Abstract
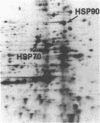
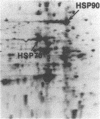
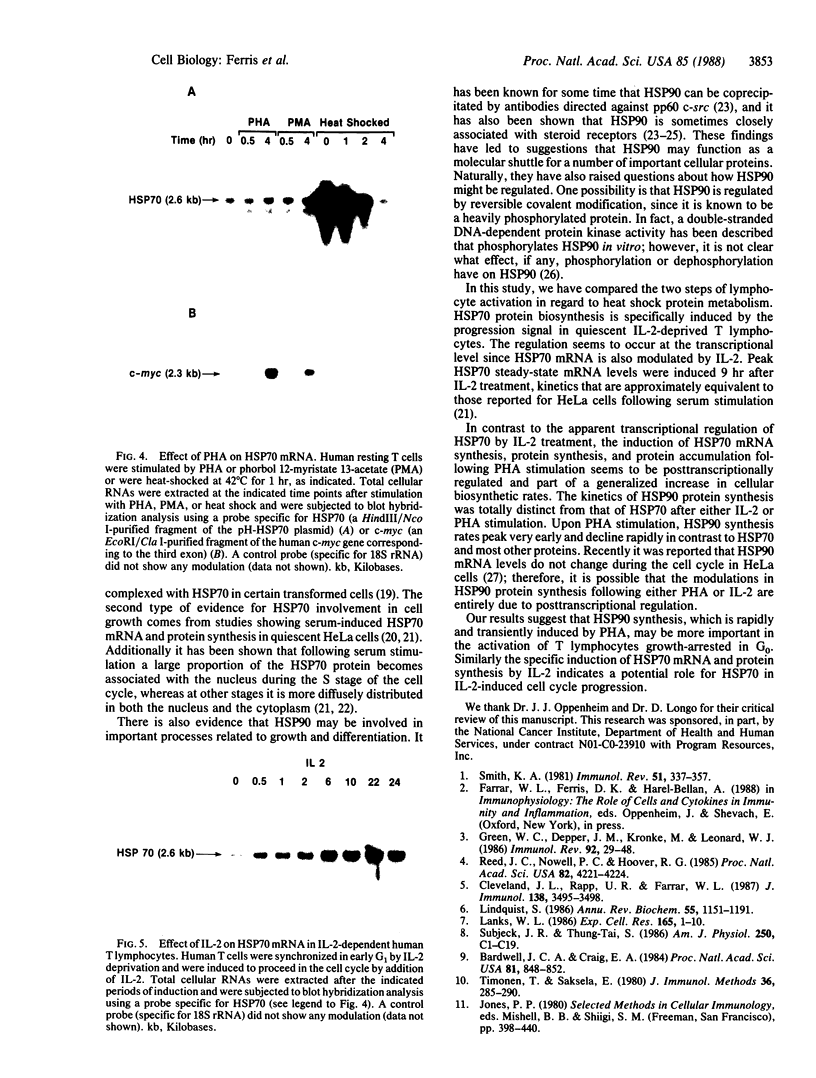
We have examined the effects of phytohemagglutinin (PHA) and the polypeptide growth factor interleukin 2 (IL-2) on the synthesis of the 70- and 90-kDa heat shock proteins (HSP70 and HSP90, respectively) in human T lymphocytes. Resting T cells (G0) stimulated with PHA responded with a generalized increase in protein synthesis that included HSP70. Gel blot analysis indicated that steady-state levels of HSP70 mRNA were not specifically modulated by PHA. Synthesis of HSP90 protein, however, peaked very rapidly following PHA stimulation and decreased sharply after 1 hr. When IL-2-dependent human T cells, synchronized by IL-2 deprivation, were treated with IL-2, synthesis of HSP70 mRNA was increased as much as 15-fold. HSP70 and HSP90 protein synthesis increased significantly upon IL-2 stimulation of human T lymphocytes. Two distinct members of the ancient family of heat shock genes, HSP70 and HSP90, are shown to be stimulated at the activation and progression stages of lymphocyte mitogenesis, which suggests that genetic mechanisms evolved from primitive stress/adaptation responses may be conserved in mammalian cellular activation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Jung-Testas I., Renoir J. M., Baulieu E. E., Feramisco J. R., Welch W. J. The common 90-kd protein component of non-transformed '8S' steroid receptors is a heat-shock protein. EMBO J. 1985 Dec 1;4(12):3131–3135. doi: 10.1002/j.1460-2075.1985.tb04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland J. L., Rapp U. R., Farrar W. L. Role of c-myc and other genes in interleukin 2 regulated CT6 T lymphocytes and their malignant variants. J Immunol. 1987 May 15;138(10):3495–3504. [PubMed] [Google Scholar]
- Greene W. C., Depper J. M., Krönke M., Leonard W. J. The human interleukin-2 receptor: analysis of structure and function. Immunol Rev. 1986 Aug;92:29–48. doi: 10.1111/j.1600-065x.1986.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Kingston R. E., Baldwin A. S., Jr, Sharp P. A. Regulation of heat shock protein 70 gene expression by c-myc. Nature. 1984 Nov 15;312(5991):280–282. doi: 10.1038/312280a0. [DOI] [PubMed] [Google Scholar]
- Lanks K. W. Modulators of the eukaryotic heat shock response. Exp Cell Res. 1986 Jul;165(1):1–10. doi: 10.1016/0014-4827(86)90528-8. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Milarski K. L., Morimoto R. I. Expression of human HSP70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9517–9521. doi: 10.1073/pnas.83.24.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Pinhasi-Kimhi O., Michalovitz D., Ben-Zeev A., Oren M. Specific interaction between the p53 cellular tumour antigen and major heat shock proteins. Nature. 1986 Mar 13;320(6058):182–184. doi: 10.1038/320182a0. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Nowell P. C., Hoover R. G. Regulation of c-myc mRNA levels in normal human lymphocytes by modulators of cell proliferation. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4221–4224. doi: 10.1073/pnas.82.12.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez E. R., Housley P. R., Pratt W. B. The molybdate-stabilized glucocorticoid binding complex of L-cells contains a 98-100 kdalton steroid binding phosphoprotein and a 90 kdalton nonsteroid-binding phosphoprotein that is part of the murine heat-shock complex. J Steroid Biochem. 1986 Jan;24(1):9–18. doi: 10.1016/0022-4731(86)90025-7. [DOI] [PubMed] [Google Scholar]
- Schuh S., Yonemoto W., Brugge J., Bauer V. J., Riehl R. M., Sullivan W. P., Toft D. O. A 90,000-dalton binding protein common to both steroid receptors and the Rous sarcoma virus transforming protein, pp60v-src. J Biol Chem. 1985 Nov 15;260(26):14292–14296. [PubMed] [Google Scholar]
- Simon M. C., Kitchener K., Kao H. T., Hickey E., Weber L., Voellmy R., Heintz N., Nevins J. R. Selective induction of human heat shock gene transcription by the adenovirus E1A gene products, including the 12S E1A product. Mol Cell Biol. 1987 Aug;7(8):2884–2890. doi: 10.1128/mcb.7.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Subjeck J. R., Shyy T. T. Stress protein systems of mammalian cells. Am J Physiol. 1986 Jan;250(1 Pt 1):C1–17. doi: 10.1152/ajpcell.1986.250.1.C1. [DOI] [PubMed] [Google Scholar]
- Timonen T., Saksela E. Isolation of human NK cells by density gradient centrifugation. J Immunol Methods. 1980;36(3-4):285–291. doi: 10.1016/0022-1759(80)90133-7. [DOI] [PubMed] [Google Scholar]
- Walker A. I., Hunt T., Jackson R. J., Anderson C. W. Double-stranded DNA induces the phosphorylation of several proteins including the 90 000 mol. wt. heat-shock protein in animal cell extracts. EMBO J. 1985 Jan;4(1):139–145. doi: 10.1002/j.1460-2075.1985.tb02328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem. 1984 Apr 10;259(7):4501–4513. [PubMed] [Google Scholar]
- Welch W. J., Suhan J. P. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol. 1986 Nov;103(5):2035–2052. doi: 10.1083/jcb.103.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B. J., Hurst H. C., Jones N. C., Morimoto R. I. The E1A 13S product of adenovirus 5 activates transcription of the cellular human HSP70 gene. Mol Cell Biol. 1986 Aug;6(8):2994–2999. doi: 10.1128/mcb.6.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B. J., Morimoto R. I. Transcription of the human hsp70 gene is induced by serum stimulation. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6070–6074. doi: 10.1073/pnas.82.18.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Hunt C., Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol. 1985 Feb;5(2):330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]