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M., Itikawa H. Participation of Escherichia coli K-12 groE gene products in the synthesis of cellular DNA and RNA. J Bacteriol. 1984 Feb;157(2):694–696. doi: 10.1128/jb.157.2.694-696.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. H., Bertsch L. L., Kornberg A. The deoxyribonucleic acid unwinding protein of Escherichia coli. Properties and functions in replication. J Biol Chem. 1975 Mar 25;250(6):1972–1980. [PubMed] [Google Scholar]
- Williams K. R., Murphy J. B., Chase J. W. Characterization of the structural and functional defect in the Escherichia coli single-stranded DNA binding protein encoded by the ssb-1 mutant gene. Expression of the ssb-1 gene under lambda pL regulation. J Biol Chem. 1984 Oct 10;259(19):11804–11811. [PubMed] [Google Scholar]