Abstract
Sulphate assimilation provides reduced sulphur for the synthesis of cysteine, methionine, and numerous other essential metabolites and secondary compounds. The key step in the pathway is the reduction of activated sulphate, adenosine 5′-phosphosulphate (APS), to sulphite catalysed by APS reductase (APR). In the present study, [35S]sulphur flux from external sulphate into glutathione (GSH) and proteins was analysed to check whether APR controls the flux through the sulphate assimilation pathway in poplar roots under some stress conditions and in transgenic poplars. (i) O-Acetylserine (OAS) induced APR activity and the sulphur flux into GSH. (ii) The herbicide Acetochlor induced APR activity and results in a decline of GSH. Thereby the sulphur flux into GSH or protein remained unaffected. (iii) Cd treatment increased APR activity without any changes in sulphur flux but lowered sulphate uptake. Several transgenic poplar plants that were manipulated in sulphur metabolism were also analysed. (i) Transgenic poplar plants that overexpressed the γ-glutamylcysteine synthetase (γ-ECS) gene, the enzyme catalysing the key step in GSH formation, showed an increase in sulphur flux into GSH and sulphate uptake when γ-ECS was targeted to the cytosol, while no changes in sulphur flux were observed when γ-ECS was targeted to plastids. (ii) No effect on sulphur flux was observed when the sulphite oxidase (SO) gene from Arabidopsis thaliana, which catalyses the back reaction of APR, that is the reaction from sulphite to sulphate, was overexpressed. (iii) When Lemna minor APR was overexpressed in poplar, APR activity increased as expected, but no changes in sulphur flux were observed. For all of these experiments the flux control coefficient for APR was calculated. APR as a controlling step in sulphate assimilation seems obvious under OAS treatment, in γ-ECS and SO overexpressing poplars. A possible loss of control under certain conditions, that is Cd treatment, Acetochlor treatment, and in APR overexpressing poplar, is discussed.
Keywords: APS synthetase, flux measurements, glutathione, poplar, roots, sulphate uptake, sulphite oxidase
Introduction
Plants meet their demand for the essential nutrient sulphur by taking up inorganic sulphate from the soil, reducing it to sulphide, and incorporating it into cysteine (Cys) in the sulphate assimilation pathway (for reviews, see Leustek et al., 2000; Kopriva, 2006). Cys can be further incorporated into proteins or used as a sulphur donor for methionine and glutathione (GSH) synthesis and for a wide range of co-factors and secondary metabolites. Since sulphate is chemically very stable, it has to be activated before reduction by adenylation to adenosine 5′-phosphosulphate (APS) in a reaction catalysed by ATP sulphurylase (ATPS). APS is reduced to sulphite by APS reductase (APR) and further to sulphide by sulphite reductase (SiR). Cys is synthesized by O-acetylserine(thiol)lyase from sulphide and O-acetylserine (OAS), which is formed in a reaction between serine and acetyl-CoA catalysed by serine acetyltransferase (SAT). A major sink for Cys is the tripeptide GSH, an essential component of the plant stress response and redox homeostasis (Foyer and Rennenberg, 2000; Foyer and Noctor, 2005; Meyer and Hell, 2005; Mullineaux and Rausch, 2005; Cairns et al., 2006; Meyer, 2008). GSH is synthesized in two consecutive enzymatic steps. The first step, catalysed by γ-glutamylcysteine synthetase (γ-ECS), joins Cys with glutamate. In the second step, glycine is added to γ-glutamylcysteine (γ-EC) by the glutathione synthetase (GSHS).
The synthesis of Cys represents a merging point of sulphate assimilation with nitrogen and carbon metabolism and it is thus not surprising that the pathway is strongly regulated by both nitrogen and carbohydrate availability (Reuveny et al., 1980; Brunold and Suter, 1984; Kopriva et al., 1999; Kopriva and Rennenberg, 2004). Sulphate assimilation can also respond to the availability of sulphur in a demand-driven manner (Lappartient and Touraine, 1996; Herschbach et al., 2000). Accordingly, the mRNA levels of most components of the sulphate assimilation pathway are increased in response to sulphur starvation (Hirai et al., 2003; Maruyama-Nakashita et al., 2003; Nikiforova et al., 2003), feeding with OAS (Neuenschwander et al., 1991; Hirai et al., 2003), or after exposure to heavy metals, which induce synthesis of phytochelatins (PCs) and thus a rapid drain of reduced sulphur pools (Nussbaum et al., 1988). On the other hand, when plants are provided with reduced sulphur either by fumigation with hydrogen sulphide (Westerman et al., 2001) or by feeding with Cys or GSH (Vauclare et al., 2002), the pathway is repressed. Regulation of sulphate assimilation is well described and understood at the level of transcripts, enzyme activities, and metabolites, using system approaches as well as targeted analyses (Takahashi et al., 1997; Herschbach et al., 2000; Koprivova et al., 2000; Hopkins et al., 2004; Kawashima et al., 2005; Hirai et al., 2005). Analysis of metabolite composition and enzyme activities describes only a steady state. However, determination of incorporated labelled precursors into products (ap Rees and Hill, 1994) provides information about changes in the flux through a metabolic pathway that is important to understand its control (Fell, 1998). The controlling step and the regulation of this step, that is of the enzyme that controls the flux through a metabolic pathway, have to be distinguished (ap Rees and Hill, 1994). The controlling step means the enzymatic reaction by which the flux through the pathway is limited. APR seems to be the controlling step of assimilatory sulphate reduction in Arabidopsis roots (Vauclare et al., 2002; Kopriva and Rennenberg, 2004). Regulation of the controlling step indicates the mechanism(s) that influence(s) its reaction. Feedback inhibition, covalent modification of enzymes, as well as control of enzyme synthesis and degradation are mechanisms that can alter the flux through a metabolic pathway (Fell, 1992). For assimilatory sulphate reduction many studies revealed a regulation of APR at the transcriptional level by thiols (Bick et al., 2001; Vauclare et al., 2002; Hartmann et al., 2004), amino compounds (Brunold and Suter, 1984; Neuenschwander et al., 1991; Koprivova et al., 2000; Hesse et al., 2004; Hopkins et al., 2005), carbohydrates (Neuenschwander et al., 1991; Kopriva et al., 1999, 2002; Hesse et al., 2003), and hormones (Harada et al., 2000; Ohkama et al., 2002). More recently, APR was also found to be under post-transcriptional control (Bick et al., 2001; Koprivova et al., 2008).
Flux through sulphate assimilation can be estimated by incubation of plants with [35S]sulphate and measurement of the radioactivity in proteins and thiols (Neuenschwander et al., 1991; Kopriva et al., 1999, 2002; Koprivova et al., 2000; Vauclare et al., 2002). Flux data can be utilized together with enzyme activity data for flux control analyses by calculating ‘flux control coefficients’ of individual components of the pathway (Fell, 1998). These ‘flux control coefficients’ represent a quantitative measure of the contribution of individual enzymes to the control of the flux through the pathway. The degree of control of flux through the metabolic pathway has been addressed for several enzymes of carbon metabolism (for review, see Fell, 1992; ap Rees and Hill, 1994) and is a developing field for analysing metabolic networks (Ratcliffe and Shachar-Hill, 2006; Libourel and Shachar-Hill, 2008). Using this framework and Arabidopsis root cultures treated with Cys or GSH, Vauclare et al. (2002) calculated that APR possesses 92% control over the pathway from internal sulphate and shares the control with sulphate transporters when sulphate uptake is taken into account.
In order to test whether APR controls the flux through sulphate assimilation under different environmental conditions, several treatments known to affect APR activity strongly by increasing the demand for reduced sulphur were selected. These treatments included (i) OAS exposure which greatly promotes Cys synthesis (Neuenschwander et al., 1991); (ii) Cd exposure that induces PC synthesis from GSH (Nussbaum et al., 1988); and (iii) exposure to Acetochlor, a pesticide which is detoxified via conjugation to GSH by glutathione S-transferase (GST; Jablonkai and Hatzios, 1991). Poplar was chosen because (i) this tree species is discussed as a plant that could be useful in phytoremediation of heavy metals and herbicides (Peuke and Rennenberg, 2005a, b); (ii) transgenic lines overexpressing γ-ECS that possess increased amounts of GSH are available; and (iii) sulphate assimilation is well understood in this species (Strohm et al., 1995; Arisi et al. 1997; Noctor et al., 1998; Hartmann et al., 2004; Kopriva et al., 2004; Rennenberg et al., 2007). In the present study, the 35S flux from sulphate taken up by excised roots of wild type (WT) poplar into GSH was determined under different environmental conditions. As a more precise calculation of APR flux control coefficients requires a wide range of changes in the enzyme activity, Lemna minor APR was overexpressed in poplar. In addition, the effects on the 35S flux of modulated levels of sulphite oxidase (SO), an enzyme which counteracts sulphite production via APR by producing sulphate from sulphite (Eilers et al., 2001; Hänsch et al., 2006; 2007), were analysed. The effects of these treatments and metabolic modifications on the flux through sulphate assimilation in excised poplar roots and the consequences for the contribution of APR to flux control of this pathway are reported.
Materials and methods
Plant material and growth conditions
Cuttings of the poplar hybrid Populus tremula×P. alba, clone 717 1B4 (Institut National de la Recherche Agronomique, INRA) and all transgenic lines were micropropagated as described by Strohm et al. (1995) and Noctor et al. (1996). After 4 weeks, cuttings were transferred onto quartz sand (0.7–2 mm, Götz & Moritz, Freiburg, Germany) and were grown in a greenhouse (26±5°C) under long day conditions. Seedlings were watered with a modified Hoagland solution consisting of 0.125 mM KNO3, 0.25 mM Ca(NO3)2, 0.05 mM MgSO4, 0.45 mM MgCl2, 0.05 mM KH2PO4, 2 μM MnSO4, 10 μM H3BO3, 0.2 μM CuSO4, 0.2 μM ZnSO4, 0.2 μM Na2MoO4, 0.04 μM CoSO4, 0.1 mM FeSO4, and 0.095 mM NaEDTA.
Transgenic poplar lines
Transgenic poplar lines that express the (γ-ECS) gene from Escherichia coli and target the protein either to the cytosol (line ggs28) or to plastids (lines Lggs6, Lggs12, and Lggs20) were described previously (Arisi et al., 1997; Noctor et al., 1998). APR is considered the key enzyme of the sulphate assimilation pathway (reviewed in Kopriva and Koprivova, 2004). To analyse the impact of increased APR activity on S metabolism in poplar, APR cDNA from Lemna minor (Suter et al., 2000) was overexpressed in the 717 1B4 clone of P. tremula×P. alba under the control of the constitutive 35S promoter. Transgenic poplar lines overexpressing APR (lines 303, 304, 391, and 404) were constructed as follows: the cDNA encoding the complete APR open reading frame was amplified from total RNA of Lemna minor and cloned into the pCR2 plasmid carrying EcoRI and BamHI restriction sites at the 5’ and 3’ end, respectively (Suter et al., 2000). The restriction sites were used to prepare a translational fusion with the 35S promoter in the plasmid pCK/2×35S. Transgenic lines that overexpressed the SO gene (cDNA) from Arabidopsis thaliana under the control of either the constitutive cauliflower mosaic virus (CaMV) 35S promoter (lines 158-2, 159, and 161) or the leaf-specific promoter from tobacco (ST-LS1 promoter; Stockhaus et al., 1987) (lines 93, 150-1, and 185) were created by PCR amplification of SO from pQE60-SO (Eilers et al., 2001). XhoI and BamHI restriction sites at the 5’ and 3’ end were used to clone the PCR fragment into the plasmids pCK/2×35S; BamHI restriction sites at both ends are used for the creation of a translational fusion of the gene in sense orientation with the ST-LS1 promoter. HindIII fragments containing the chimeric genes consisting of the promoter, the coding region of the genes, and 35S terminator were cloned into the binary vector pBin19 (Bevan, 1984).
WT poplars (clone 717 1B4) were transformed with these constructs using Agrobacterium-mediated transformation, according to published protocols (Leplé et al., 1992). Regenerated kanamycin-resistant plants were tested for the presence of the transgenes by genomic PCR, for the amount of protein by western blot, and for enzyme activity. Plants with the highest activity compared with WT poplars were selected, propagated, and analysed further.
[35S]Sulphate feeding
Fine roots were sampled from 2- to 5-month-old poplar seedlings grown in sand. The roots were washed with water to remove sand particles. Fine roots up to a diameter of 1 mm were separated from the poplar root system and transferred into 10 ml of an incubation solution (Hoagland; see above) adjusted to 0.1 mM SO42–. After 2 h pre-incubation, the incubation solution was exchanged and sulphate uptake measurement was started by adding 150 μCi (5.55×106Bq) of carrier-free [35S]SO42– (Hartmann Analytic, Braunschweig, Germany). After 4 h, sulphate uptake was stopped by washing roots three times with the incubation medium. Root samples were frozen in liquid nitrogen and stored until analyses at –20°C.
Treatments
OAS at 1 mM was applied to fine roots during the whole incubation, that is during the 2 h pre-incubation and 4 h incubation with [35S]sulphate. Cd treatment was performed by adding 0.5 mM CdCl2 to the watering solution for 4 weeks prior to the experiment, but was omitted in the incubation solutions. The herbicide Acetochlor was added to the watering solution for 2 d (66 μg ml−1) before [35S]sulphate uptake experiments were started.
35S analyses
[35S]Sulphate uptake was determined by measurement of radioactivity in 20 mg of root tissue (powdered under liquid nitrogen) as reported by Herschbach and Rennenberg (1996). After solubilization with a tissue solubilizer (Soluene 350, Packard Instruments, Frankfurt, Germany) root samples were bleached with 200 μl of H2O2 (30%) overnight. After adding 5 ml of scintillation fluid (HiSafe 2, Packard Instruments, Frankfurt, Germany), radioactivity was determined by scintillation counting (Wallac System 1409, Wallac, Turku, Finland) and was corrected for quenching.
35S metabolite analyses
Thiols were determined as described by Hartmann et al. (2000). A 30 mg aliquot of fine root tissue (powdered under liquid nitrogen) was homogenized in 750 μl of 0.1 M HCl that contained 50 mg of insoluble polyvinylpolypyrrolidone (PVPP). Root samples were centrifuged and 120 μl of the clear supernatant were added to 180 μl of CHES buffer (200 mM, pH 9.3). Reduction of thiols was performed with dithiothreitol (DTT; 30 μl, 15 mM) for 1 h at room temperature. Thiols were derivatized with monobromobimane (20 μl, 30 mM) and stabilized by adding 240 μl of acetic acid (10%, v/v) after 15 min of derivatization. Aliquots of 150 μl were taken to separate bimane conjugates by HPLC (SUPERCOSILTM LC-18, 25 cm×4.6 mm, 5 μm, Sigma-Aldrich) according to Schupp and Rennenberg (1988) using 10% (v/v) methanol, 0.25% (v/v) acetic acid (pH 3.9) as solvent A and 90% (v/v) methanol, 0.25% (v/v) acetic acid (pH 3.9) as solvent B. Bimane derivatives were detected by fluorescence detection (Schupp and Rennenberg, 1988). To determine the amount of 35S in thiols, 1 ml fractions of the eluate were collected with a fraction collector; after adding 4 ml of scintillation fluid (HiSafe 3, Packard Instruments, Frankfurt, Germany) the radioactivity was determined by liquid scintillation counting and classified as GSH by comparison with the fluorescent detector output. Acid-insoluble 35S was determined as reported by Herschbach and Rennenberg (1996). Sulphate was extracted and determined from 50 mg of root tissue (powdered under liquid nitrogen) by anion exchange chromatography (Herschbach et al., 2000). Radioactivity in sulphate was determined in 1.2 ml fractions collected after anion exchange chromatography.
APR measurements and protein determination
Fresh root material (∼70–90 mg) was homogenized in 1ml of extraction buffer (50 mM sodium/potassium phosphate buffer pH 8 with additions of 30 mM Na2SO3, 0.5 mM AMP, and 10 mM DTT) in a mortar. APR activity was measured using 20 μl of the extract by measuring the acid-volatile 35S formed in the presence of [35S]APS and DTT according to Brunold and Suter (1990).
The amount of protein was determined as described by Bradford (1976) using bovine serum albumin (BSA) as a standard. For determination of radioactivity in protein, 30 mg of frozen root powder of root tissue was homogenized in 1 ml of extraction buffer. Protein was precipitated in 10% (v/v) trichloroacetic acid (TCA) for 30 min on ice, washed, and re-dissolved in 0.2 M NaOH as described (Vauclare et al., 2002). The amount of 35S incorporated into protein was determined in the re-dissolved protein by liquid scintillation counting after adding 5 ml of liquid scintillation fluid (HiSafe 2).
Calculation of flux control coefficients
In the present study, labelled [35S]sulphate was used and its flow into thiols and proteins of excised poplar roots was analysed in relation to treatments or genetic modifications affecting APR activity. The ‘metabolic control analysis’ described by Vauclare et al. (2002) and Fell (1998) was used to calculate the flux control coefficient for this enzyme. For this purpose, the equation of Vauclare et al. (2002) was applied:
where CJAPR=the flux control coefficient; ΔlnJ=changes in flux, ΔlnEAPR=changes in APR activity, and ΔlnAPS=changes in APS. The minimum value of the control coefficient would be obtained when the elasticity (ε) of APR to a change in APS is 1. Changes in APS are assumed to be proportional to changes in internal SO42– (the latter is used to calculate the flux control coefficient in the present study as described by Vauclare et al., 2002), when the first enzyme of the sulphate assimilation, the ATPS, was near equilibrium and exerted little control (Vauclare et al., 2002). This seems to be the case in poplar roots tested under nitrogen and sulphur deficiency (Kopriva et al., 2004). The interpretation is based on the summation theorem (Kacser and Burns, 1973), that is the sum of the flux control coefficients of the enzymes involved in a metabolic system is assumed to be 1. Thus for calculating the flux control coefficients in the present study, the calculation was started at internal sulphate and ended at the sulphate assimilation into GSH and protein (Vauclare et al., 2002). Significant differences between the control (WT) and the treatment or the transgenic lines were analysed according to the Student's t-test for P <0.05.
Results
Changes in sulphur metabolism due to different treatments
In order to address the dynamics of sulphate assimilation under different environmental conditions in poplar roots, the flux through the pathway was analysed. For this purpose, [35S]sulphate was fed to excised fine roots, and sulphate uptake rates, total sulphate, GSH and protein contents were determined, as well as the 35S flux into the sulphate, GSH, and protein pools. In addition, the activity of the key enzyme of the pathway, APR, was determined. After 4 h incubation with [35S]sulphate, radioactivity was detected in all fractions, that is in sulphate, GSH, and proteins. The specific activity of the sulphate pool was always lower than the specific 35S activity in the GSH fraction (Table 1).
Table 1.
Sulphate uptake rates, specific 35S activities of the sulphate and GSH fraction, and the ratio between soluble and insoluble 35S
| Sulphate uptake (nmol g−1 FW h−1) | Specific activity of sulphate (dpm nmol−1) | Specific activity of glutathione (dpm nmol−1) | 35S soluble/35S insoluble | |
| OAS control | 25.5±17.2 | 793±332 | 3961±2495 | 2.6±0.9 |
| OAS | 24.2±8.8 | 1137±615 | 6331±3581 | 8.0±3.5* |
| Cd control | 6.8±2.0 | 907±505 | 3388±972 | 4.8±1.8 |
| Cd | 4.6±1.9* | 1063±277 | 2909±1486 | 3.3±1.0* |
| Acetochlor control | 12.4±9.3 | 1329±1058 | 3065±958 | 8.7±2.7 |
| Acetochlor | 11.8±7.9 | 1018±576 | 4424±2192 | 7.3±2.3 |
| Wild type | 21.8±11.6 | 2167±596 | 4802±2091 | 2.3±0.7 |
| APR sense | 17.3±12.0 | 1224±913 | 3422±1462 | 2.3±0.8 |
| Wild type | 12.9±3.8 | 2737±2333 | 5050±2487 | 2.5±1.2 |
| ggs28 | 33.1±24.3 | 6285±4121 | 6170±386 | 4.4±1.0* |
| Lggs | 12.3±6.3 | 1712±1018 | 3577±1453 | 1.5±0.5* |
| Wild type | 22.5±10.6 | 1818±946 | 1816±761 | 6.5±2.4 |
| SO CaMV 35S | 19.4±7.3 | 1811±713 | 2582±1185 | 4.2±2.1 |
| SO ST-LS | 31.5±14.9 | 2434±1174 | 2662±1422 | 5.5±3.2 |
Significant differences from the control treatment or from the wild type poplar trees at P <0.05.
OAS treatment:
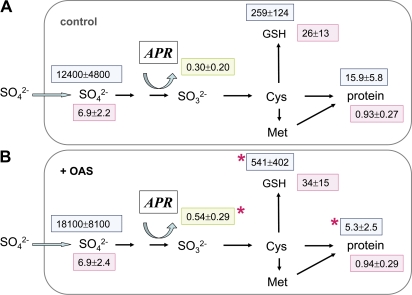
OAS treatment of excised fine roots resulted in a 1.8-fold increased APR activity, but it did not affect the sulphate uptake rate in the present experiment. Simultaneously, the metabolite contents, that is sulphate, GSH, and protein contents, did not change due to OAS exposure. Nevertheless, an ∼2-fold higher flux of 35S into the GSH pool and a reduced flux into the protein pool was found (Fig. 1). This was accompanied by a 3-fold increase in the ratio of soluble to insoluble [35S]sulphur (Table 1) and by a higher specific 35S activity of the GSH pool, although this increase was not significant at the P=0.05 level.
Fig. 1.
Sulphur metabolite contents and 35S flux into different metabolite pools of OAS-treated poplar roots. Excised fine roots from poplar trees were pre-treated without (A, n=12) or with 1 mM OAS (B, n=12) for 2 h and subsequently exposed to [35S]sulphate plus 1 mM OAS for 4 h for sulphate uptake and 35S flux measurements. Sulphate (μmol g−1 FW), GSH (nmol g−1 FW), and protein (mg g−1 FW) contents were determined (pink squares). 35S flux into internal sulphate, GSH, and protein is given as pmol 35S g−1 FW h−1 (light blue squares). APR activity is indicated in a green square as nmol mg−1 protein min−1. Significant differences at P <0.05 from control roots without OAS are indicated by asterisks.
Cd treatment:
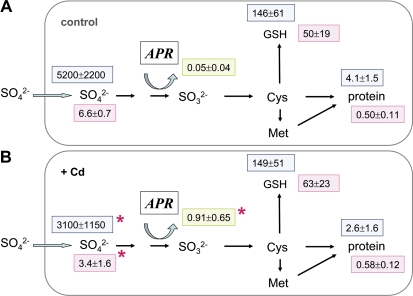
Poplar trees were pre-treated with 0.5 mM CdCl2 added to the Hoagland nutrient solution during watering for 4 weeks. This treatment resulted in an 18-fold increase in APR activity of the fine roots. Protein and GSH contents were not affected, while the sulphate content was reduced by ∼50% (Fig. 2). Accordingly, sulphate uptake in Cd-treated plants was significantly reduced by 22% (Table 1). However, 35S flux into GSH or proteins was not affected, while the flux into the internal sulphate pool was reduced (Fig. 2). In addition, the ratio between soluble and insoluble 35S was diminished due to the Cd treatment (Table 1).
Fig. 2.
Sulphur metabolite contents and 35S flux into different metabolite pools of fine roots excised from poplar plants pre-treated with Cd. Fine roots were excised from poplar trees watered without (A, n=10) or with 0.5 mM Cd (B, n=10) in the nutrient solution for 4 weeks. The excised fine roots were pre-incubated for 2 h and subsequently exposed to [35S]sulphate for 4 h to measure sulphate uptake and 35S flux into different metabolite pools. Sulphate (μmol g−1 FW), GSH (nmol g−1 FW), and protein (mg g−1 FW) contents were determined (pink squares). 35S flux into internal sulphate, GSH, and protein is given as pmol 35S g−1 FW h−1 (light blue squares). APR activity is indicated in a green square as nmol mg−1 protein min−1. Significant differences at P <0.05 from control roots without Cd pre-treatment are indicated by asterisks.
Acetochlor treatment:
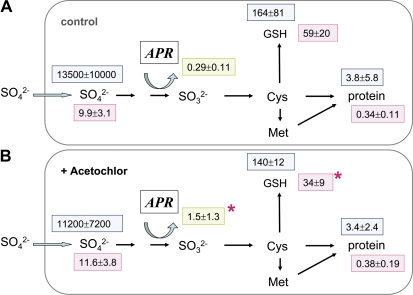
Application of the herbicide Acetochlor to poplar plants 2 d prior the experiment did not affect sulphate uptake (Table 1) or the sulphate content in fine roots (Fig. 3). Also the 35S flux into the internal sulphate pool was comparable in both Acetochlor-treated and control roots. However, a 42% reduction of GSH levels was observed although APR activity increased 5-fold. Surprisingly, 35S flux into GSH and protein remained unaffected by the herbicide treatment.
Fig. 3.
Sulphur metabolite contents and 35S flux into different metabolite pools of fine roots excised from poplar plants pre-treated with Acetochlor. Fine roots were excised from poplar trees watered without (A, n=9) or with 66 μg ml−1 Acetochlor (B, n=9) in the nutrient solution for 2 days. The excised fine roots were pre-incubated for 2 h and subsequently exposed to [35S]sulphate for 4 h to measure sulphate uptake and 35S flux into different metabolite pools. Sulphate (μmol g−1 FW), GSH (nmol g−1 FW), and protein (mg g−1 FW) contents were determined (pink squares). 35S flux into internal sulphate, GSH, and protein is given as pmol 35S g−1 FW h−1 (light blue squares). APR activity is indicated in a green square as nmol mg−1 protein min−1. Significant differences at P <0.05 from control roots without Acetochlor pre-treatment are indicated by asterisks.
Changes in sulphur metabolism due to manipulation of gene expression
γ-ECS overexpression:
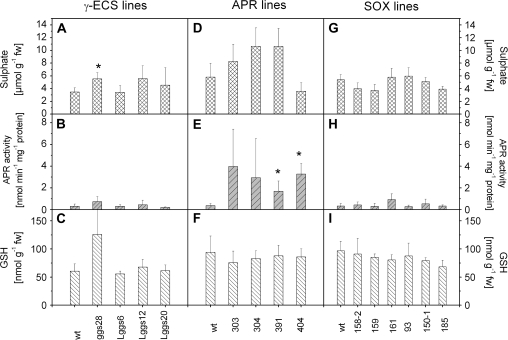
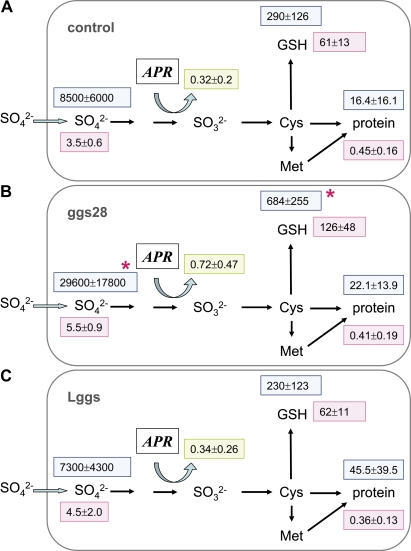
In the present study, GSH content was found to be 2-fold higher in the fine roots of line ggs28 (Figs 4C, 5B) and APR activity was also increased. As expected, overexpression of γ-ECS led to a significant increase in 35S flux into the GSH pool while the flux to proteins was not different compared with the WT control (Fig. 5B). Sulphate uptake was enhanced in ggs28 plants (Table 1), thus leading to increased 35S flux into the internal sulphate pool (Fig. 5). Consequently, the ratio between soluble and insoluble 35S was significantly enhanced.
Fig. 4.
Characteristics of γ-ECS, APR, and SO overexpressing poplar plants. Three to four independent lines expressing the γ-ECS gene from E. coli and targeting the protein either to the cytosol (line ggs28) or to plastids (lines Lggs6, Lggs12, and Lggs20) (A–C), four independent transgenic lines expressing APR from Lemna minor under the control of the 35S promoter (D–F), and six independent lines expressing SO (G–I) under the control of the 35S promoter (158-2, 159, and 161) and under the control of ST-LS1 (93, 150-1, and 185) were analysed. Sulphate contents (A, D, G), APR activity (B, E, H), and GSH contents (C, F, I) were determined in roots. The data presented are means ±SD from 3–7 independent measurements. Significant differences between the WT and transgenic lines at P <0.05 are indicated by asterisks.
Fig. 5.
Sulphur metabolite contents and 35S flux into different metabolite pools of fine roots from poplar plants overexpressing bacterial γ-ECS. Excised fine roots from the WT (A, n=6) and from transgenic poplar lines overexpressing γ-ECS targeted into either the cytosol (B, n=3) or plastids (C, n=11) were selected. Roots were exposed to [35S]sulphate for 4 h to measure sulphate uptake and 35S flux into different metabolite pools. Sulphate (μmol g−1 FW), GSH (nmol g−1 FW), and protein (mg g−1 FW) contents were determined (pink squares). 35S flux into internal sulphate, GSH, and protein is given as pmol 35S g−1 FW h−1 (light blue squares). APR activity is indicated in a green square as nmol mg−1 protein min−1. Significant differences between WT and transgenic poplar roots at P <0.05 are indicated by asterisks.
Targeting γ-ECS to the plastids in another set of transgenic lines (Lggs) increases GSH levels in leaves to the same extent as in the ggs lines where the enzyme is targeted to the cytosol (Noctor et al., 1998). However, flux analysis in fine roots of these lines did not reveal any differences from WT plants (Fig. 5C). Sulphate uptake (Table 1), 35S fluxes, APR activity, and metabolite levels were not different in Lggs and WT plants. Nevertheless, a significant reduction in the ratio between soluble and insoluble 35S was detected (Table 1).
APR overexpression:
Fifty independent kanamycin-resistant plantlets that overexpressed Lemna minor APR were grown in tissue culture and were tested for APR activity in the leaves. Four lines (303, 304, 391, and 404) with 17- to 72-fold increased foliar APR activity (data not shown) were selected and further propagated. Initially, the plants did not show any obvious morphological differences compared with WT poplars when grown on sandy soil, although with prolonged growth on perlite, sand, and humus soil, some alterations in leaf shape and increased branching were observed (Rennenberg et al., 2007). The increase in foliar APR activity was accompanied by a significant increase in foliar thiol levels (data not shown). In leaves, Cys and GSH were increased 2- to 4-fold in the transgenic plants, with the highest thiol levels in line 303 that also possessed the highest APR activity (data not shown). No consistent changes in the levels of sulphate were detected (data not shown). While the leaves of line 391 accumulated ∼50% more sulphate than the WT, sulphate levels in roots seemed, although not significantly, doubled in line 304 and 391, but remained unaffected in the other lines (Fig. 4D). All transgenic lines revealed constant GSH contents in fine roots (Fig. 4F) although the APR activity was up to 10-fold higher compared with the WT (Figs 4E, 6). Surprisingly, the high APR activity did not affect the flux of 35S into sulphate, thiols, and proteins. Also sulphate uptake rates of roots from APR overexpressing poplars did not differ from those of the WT (Fig. 6, Table 1).
Fig. 6.
Sulphur metabolite contents and 35S flux into different metabolite pools of fine roots from poplar plants overexpressing APR from Lemna. Excised fine roots from the WT (A, n=4) and from different transgenic poplar lines overexpressing Lemna APR (B, n=16) were selected and exposed to [35S]sulphate for 4 h to measure sulphate uptake and 35S flux into different metabolite pools. Sulphate (μmol g−1 FW), GSH (nmol g−1 FW), and protein (mg g−1 FW) contents were determined (pink squares). 35S flux into internal sulphate, GSH, and protein is given as pmol 35S g−1 FW h−1 (light blue squares). APR activity is indicated in a green square as nmol mg−1 protein min−1. Significant differences between WT and transgenic poplar roots at P <0.05 are indicated by asterisks.
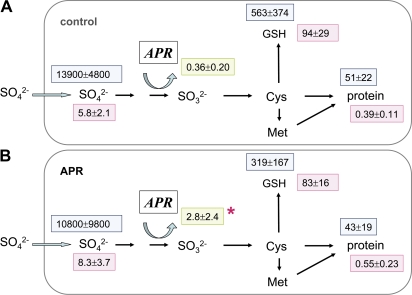
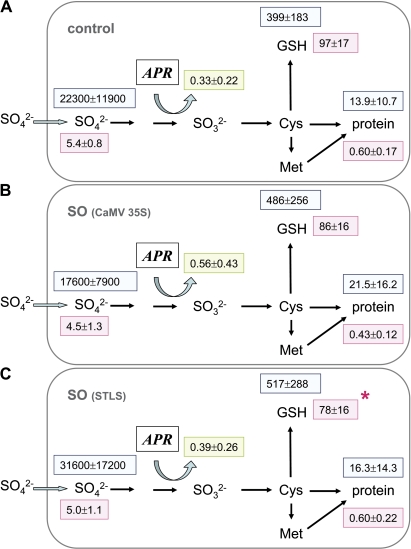
SO overexpression:
Overexpression of SO under the control of the constitutive CaMV 35S promoter (lines 158-2, 159, and 161) or under the control of a leaf-specific promoter (ST-LS1; Stockhaus et al., 1987) (lines 93, 150-1, and 185) did not lead to changes in sulphate uptake of the fine roots (Table 1). Similarly, 35S flux into the internal pools of sulphate, GSH, and protein was not different between transgenic poplar lines and WT controls (Fig. 7). Other parameters such as sulphate (Fig. 4G) and protein content as well as APR activity (Fig. 4H) of the roots remained unchanged. However, the GSH content was lower than in WT plants or lines overexpressing the gene under the control of the 35S promoter when the roots of all transgenic lines overexpressing SO under the control of the leaf-specific promoter ST-LS1 were combined (Fig. 7).
Fig. 7.
Sulphur metabolite contents and 35S flux into different metabolite pools of fine roots from poplar plants overexpressing SO. Excised fine roots from the WT (A, n=9) and from transgenic poplar lines overexpressing SO under the control of either the constitutive CaMV 35S promoter (B, n=9) or the leaf-specific promoter ST-LS1 (C, n=9) were selected. Roots were exposed to [35S]sulphate for 4 h to measure sulphate uptake and 35S flux into different metabolite pools. Sulphate (μmol g−1 FW), GSH (nmol g−1 FW), and protein (mg g−1 FW) contents were determined (pink squares). 35S flux into internal sulphate, GSH, and protein is given as pmol 35S g−1 FW h−1 (light blue squares). APR activity is indicated in a green square as nmol mg−1 protein min−1. Significant differences between WT and transgenic poplar roots at P <0.05 are indicated by asterisks.
Flux control coefficients
Flux control coefficients were calculated from the data of the presented experiments. Several conditions revealed that APR indeed is the main controller of sulphate assimilation. This was obvious from (i) OAS treatment (CJAPR=0.72); (ii) from transgenic poplars overexpressing γ-ECS and targeting the protein either to the cytosol (CJAPR=0.41) or to plastids (CJAPR=1.11); and (iii) from transgenic poplars overexpressing SO, either under the CaMV 35S promoter (CJAPR=0.71) or under the ST-LS1 promoter (CJAPR=0.49). However, this was not evident for certain stress conditions. Cd (CJAPR=0.003) and Acetochlor (CJAPR=–0.11) treatments and also analyses of roots from APR overexpressing poplar plants (CJAPR=–0.29) revealed that the flux control of sulphate assimilation via APR may be disturbed.
Discussion
There are four main ways by which the maximum catalytic activity of a given enzyme can be altered: inhibitors, induction/repression, mutation, and genetic manipulation (ap Rees and Hill, 1994). In the present S flux analysis study, APR activity was induced by OAS, Cd, and Acetochlor treatment, and several transgenic lines that overexpressed genes of the sulphur metabolism were used. The activity of the key enzyme of the sulphate assimilation pathway, APR, was determined to allow calculation of the flux control coefficient for the 35S flux into GSH and protein (Vauclare et al., 2002). After 4 h of incubation with [35S]sulphate, radioactivity was detected in all metabolic fractions, that is in sulphate, GSH, and proteins. The specific activity of the sulphate pool was always lower than the specific 35S activity in the GSH pool (Table 1), since the cytosolic [35S]sulphate pool was apparently diluted during extraction by sulphate from the vacuole. These results clearly show that vacuolar sulphate is not involved in GSH formation in poplar roots in the present study. The higher specific activity of the GSH pool can only be achieved when the [35S]sulphate taken up into the cytosol is immediately transported into plastids for sulphate assimilation and further GSH synthesis. Thus, for the model to calculate the flux control coefficient used in the present study, consideration of compartmentation of metabolites is unnecessary. However, these results support the assumption of a defined pathway leading from external sulphate into Cys that legitimizes the calculation of flux control coefficients. Cys itself is the first product and a branching point from which methionine, proteins, GSH, and secondary sulphur-containing compounds are produced (Bergmann and Rennenberg, 1993; Schnug, 1993). This is an essential pre-requisite for flux analyses after ap Rees and Hill (1994).
Control of sulphate assimilation by APR
The flux control coefficient calculated in the present study from OAS treatment indicates that sulphate assimilation in poplar roots is controlled by APR. OAS stimulates sulphate assimilation in potato (Hopkins et al., 2005) and in Arabidopsis (Koprivova et al., 2000; Hesse et al., 2003), and the flux of 35S into both GSH and protein in Arabidopsis roots (Koprivova et al., 2000). Also in the present experiments with excised poplar roots, OAS enhanced the 35S flux into GSH and protein, which correlated with higher 35S in the acid-soluble fraction and increased APR activity. The flux control coefficient estimated a control of the sulphur flux through the sulphate reduction and assimilation pathway by APR of 72%. This strong control is consistent with findings of Koprivova et al. (2000) on the effect of feeding various nitrogen compounds to N-starved Arabidopsis plants. The closer the N source was related metabolically to OAS the higher was the incorporation of 35S into GSH and protein. However, OAS can also act as a positive regulator of sulphate transporter transcription and sulphate uptake (Smith et al., 1997; Hopkins et al., 2005; Hawkesford and De Kok, 2006). In the present study, sulphate uptake was not induced in OAS-treated poplar roots. This observation agrees with findings from Arabidopsis. There it was shown that the expression of AtSULTR1;1 and AtSULTR1;2 did not reveal a relationship to the internal OAS content (Rouached et al., 2008). Thus, sufficient sulphate supply to the roots can be assumed under the experimental conditions, that is despite low N supply.
GSH levels cannot only be modulated by external application of metabolites, but also by genetic manipulation of enzymes involved in sulphate assimilation and GSH synthesis (reviewed in Rennenberg et al., 2007). Increased GSH contents in poplar were observed in glutathione reductase (GR) (Foyer et al., 1995), γ-ECS (Strohm et al., 1995; Noctor et al., 1996, 1998; Arisi et al., 1997), and APR overexpressing poplar (Rennenberg et al., 2007). The best characterized example of transgenic plants with increased GSH levels are poplars expressing the bacterial GSH biosynthesis genes (Strohm et al., 1995; Arisi et al., 1997; Noctor et al., 1998). Overexpression of γ-ECS and targeting the protein to the cytosol was associated with a 2- to 4-fold increase in GSH levels in leaves and in roots (Arisi et al., 1997; Herschbach et al. 2000; Hartmann et al., 2004).
Overexpression of γ-ECS, the control step in GSH synthesis (Strohm et al., 1995; Noctor et al., 1996), and targeting the protein to the cytosol led to increased GSH contents in roots (Fig. 4C, line ggs28) as also found in previous studies for the line ggs28 (Herschbach et al., 2000; Hartmann et al., 2004). As Cys was shown to limit GSH synthesis in leaves (Noctor et al., 1996), one pre-requisite for enhanced GSH synthesis is sufficient Cys formation. Hence, a higher sulphur flux through the sulphate assimilation pathway is required. This was indeed observed in the present experiments. 35S flux into GSH and proteins of transgenic poplar roots was significantly enhanced. Although poplar plants were grown at very low nitrogen nutrition, OAS seems not to be a limiting factor in this transgenic line. Thus APR activity increased and the flux control coefficient calculated for the APR activity revealed a control of only ∼40%. Vauclare et al. (2002) have already demonstrated the importance of sulphate uptake as a controlling step in sulphate assimilation. Sulphate uptake rates were not enhanced with the numbers of replicates in the present study. However, increased sulphate uptake rates have been demonstrated in an earlier experiment (Herschbach et al., 2000). The high variance of sulphate uptake rates by roots excised from poplar plants was observed across all experiments and results from a high standard deviation (Table 1). Nevertheless, the higher specific activity of the sulphate pool and the increase in soluble 35S indicate a higher sulphate uptake for ggs28 also in the present study. Thus, sulphate assimilation in roots of the transgenic poplar line ggs28 seems to be controlled by both sulphate uptake and APR activity.
In contrast, when γ-ECS was targeted to the natural subcellular localization of APR, the plastids (Kopriva, 2006), the calculated flux control coefficient of APR was slightly above 1. To the best of our knowledge no flux control coefficient higher than 1 has been published and it is also unrealistic, though theoretically possible where pathways branch or cycle (Fell, 1992). It may therefore be possible that the pathway of sulphate assimilation up to GSH considered in this study neglected one or more enzymatic step(s). When γ-ECS is targeted to plastids it may be speculated that the reverse reaction from sulphite to sulphate probably via SO counteracts APR activity in vivo.
Nevertheless, the present results from Lggs transgenic poplar lines indicate a very strong control of the sulphur flux through the sulphate assimilation pathway by APR in the roots. Since neither the flux nor the APR activity was significantly different between transgenic and WT roots, it seems that the high flux control coefficient reflects a high control of the pathway already in the WT. The fact that, in contrast to the ggs28 line, APR is not affected in the roots of Lggs plants may explain why the flux of sulphate into GSH is higher in the former than in WT and Lggs roots. Traditionally, it is thought that GSH is synthesized in both the plastids and the cytosol. However, Wachter et al. (2005) showed that at least in Arabidopsis, γ-ECS is exclusively localized in the plastids while a major part of GSHS is also found in the cytosol. Thus, it can be assumed that γ-EC is synthesized in plastids and is transported into the cytosol to be converted into GSH (Mullineaux and Rausch, 2005; Wachter et al., 2005; Pasternak et al., 2008). If this is also true for poplar roots, the export of γ-EC from plastids could limit GSH synthesis in Lggs transgenic poplar plants whereas targeting γ-ECS to the cytosol is not connected with such a barrier and allows increased GSH synthesis. Consequently, APR activity is increased when γ-ECS is targeted to the cytosol to provide the reduced sulphur necessary to sustain the increased GSH synthesis, but not when the γ-ECS is targeted to the plastids.
SO catalyses the back reaction from sulphite to sulphate, however, localized in peroxisomes. Thus, this enzyme is clearly separated spatially from the sulphate reduction pathway (Nowak et al., 2004; Hänsch and Mendel, 2005), in particular from APR which is located exclusively in plastids (Kopriva and Koprivova, 2004). The biological function of SO is still under debate (Hänsch et al., 2007). One established function is the detoxification of sulphite that is important especially under high atmospheric SO2 (Lang et al., 2007). However, SO is constitutively expressed in all tested tissues without any pronounced diurnal rhythm (Hänsch et al., 2007) and, thus, SO may be an essential housekeeping protein. Theoretically SO overexpression enforces the back reaction of APR (Nowak et al., 2004; Hänsch and Mendel, 2005). As SO may act against the normal flow of sulphate assimilation, overexpression of SO may diminish the availability of free sulphite for further reduction and Cys formation. Nevertheless, the flux control coefficients calculated from analyses of roots from transgenic poplar overexpressing SO revealed a control via APR of 72% and 49% for the sulphur flux through the sulphate assimilation pathway. Thus, the present results did not provide any indication that SO significantly disturbed or reduced the control of sulphate assimilation by APR. A further approach to test the capacity of SO keeping sulphite at non-toxic levels could be a simultaneous overexpression of both APR and SO.
Possible disturbance of the control of sulphate assimilation by APR
Despite numerous previous reports and the present results with γ-ECS or SO overexpressing plants, APR was found not to be important for the pathway control under several environmental conditions. When APR was overexpressed in poplar and when WT plants were treated with Cd or Acetochlor, negative flux control coefficients were calculated for APR. It seems that in these cases the control of sulphate assimilation via APR activity is disturbed. The common reason could be that under these conditions APR activity was at least 5-fold enhanced compared with the controls. Nevertheless, Cd treatment and APR overexpression did not enhance GSH levels in root tissues. In leaves of APR overexpressing poplars GSH levels enhanced to a similar extent were found when other enzymes of the sulphur assimilation pathway were overexpressed (reviewed in Rennenberg et al., 2007). Thus, the 35S flux through sulphate assimilation into GSH seems to be subject to an additional control. When Pseudomonas aeruginosa APR (PaAPR) was overexpressed in Arabidopsis, a strong de-regulation of sulphate assimilation, evident from increased levels of sulphite, thiosulphate, Cys, γ-EC, and GSH, was observed and led to plant injury (Tsakraklides et al., 2002). Increasing GSH contents were also found in Zea mays overexpressing the same gene (Martin et al., 2005), but not in roots of transgenic poplar lines overexpressing Lemna APR, although leaf Cys and GSH contents increased (C Herschbach, unpublished results). As Cys formation in transgenic Arabidopsis overexpressing PaAPR might be limited by the availability of OAS (Tsakraklides et al., 2002), nitrogen limitation in the present experiment may also limit OAS formation in poplar roots and, as a consequence, Cys and GSH formation. Therefore, at very high APR activity sulphate assimilation seems to be controlled at another metabolic step. From the results presented for the OAS treatment of poplar roots (increased flux into GSH, see above) the most likely step that may control Cys and GSH synthesis in APR overexpressing poplar roots seems to be the synthesis of OAS via serine acetyltransferase.
After long-term Cd exposure, APR activity increased and the flux of 35S through sulphate assimilation seems not to be controlled by APR, as indicated by the flux control coefficient. Similar to the findings of the present study, APR activity was also found to be increased upon Cd treatment in excised roots from Brassica juncea (Lee and Leustek, 1999). The increased APR activity indicates a high need for reduced sulphur. Surprisingly, the flux of 35S into GSH and protein of poplar roots was not affected. Moreover a diminished rate of sulphate uptake resulted in a reduction of 35S in the soluble fraction inside the roots. Cd can react with thiol groups of proteins and may inhibit sulphate uptake by a deactivation of sulphate transporter protein as a consequence of covalent binding. In contrast, increased uptake of sulphate and higher expression of SULTR genes have been observed in Zea mays upon Cd exposure (Nocito et al., 2002, 2006). In the present study, 35S flux into GSH of Cd-treated poplar roots was not affected at reduced sulphate uptake. This indicates the preferential channelling of the sulphate that has been taken up into GSH synthesis. Under these conditions, the selected pathway with GSH and protein as an end-product does not take into consideration synthesis of PCs as additional end-products. Thus, under Cd exposure, flux analyses should be extended to PCs for the correct calculation of the flux control coefficient. This assumption is consistent with the observation that the GSH content was unchanged in poplar roots upon Cd exposure, as also found in previous studies (Koprivova et al., 2002). In these studies, long-term Cd treatment left the GSH content unaffected, but increased the PC content in Brassica juncea (Zhu et al., 1999a, b) and poplar (Koprivova et al., 2002), whereas a decline in GSH after short-term Cd exposure corresponded to enhanced PC contents in maize roots (Nocito et al., 2002, 2006). PCs are involved in heavy metal detoxification (Rauser, 1995, 1999; Cobbett, 2000a, b), and sulphur flux into PCs may be an additional sink of 35S taken up by the roots under Cd exposure. It may therefore be assumed that taking into consideration 35S flux into PCs can restore the control via APR, or that phytochelatin synthase constitutes a controlling step of sulphate assimilation under Cd exposure. In addition, the low nitrogen supply during plant growth may limit Cys synthesis via OAS availability. However, since all enzymes of the sulphate reduction pathway can be affected by Cd treatment (Ernst et al., 2008), it cannot be excluded that the loss of control of sulphur flux through the sulphate assimilation pathway by APR is a consequence of the interaction of Cd with other enzymes.
Short-term application of Acetochlor resulted in a low contribution of APR to the control of sulphate assimilation. Although APR activity increased dramatically and the GSH content decreased, changes in the flux of 35S into GSH, sulphate, or proteins were not found. The slight increase in the specific 35S activity of the GSH pool may be an indication for a preferential channelling of the sulphate taken up into GSH for Acetochlor detoxification. Thus, processes involved in Acetochlor conjugation with GSH and the degradation of the conjugation product such as the reactions catalysed by GST (Edwards et al., 2000) and/or phytochelatin synthase (Grzam et al., 2006; Blum et al., 2007) may execute flux control under these conditions. GST consumes GSH by its conjugation with xenobiotics and thereby contributes to herbicide detoxification. Thus, synthesis of GSH conjugates may have reduced the GSH content in poplar roots exposed to Acetochlor and may have removed GSH from the pools measured for flux determination. GSH conjugates, synthesized when Acetochlor is bound to GSH via GST (Jablonkai and Hatzios, 1991; Mezzari et al., 2005; Cho and Kong, 2008), are thought to be transported into the vacuole for further metabolism (Coleman et al., 1997a, b). Recently phytochelatin synthase was shown to be involved in this metabolism (Grzam et al., 2006; Blum et al., 2007). Hence, both enzymes, GST and phytochelatin synthase, may take over the control of the flux through the sulphate assimilation pathway during xenobiotic exposure. On the other hand, as mentioned above, calculation of 35S flux rates into PCs and GSH conjugates may restore the control via APR. However, also during Acetochlor treatment OAS limitation due to the low nitrogen supply may be limiting Cys synthesis under high demand.
Experimental constraints of flux analyses through the sulphate assimilation pathway
For estimation of the flux control coefficient of APR, Vauclare et al. (2002) defined several assumptions: (i) for a given treatment there are no routes other than their effect on APR. This means that the change in APR activity must be specific for the treatments, that is for the present study for OAS, Cd, or Acetochlor exposure. This assumption was not confirmed in the current experiments with Cd and Acetochlor exposure. In these experiments, further metabolic steps that include PC synthesis and GSH–Acetochlor conjugation must be taken into account when the control via APR is discussed. (ii) The sulphate assimilation pathway starts at internal sulphate. Even Vauclare et al. (2002) calculated that sulphate uptake contributes significantly to the control of sulphur flux through the sulphate assimilation pathway. Hence under Cd treatment where sulphate uptake was reduced and in the transgenic poplar line ggs28 that revealed a higher sulphate uptake, the sulphate uptake step has to be taken into account for metabolic control analysis. (iii) ATPS is near equilibrium and exerts little control, so that the internal sulphate reflects the APS concentration.
Other important assumptions concerning application of metabolic control analysis have been summarized by ap Rees and Hill (1994). The metabolites are distributed evenly throughout the tissue, cells, and compartments. The sulphate pool measured includes the sulphate from the cytosol and all organelles including the vacuole. As the vacuolar sulphate does not seem to contribute significantly to the flux through sulphate assimilation up to GSH, metabolic control analysis can be applied. Furthermore, caution is advisable when transgenic plants are analysed (ap Rees and Hill, 1994). It is crucial that only the enzyme in question is changed. In the case of APR overexpression it has not been shown that other enzymes of sulphate assimilation are affected. In the case of γ-ECS overexpression either in the cytosol (ggs28) or in plastids (Lggs), and in the case of SO overexpression, APR activity was not affected. In conclusion, calculation of the flux control coefficient in the present study revealed some critical aspects, but nevertheless provided important information on the control of sulphur flux through the sulphate assimilation pathway.
Conclusion
The presented results show that the control of sulphur flux through the sulphate assimilation pathway in poplar roots was not apparent under certain conditions. This seems to be the case during Cd and Acetochlor treatment as well as in transgenic poplar overexpressing Lemna APR. Sulphur flux through the sulphate assimilation pathway may also be controlled by ATPS (Lappartient et al., 1999) or by the serine acetyltransferase/O-acetylserine(thiol)lyase complex (Wirtz and Hell, 2006). Furthermore, whether the primary site for Cys synthesis is in the mitochondria (Heeg et al., 2008) or in the cytosol (Krueger et al., 2009) is still under discussion. Therefore, differentiated analyses of the sulphur flux through the sulphate assimilation pathway that include separation of the sulphur pools of each compartment are required. A complete realistic view thus can only be obtained if all enzymatic steps of the sulphate assimilation pathway with their corresponding flux control coefficients are determined and if all metabolites that integrate 35S are analysed in further studies. This is of great importance because analyses of APR overexpression showed complete loss of control of sulphur flux via APR, whereas this was not the case when γ-ECS was overexpressed. Hence other component(s) of sulphate assimilation seem to take over the control in APR overexpressing poplar. On the other hand, APR overexpression and also γ-ECS overexpression did not affect sulphate assimilation only in roots but also in the shoot (Hartmann et al., 2004). GSH can be transported in the phloem to the roots (Hartmann et al., 2000). Thus, in addition, whole plant interactions, most importantly shoot to root signalling, may also influence the sulphur flux through the sulphate assimilation pathway in roots. This may also be important during Cd exposure when GSH and PCs increased in leaves (Koprivova et al., 2002). Furthermore, under conditions where GSH is increasingly used for other metabolic pathways such as PC synthesis and herbicide detoxification via GST, the sulphur flux into these compounds needs to be investigated.
Acknowledgments
This work was financially supported by the Deutsche Forschungsgemeinschaft (DFG) under contract numbers RE 515/11, RE 515/32-1, and the GRK 1305. The authors thank Professor David Fell (Oxford Brookes University) for very helpful discussions on the calculation of flux control coefficients.
References
- ap Rees T, Hill SA. Metabolic control analysis of plant metabolism. Plant, Cell and Environment. 1994;17:587–599. [Google Scholar]
- Arisi A-CM, Noctor G, Foyer CH, Jouanin L. Modification of thiol contents in poplars (Populus tremula× P. alba) overexpressing enzymes involved in glutathione synthesis. Planta. 1997;203:362–372. doi: 10.1007/s004250050202. [DOI] [PubMed] [Google Scholar]
- Bergmann L, Rennenberg H. Glutathione metabolism in plants. In: De Kok LJ, Stulen I, Rennenberg H, Brunold C, Rauser W, editors. Sulphur nutrition and assimilation in higher plants. The Hague: SPB Academic Publishing; 1993. pp. 109–123. [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Research. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J-A, Setterdahl AT, Lnaff DB, Chen Y, Pitcher LH, Zilinskas BA, Leustek T. Regulation of the plant-type 5’ adenylyl sulphate reductase by oxidative stress. Biochemistry. 2001;40:9040–9048. doi: 10.1021/bi010518v. [DOI] [PubMed] [Google Scholar]
- Blum R, Beck A, Korte A, Stengel A, Letzel T, Lenzian K, Grill E. Function of phytochelatin synthase in catabolism of glutathione-conjugates. The Plant Journal. 2007;49:740–749. doi: 10.1111/j.1365-313X.2006.02993.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brunold C, Suter M. Regulation of sulphate assimilation by nitrogen nutrition in the duckweed Lemna minor L. Plant Physiology. 1984;76:579–583. doi: 10.1104/pp.76.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunold C, Suter M. Adenosine 5′-phosphosulphate sulfotransferase. In: Lea P, editor. Methods in plant biochemistry. Vol. 3. London: Academic Press; 1990. pp. 339–343. [Google Scholar]
- Cairns NG, Pasternak M, Wachter A, Cobbett CS, Meyer AJ. Maturation of Arabidopsis seeds is dependent on glutathione biosynthesis within the embryo. Plant Physiology. 2006;141:446–455. doi: 10.1104/pp.106.077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H-Y, Kong K- H. Study on the biochemical characterization of herbicide detoxification enzyme, glutathione S-transferase. Biofactors. 2008;30:281–287. doi: 10.1002/biof.5520300410. [DOI] [PubMed] [Google Scholar]
- Cobbett CS. Phytochelatins and their roles in heavy metal detoxification. Plant Physiology. 2000a;123:825–832. doi: 10.1104/pp.123.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett CS. Phytochelatin biosynthesis and function in heavy-metal detoxification. Current Opinion in Plant Biology. 2000b;3:211–216. [PubMed] [Google Scholar]
- Coleman JOD, Blake-Kalff MMA, Davies TGE. Detoxification of xenobiotics by plants: chemical modification and vacuolar compartmentation. Trends in Plant Science. 1997a;2:144–151. [Google Scholar]
- Coleman JOD, Randall R, Blake-Kalff MMA. Detoxification of xenobiotics in plant cells by glutathione conjugation and vacuolar compartimentalization: a fluorescent assay using monochlorobimane. Plant, Cell and Environment. 1997b;20:449–460. [Google Scholar]
- Edwards R, Dixon DP, Walbot V. Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends in Plant Science. 2000;5:193–198. doi: 10.1016/s1360-1385(00)01601-0. [DOI] [PubMed] [Google Scholar]
- Eilers T, Schwarz G, Brinkmann H, Witt C, Richter T, Nieder J, Koch B, Hille R, Hänsch R, Mendel RR. Identification and biochemical characterization of Arabidopsis thaliana sulphite oxidase. A new player in plant sulphur metabolism. Journal of Biological Chemistry. 2001;276:46989–46994. doi: 10.1074/jbc.M108078200. [DOI] [PubMed] [Google Scholar]
- Ernst WHO, Krauss G-J, Verkleij JAA, Wesenberg D. Interaction of heavy metals with the sulphur metabolism in angiosperms from an ecological point of view. Plant, Cell and Environment. 2008;31:123–143. doi: 10.1111/j.1365-3040.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- Fell DA. Metabolic control analysis: a survey of its theoretical and experimental development. Biochemical Journal. 1992;286:313–330. doi: 10.1042/bj2860313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell DA. Increasing the flux in metabolic pathways: a metabolic control analysis perspective. Biotechnology and Bioengineering. 1998;58:121–124. doi: 10.1002/(sici)1097-0290(19980420)58:2/3<121::aid-bit2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Foyer C, Noctor G. Redox homeostasis and antioxidant signalling: a metabolic interface between stress perception and physiological responses. The Plant Cell. 2005;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C, Rennenberg H. Regulation of glutathione synthesis and its role in abiotic and biotic stress defence. In: Brunold C, Rennenberg H, De Kok LJ, Stulen I, Davidian JC, editors. Sulphur nutrition and sulphur assimilation in higher plants: molecular, biochemical and physiological aspects. Bern, Switzerland: Paul Haupt Publishers; 2000. pp. 127–153. [Google Scholar]
- Foyer C, Souriau N, Lelandais M, Kunert K-J, Pruvost C, Jouanin L. Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiology. 1995;109:1047–1057. doi: 10.1104/pp.109.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzam A, Tennstedt P, Clemens S, Hell R, Meyer AJ. Vacuolar sequestration of glutathione S-conjugates outcompetes a possible degradation of the glutathione moiety by phytochelatin synthase. FEBS Letters. 2006;580:6384–6390. doi: 10.1016/j.febslet.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Hänsch R, Lang C, Rennenberg H, Mendel RR. Significance of plant sulphite oxidase. Plant Biology. 2007;9:589–595. doi: 10.1055/s-2007-965433. [DOI] [PubMed] [Google Scholar]
- Hänsch R, Lang C, Wüstefeld Y, Riebeseel E, Lindigkeit R, Gessler A, Rennenberg H, Mendel RR. Plant sulphite oxidase as a novel producer of H2O2: combination of enzyme catalysis with a subsequent non-enzymatic reaction step. Journal of Biological Chemistry. 2006;281:6884–6888. doi: 10.1074/jbc.M513054200. [DOI] [PubMed] [Google Scholar]
- Hänsch R, Mendel RR. Sulphite oxidation in plant peroxisomes. Photosynthesis Research. 2005;86:337–343. doi: 10.1007/s11120-005-5221-x. [DOI] [PubMed] [Google Scholar]
- Harada E, Kusano T, Sano H. Differential expression of genes encoding enzymes involved in sulphur assimilation pathways in response to wounding and jasmonate in Arabidopsis thaliana. Journal of Plant Physiology. 2000;156:272–276. [Google Scholar]
- Hartmann T, Hönicke P, Wirtz M, Hell R, Rennenberg H, Kopriva S. Sulphate assimilation in poplars (Populus tremula× P. alba) overexpressing γ-glutamylcysteine synthetase in the cytosol. Journal of Experimental Botany. 2004;55:837–845. doi: 10.1093/jxb/erh094. [DOI] [PubMed] [Google Scholar]
- Hartmann T, Mult S, Suter M, Rennenberg H, Herschbach C. Leaf age-dependent differences in sulphur assimilation and allocation in poplar (Populus tremula× P. alba) leaves. Journal of Experimental Botany. 2000;51:1077–1088. doi: 10.1093/jexbot/51.347.1077. [DOI] [PubMed] [Google Scholar]
- Hawkesford MJ, De Kok LJ. Managing sulphur metabolism in plants. Plant, Cell and Environment. 2006;29:382–395. doi: 10.1111/j.1365-3040.2005.01470.x. [DOI] [PubMed] [Google Scholar]
- Heeg C, Kruse C, Jost R, Gutensohn M, Ruppert T, Wirtz M, Hell R. Analysis of the Arabidopsis O-acetylserine(thiol)lyase gene family demonstrates compartment-specific differences in the regulation of cysteine synthesis. The Plant Cell. 2008;20:168–185. doi: 10.1105/tpc.107.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschbach C, Rennenberg H. Storage and remobilization of sulphur in beech trees (Fagus sylvatica) Physiologia Plantarum. 1996;98:125–132. [Google Scholar]
- Herschbach C, van der Zalm E, Schneider A, Jouanin L, De Kok LJ, Rennenberg H. Regulation of sulphur nutrition in wild-type and transgenic poplar over-expressing γ-glutamylcysteine synthetase in the cytosol as affected by atmospheric H2S. Plant Physiology. 2000;124:461–473. doi: 10.1104/pp.124.1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse H, Nikiforova V, Galière B, Hoefgen R. Molecular analysis and control of cysteine biosynthesis: integration of nitrogen and sulphur metabolism. Journal of Experimental Botany. 2004;55:1283–1292. doi: 10.1093/jxb/erh136. [DOI] [PubMed] [Google Scholar]
- Hesse H, Trachsel N, Suter M, Kopriva S, von Ballmoos P, Rennenberg H, Brunold C. Effect of glucose on assimilatory sulphate reduction in roots of Arabidopsis thaliana. Journal of Experimental Botany. 2003;54:1701–1709. doi: 10.1093/jxb/erg177. [DOI] [PubMed] [Google Scholar]
- Hirai MY, Fujiwara T, Awazuhara M, Kimura T, Noji M, Saito K. Global expression profiling of sulphur-starved Arabidopsis by DNA macroarray reveals the role of O-acetyl-l-serine as a general regulator of gene expression in response to sulphur nutrition. The Plant Journal. 2003;33:651–663. doi: 10.1046/j.1365-313x.2003.01658.x. [DOI] [PubMed] [Google Scholar]
- Hirai MY, Fujikawa Y, Yano M, et al. Clustering of sulphur-assimilation gene-family members for comprehensive prediction of their functions. In: Saito K, DeKok LJ, Stulen I, Hawkesford MJ, Schnug E, Sirko A, Rennenberg H, editors. Sulphur transport and assimilation in plants in the post genomic era. Leiden, The Netherlands: Backhuys Publishers; 2005. pp. 183–186. [Google Scholar]
- Hopkins L, Parmar S, Blaszczyk A, Hesse H, Hoefgen H, Hawkesford MJ. O-Acetylserine and the regulation of expression of genes encoding components for sulphate uptake and assimilation in potato. Plant Physiology. 2005;138:433–440. doi: 10.1104/pp.104.057521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins L, Parmar S, Bouranis DL, Howarth JR, Hawkesford MJ. Coordinated expression of sulphate uptake and components of the sulphate assimilatory pathway in maize. Plant Biology. 2004;6:408–414. doi: 10.1055/s-2004-820872. [DOI] [PubMed] [Google Scholar]
- Jablonkai I, Hatzios KK. Role of glutathione and glutathione S-transferase in the selectivity of acetochlor in maize and wheat. Pesticide Biochemistry and Physiology. 1991;41:221–231. [Google Scholar]
- Kacser H, Burns JA. The control of flux. Symposium of the Society of Experimental Biology. 1973;27:65–104. [reprinted in Biochemical Society Transactions23, 341–366 (1995)] [PubMed] [Google Scholar]
- Kawashima CG, Berkowitz O, Hell R, Noji M, Saito K. Characterization and expression analysis of a serine acetyltransferase gene family involved in a key step of the sulphur assimilation pathway in Arabidopsis. Plant Physiology. 2005;137:220–230. doi: 10.1104/pp.104.045377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopriva S. Regulation of sulphate assimilation in Arabidopsis and beyond. Annals of Botany. 2006;97:479–495. doi: 10.1093/aob/mcl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopriva S, Hartmann T, Massaro G, Hönicke P, Rennenberg H. Regulation of sulphate assimilation by nitrogen and sulphur nutrition in poplar trees. Trees. 2004;18:320–326. [Google Scholar]
- Kopriva S, Koprivova A. Plant adenosine 5′-phosphosulphate reductase: the past, the present, and the future. Journal of Experimental Botany. 2004;55:1775–1783. doi: 10.1093/jxb/erh185. [DOI] [PubMed] [Google Scholar]
- Kopriva S, Muheim R, Koprivova A, Trachsel N, Catalano C, Suter M, Brunold C. Light regulation of assimilatory sulphate reduction in Arabidopsis thaliana. The Plant Journal. 1999;20:37–44. doi: 10.1046/j.1365-313x.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- Kopriva S, Rennenberg H. Control of sulphate assimilation and glutathione synthesis: interaction with N and C metabolism. Journal of Experimental Botany. 2004;55:1831–1842. doi: 10.1093/jxb/erh203. [DOI] [PubMed] [Google Scholar]
- Kopriva S, Suter M, von Ballmoos P, Hesse H, Krähenbühl U, Rennenberg H, Brunold C. Interaction of sulphate assimilation with carbon and nitrogen metabolism in Lemna minor. Plant Physiology. 2002;130:1406–1413. doi: 10.1104/pp.007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivova A, Kopriva S, Jäger D, Will B, Jouanin L, Rennenberg H. Evaluation of transgenic poplars over-expressing enzymes of glutathione synthesis for phytoremediation of cadmium. Plant Biology. 2002;4:664–670. [Google Scholar]
- Koprivova A, North KA, Kopriva S. Complex signaling network in regulation of adenosine 5’ phosphosulphate reductase by salt stress in Arabidopsis roots. Plant Physiology. 2008;146:1408–1420. doi: 10.1104/pp.107.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivova A, Suter M, Op den Camp R, Brunold C, Kopriva S. Regulation of sulphate assimilation by nitrogen in Arabidopsis. Plant Physiology. 2000;122:737–746. doi: 10.1104/pp.122.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger S, Niehl A, Martin MCL, Steinhauser D, Donath A, Hildebrandt T, Romero LC, Hoefgen R, Gotor C, Hesse H. Analysis of cytosolic and plastidic serine acetyltransferase mutants and subcellular metabolite distributions suggests interplay of the cellular compartments for cysteine biosynthesis in Arabidopsis. Plant, Cell and Environment. 2009;32:349–367. doi: 10.1111/j.1365-3040.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- Lang C, Popko J, Wirtz M, Hell R, Herschbach C, Kreuzwieser J, Rennenberg H, Mendel RR, Hänsch R. Sulphite oxidase as key enzyme for protecting plants against sulphur dioxide. Plant, Cell and Environment. 2007;30:447–455. doi: 10.1111/j.1365-3040.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- Lappartient AG, Touraine B. Demand-driven control of root ATP sulphurylase activity and SO42–uptake in intact canola. The role of phloem-translocated glutathione. Plant Physiology. 1996;111:147–157. doi: 10.1104/pp.111.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappartient AG, Vidmar JJ, Leustek T, Glass ADM, Touraine B. Inter-organ signaling in plants: regulation of ATP sulphurylase and sulphate transporter genes expression in roots mediated by phloem-translocated compound. The Plant Journal. 1999;18:89–95. doi: 10.1046/j.1365-313x.1999.00416.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Leustek T. The affect of cadmium on sulphate assimilation enzymes in Brassica juncea. Plant Science. 1999;141:210–207. [Google Scholar]
- Leplé JC, Brasileiro ACM, Michel MF, Delmotte F, Jouanin L. Transgenic poplars: expression of chimeric genes using four different constructs. Plant Cell Reports. 1992;11:137–141. doi: 10.1007/BF00232166. [DOI] [PubMed] [Google Scholar]
- Leustek T, Martin MN, Bick J-A, Davies JP. Pathway and regulation of sulphur metabolism revealed through molecular and genetic studies. Annual Review of Plant Physiology and Plant Molecular Biology. 2000;51:141–165. doi: 10.1146/annurev.arplant.51.1.141. [DOI] [PubMed] [Google Scholar]
- Libourel IGL, Shachar-Hill Y. Metabolic flux analysis in plants: from intelligent design to rational engineering. Annual Review of Plant Biology. 2008;59:625–650. doi: 10.1146/annurev.arplant.58.032806.103822. [DOI] [PubMed] [Google Scholar]
- Martin MN, Tarczynski MC, Shen B, Leustek T. The role of 5′-adenylylsulphate reductase in controlling sulphate reduction in plants. Photosynthesis Research. 2005;86:309–323. doi: 10.1007/s11120-005-9006-z. [DOI] [PubMed] [Google Scholar]
- Maruyama-Nakashita A, Inoue E, Watanabe-Takahashi A, Yarnaya T, Takahashi H. Transcriptom profiling of sulphur-responsive genes in Arabidopsis reveals global effects of sulphur nutrition on multiple metabolic pathways. Plant Physiology. 2003;132:597–605. doi: 10.1104/pp.102.019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AJ. The integration of glutathione homeostasis and redox signalling. Journal of Plant Physiology. 2008;165:1390–1403. doi: 10.1016/j.jplph.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Hell R. Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynthesis Research. 2005;86:435–457. doi: 10.1007/s11120-005-8425-1. [DOI] [PubMed] [Google Scholar]
- Mezzari MP, Walters K, Jelíkova M, Shih M-C, Just CL, Schnoor JL. Gene expression and microscopic analysis of Arabidopsis exposed to chloracetanilide hebicides and explosive compounds. A phytoremediation approach. Plant Physiology. 2005;138:858–869. doi: 10.1104/pp.104.056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux PM, Rausch T. Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynthesis Research. 2005;86:459–474. doi: 10.1007/s11120-005-8811-8. [DOI] [PubMed] [Google Scholar]
- Neuenschwander U, Suter M, Brunold C. Regulation of sulphate assimilation by light and O-acetyl-l-serine in Lemna minor L. Plant Physiology. 1991;97:252–258. doi: 10.1104/pp.97.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforova V, Freitag J, Kempa S, Adamik M, Hesse H, Hoefgen R. Transcriptome analysis on sulphur depletion in Arabidopsis thaliana: interlacing of biosynthetic pathways provides response specificity. The Plant Journal. 2003;33:633–647. doi: 10.1046/j.1365-313x.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- Nocito FF, Lancilli C, Crema B, Fourcroy P, Davidian J-C, Sacchi GA. Heavy metal stress and sulphate uptake in maize roots. Plant Physiology. 2006;141:1138–1148. doi: 10.1104/pp.105.076240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocito FF, Pirovano L, Cocucci M, Sacchi GA. Cadmium-induced sulphate uptake in maize roots. Plant Physiology. 2002;129:1872–1879. doi: 10.1104/pp.002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Arisi A-CM, Jouanin L, Foyer CH. Manipulation of GSH and amino acid biosynthesis in the chloroplast. Plant Physiology. 1998;118:471–482. doi: 10.1104/pp.118.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Strohm M, Juoanin L, Kunert K-J, Foyer C, Rennenberg H. Synthesis of glutathione in leaves of transgenic poplar (Populus tremula× P. alba) overexpressing γ-glutamylcysteine synthetase. Plant Physiology. 1996;112:1071–1078. doi: 10.1104/pp.112.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak K, Luniak N, Witt C, Wüstefeld Y, Wachter A, Mendel RR, Hänsch R. Peroxisomal localization of sulphite oxidase separates it from chloroplast-based sulphur assimilation. Plant and Cell Physiology. 2004;45:1889–1894. doi: 10.1093/pcp/pch212. [DOI] [PubMed] [Google Scholar]
- Nussbaum S, Schmutz D, Brunold C. Regulation of assimilatory sulphate reduction by cadmium in Zea mays L. Plant Physiology. 1988;88:1407–1410. doi: 10.1104/pp.88.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkama N, Takei K, Sakakibara H, Hayashi H, Yoneyama T, Fujiwara T. Regulation of sulphur responsive gene expression by exogenously applied cytokinins in Arabidopsis thaliana. Plant and Cell Physiology. 2002;43:1493–1501. doi: 10.1093/pcp/pcf183. [DOI] [PubMed] [Google Scholar]
- Pasternak M, Lim B, Wirtz M, Hell R, Cobbett CC, Meyer A. Restricting glutathione biosynthesis to the cytosol is sufficient for normal plant development. The Plant Journal. 2008;53:999–1012. doi: 10.1111/j.1365-313X.2007.03389.x. [DOI] [PubMed] [Google Scholar]
- Peuke A, Rennenberg H. Phytoremediation: molecular biology, requirements for application, environmental protection, public attention, and feasibility. EMBO Reports. 2005a;6:497–501. doi: 10.1038/sj.embor.7400445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuke AD, Rennenberg H. Phytoremediation with transgenic trees. Zeitschrift für Naturforschung. 2005b;60c:199–207. [PubMed] [Google Scholar]
- Ratcliffe RG, Shachar-Hill Y. Measuring multiple fluxes through plant metabolic networks. The Plant Journal. 2006;45:490–511. doi: 10.1111/j.1365-313X.2005.02649.x. [DOI] [PubMed] [Google Scholar]
- Rauser WE. Phytochelatins and related peptides. Plant Physiology. 1995;109:1141–1149. doi: 10.1104/pp.109.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauser WE. Structure and function of metal chelators produced by plants. Cell Biochemistry and Biophysics. 1999;31:19–48. doi: 10.1007/BF02738153. [DOI] [PubMed] [Google Scholar]
- Rennenberg H, Herschbach C, Haberer K, Kopriva S. Sulphur metabolism in plants: are trees different? Plant Biology. 2007;9:620–637. doi: 10.1055/s-2007-965248. [DOI] [PubMed] [Google Scholar]
- Reuveny Z, Dougall DK, Trinity PM. Regulatory coupling of nitrate and sulphate assimilation pathways in cultured tobacco cells. Proceedings of the National Academy of Sciences, USA. 1980;77:6670–6672. doi: 10.1073/pnas.77.11.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouached H, Wirtz M, Alary R, Hell R, Arpat AB, Davidian J-C, Fourcroy P, Berthomieu P. Differential regulation of the expression of two high-affinity sulphate transporters, SULTR1.1 and SULTR1.2, in Arabidopsis. Plant Physiology. 2008;147:897–911. doi: 10.1104/pp.108.118612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnug E. Physiological functions and environmental relevance of sulphur-containing secondary metabolites. In: De Kok LJ, Stulen I, Rennenberg H, Brunold C, Rauser W, editors. Sulphur nutrition and assimilation in higher plants. The Hague: SPB Academic Publishing; 1993. pp. 179–190. [Google Scholar]
- Schupp R, Rennenberg H. Diurnal changes in the glutathione concentration of spruce needles (Picea abies L.) Plant Science. 1988;57:113–117. [Google Scholar]
- Smith FW, Hawkesford MJ, Ealing PM, Clarkson DT, van den Berg PJ, Belcher AR, Warrilow AG. Regulation of expression of a cDNA from barely roots encoding a high affinity sulphate transporter. The Plant Journal. 1997;12:875–884. doi: 10.1046/j.1365-313x.1997.12040875.x. [DOI] [PubMed] [Google Scholar]
- Stockhaus J, Eckes P, Blau A, Schell J, Willmitzer L. Organ-specific and dosage-dependent expression of a leaf/stem specific gene from potato after tagging and transfer into potato and tobacco plants. Nucleic Acids Research. 1987;15:3479–91. doi: 10.1093/nar/15.8.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohm M, Jouanin L, Kunert KJ, Pruvost C, Polle A, Foyer C, Rennenberg H. Regulation of GSH synthesis in leaves of transgenic poplar (Populus tremula× P. alba) overexpressing GSH synthetase. The Plant Journal. 1995;7:141–145. [Google Scholar]
- Suter M, von Ballmoos P, Kopriva S, Op den Camp R, Schaller J, Kuhlemeier C, Schürmann P, Brunold C. Adenosine-5′-phosphosulphate sulfotransferase and adenosine 5′-phosphosulphate reductase are identical enzymes. Journal of Biological Chemistry. 2000;275:13383–13388. doi: 10.1074/jbc.275.2.930. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yamazaki M, Sasakura N, Watanabe A, Leustek T, de Almeida Engler J, Engler G, van Montagu M, Saito K. Regulation of sulphur assimilation in higher plants: a sulphate transporter induced in sulphur-starved roots plays a central role in Arabidopsis thaliana. Proceedings of National Academy of Sciences, USA. 1997;94:11102–11107. doi: 10.1073/pnas.94.20.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakraklides G, Martin M, Chalam R, Tarczynski MC, Schmidt A, Leustek T. Sulphate reduction is increased in transgenic Arabidopsis thaliana 5’ adenylylsulphate reductase from Pseudomonas aeruginosa. The Plant Journal. 2002;32:879–889. doi: 10.1046/j.1365-313x.2002.01477.x. [DOI] [PubMed] [Google Scholar]
- Vauclare P, Kopriva S, Fell D, Suter M, Sticher L, von Ballmoos P, Krähenbühl U, Op den Camp R, Brunold C. Flux control of sulphate assimilation in Arabidopsis thaliana: adenosine 5′-phosphosulphate reductase is more susceptible than ATP sulphurylase to negative control by thiols. The Plant Journal. 2002;31:729–740. doi: 10.1046/j.1365-313x.2002.01391.x. [DOI] [PubMed] [Google Scholar]
- Wachter A, Wolf S, Steininger H, Bogs J, Rausch T. Differential of GSH1 and GSH2 is achieved by multiple transcription initiation: implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. The Plant Journal. 2005;41:15–30. doi: 10.1111/j.1365-313X.2004.02269.x. [DOI] [PubMed] [Google Scholar]
- Westerman S, Stulen I, Suter M, Brunold C, De Kok LJ. Atmospheric H2S as sulphur source for Brassica oleracea: consequences for the activity of the enzymes of the assimilatory sulphate reduction pathway. Plant Physiology and Biochemistry. 2001;39:425–432. [Google Scholar]
- Wirtz M, Hell R. Functional analysis of the cyteine synthase protein complex from plants: structural, biochemical and regulatory properties. Journal of Plant Physiology. 2006;163:273–286. doi: 10.1016/j.jplph.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Zhu YL, Pilon-Smits EAH, Tarun AS, Weber SU, Jouanin L, Terry N. Overexpression of glutathione synthetase in Indian mustard enhanced cadmium accumulation and tolerance. Plant Physiology. 1999a;119:73–80. doi: 10.1104/pp.119.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YL, Pilon-Smits EAH, Tarun AS, Weber SU, Jouanin L, Terry N. Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing gamma-glutamyl-cysteine synthetase. Plant Physiology. 1999b;121:1169–1177. doi: 10.1104/pp.121.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]